2020-03-02 17:17:47
Mechanism of the ABC phenomenon
According to Dams et al. [39], in animal models, a second dose of PEGylated liposomes, injected within a time interval of 5 and 21 days, was cleared very rapidly from the blood circulation. The ABC phenomenon showed two distinct phases: the induction phase and the effectuation phase. The induction phase followed the initial dose of PEGylated liposomes in which the biological system is ‘primed’. The effectuation phase occurs at day 3–7 after the initial dose in which a subsequent dose of PEGylated liposomes is rapidly cleared from systemic circulation [42].
Dams et al. [39] demonstrated that serum transfu- sions into normal rats from rats pretreated with PEGylated liposomes also elicited the enhanced blood clearance of a first dose of PEGylated liposomes. In addition, they reported that the phenomenon was abolished either when the rat serum was preheated at 56°C for 30 min prior to transfusion, which inactivate complement system, or when antibodies in the rat serum were removed by other means. These results support the existence of a circulating opsonizing factor that is a heat labile non-antibody molecule, which results in the rapid clearance of a second dose via phagocytosis by liver macrophages [39].
Our group has been intensively studying the mechanism behind the ABC phenomenon. We have confirmed that the ABC phenomenon against PEGylated liposomes occurred in mice [43], rats [43], minipigs [44] and beagle dogs [45] for empty liposomes (containing no drug). We further showed that anti-PEG IgM production and the resulting ABC phenomenon triggered by PEGylated liposomes occurred in BALB/c nu/nu (T cell-deficient) mice, but not in SCID (T and B cells-deficient) mice. A similar observation was reported by Semple et al.
[46] with nude and SCID-Rag2 mice. They showed that B cells and immunoglobulins, but not T cells, are critical in the development of immune responses against PEGylated liposomes containing oligonucleo- tides. These data prove that anti-PEG IgM, produced by the splenic B cells that are independent of T-cell involvement, could play a significant role in the induction of the ABC phenomenon.
The so-called class-2 of thymus-independent antigens (TI-2) may provide an additional explanation for the mechanism of the ABC phenomenon induced by PEGylated liposomes. TI-2 antigens can induce an immunological response by extensively cross-linking the cell surface immunoglobulins of specific B cells, resulting in secretion of massive amounts of neutralizing antibodies including IgM and IgG from the B cells [47,48]. Cheng and co-workers [49,50] have demon- strated that anti-PEG antibody (IgM) obtained following immunization with PEGylated β-glucuronide recognizes the repeating -(O-CH2-CH2)n- subunit (16 units) of PEG. This raises the assumption that PEG polymer in PEGylated nanocarriers acts as a TI-2 antigen and the repeating subunit may be an immunogenic epitope of PEG and a binding site for the derived anti-PEG IgM.
Based on all the above-mentioned reports, we con- firmed that the ABC phenomenon is divided into two phases: the induction phase and effectuation phase. In the induction phase, the biological system is ‘primed’ by promoting the proliferation and differentiation of specific B cells (responsible for the antibody produc- tion and cell-mediated immune responses) in the marginal zone of the spleen in T-cell independent manner, resulting in anti-PEG IgM formation [51– 54]. The delayed effectuation phase is manifested from day 5 to day 21 after the initial dose, which is closely correlated with the time course for anti-PEG IgM production in response to initial dose. In this effectuation phase, a subsequent dose of PEGylated liposomes is rapidly opsonized by C3 fragments and cleared from the systemic circulation by the Kupffer cells in coordination with anti-PEG IgM and the complement system, as shown in Figure 1. Further, the magnitude of the ABC phenomenon is closely linked to the magnitude of anti-PEG IgM production by splenic B cells in response to an initial injected dose of PEGylated liposomes [55,56]. However, although removal of spleen from mice reduced anti- PEG IgM production, it failed to completely reverse the rapid clearance of PEGylated liposomes to normal control levels. This suggests that another serum factor(s) or cell(s) may contribute to this phenom- enon [43].
Once the PEGylated liposomes (first dose) reach the spleen, they bind and crosslink to surface immunoglo- bulins on reactive B cells in the splenic marginal zone and consequently trigger the production of an anti-PEG IgM that is independent of T-cell help. Upon adminis- tration of the second dose, if anti-PEG IgM, produced in response to the first dose, still exists in the blood circulation, it binds to the PEG on the liposomes, and subsequently activates the complement system, result- ing in opsonization by C3 fragments and enhanced uptake by Kupffer cells via complement receptor- mediated endocytosis.
Factors affecting the ABC phenomenon
There are many factors that have an impact on the ABC phenomenon including: animal species, lipid dose, time interval between injections, nanoparticle physicochemical properties and type of encapsulated drug (Figure 2).
Animal species
In various types of animal models (rats, rabbits, mice, guinea pigs, minipigs and beagle dogs), repeated intra- venous injection of PEGylated liposomes has been reported to elicit the ABC phenomenon [39,42]. However, the magnitude of the elicited ABC phenom- enon varied with the animal species. Suzuki et al. [44] reported that anti-PEG IgM production and the ABC phenomenon were not detected in mice treated with Doxil® (2 and 20 mg DXR/m2), while in minipigs trea- ted with Doxil® (2 mg DXR/m2), anti-PEG IgM produc- tion and the ABC phenomenon were significantly increased. Beagle dogs seemed to be more sensitive to Doxil® than rodents in triggering anti-PEG IgM and the ABC phenomenon [45]. This might be due to variations in the sensitivity of the immune system between dogs and rodents or due to differences in the pharmacoki- netics of the initial dose of PEGylated liposomes [45].
Lipid dose
There appears to be a strong inverse relationship between the extent of the ABC phenomenon and the phospholi- pid dose for the initial dose of PEGylated liposomes, regardless of liposomal phospholipid composition. The higher the lipid dose, the lower the ABC phenomenon [57,58]. We speculate that low concentrations of phos- pholipid could activate marginal zone B cells (MZ-B), and trigger anti-PEG IgM production, while higher initial doses of PEGylated liposomes could cause MZ-B to elicit immunological tolerance or anergy [59,60]. MZ- B cells are a specialized population of B cells that are located in the marginal zone of the spleen. They secrete antibodies that help to protect against blood-borne viruses and bacteria. Rats injected intravenously with doses higher than 5 μmol phospholipid/kg did not exhi- bit increased levels of anti-PEG IgM and the subsequent ABC phenomenon. On the other hand, the ABC phe- nomenon was significantly increased at phospholipid doses less than 1 μmol phospholipid/kg [58,61,62]. Such difference also might be due to difference in the pharmacokinetics of PEGylated liposomes and/or reac- tivity of MZ-B with PEGylated liposomes in different species as described above.
Time interval between injections
The induction and the magnitude of the ABC phenom- enon were dependent on the time between injections. Some reports demonstrated that no alteration in the clearance of encapsulated drugs was observed with repeat injections of PEGylated liposomes when the interval between the initial and subsequent injection was less than 2 days or more than 4 weeks [63,64]. Actually, Dams et al. [39] and our group [51,53,58] showed that the extent of the ABC phenomenon was highest when the interval between two injections was from 4 to 7 days. This could be explained by the fact that the production of anti-PEG IgM occurred by 3–4 days after the initial dose [54,57] and the IgM disappeared within its biological half-life of 3 weeks [65].
Physicochemical properties of liposomes
The physicochemical properties of PEGylated liposomes such as size, lipid composition, extent of PEGylation and surface charge, all affected the extent of the ABC phenomenon. It has been reported that ‘classical’ (non- PEGylated) liposomes also induced the ABC phenom- enon when PEGylated liposomes were given as a subsequent dose [66]. We investigated the influence of surface charge and liposome size on the occurrence of the ABC phenomenon in mice. The ABC phenomenon was not induced when the initial dose of liposomes exhibited three different mean particle sizes (100, 400 and 800 nm) and three different surface potentials (+13,15, – 46.15 and −1.51 mV) [66]. On the other hand, the lipid composition of the liposomes did affect the appearance of the ABC phenomenon, e.g. use of phospholipids with long satu- rated acyl chains and inclusion of membrane stabilizers such as cholesterol and PEGylated lipid. In rats, the effect of phospholipid types in the initial dose of PEGylated liposomes on the ABC phenomenon was investigated using five different types of phospholipids (hydrogenated soy phosphatidylcholine, egg sphingomyelin, soybean phosphatidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phos- phocholine and egg phosphatidylcholine). All PEGylated liposomes composed of different phospholipids signifi- cantly induced the ABC phenomenon. The PEGylated liposomes composed of unsaturated phospholipid induced the phenomenon more than the PEGylated lipo- somes composed of saturated phospholipid [66,67].
Encapsulated drug
It has been reported that the drugs encapsulated in PEGylated liposomes strongly affected the ABC phe- nomenon. PEGylated liposomes containing anticancer drugs such as DXR, oxaliplatin (l-OHP) and mitoxan- trone result in no induction of the ABC phenomenon [68–71]. We can hypothesize that chemotherapeutic agents encapsulated in liposomes accumulate in immune cells and hinder anti-PEG IgM production by inhibiting B cell proliferation and/or by damaging B cells in the marginal zone [70]. Indeed, there is no evidence that the clinical use of Doxil® causes the ABC phenomenon. However, Suzuki et al. [44] reported that intravenous injection of Doxil® at very tiny doses (0.2 mg/m2) triggers the ABC phenomenon in various animal species. These small doses contain very low total doses of lipids and low total doses of the drug. The low levels of DXR in small amounts of injected Doxil® may be below the threshold for inhibiting B cell secretion of anti-PEG IgM. A similar observation was reported by Nagao et al. [57] who demonstrated that intravenous injection of l-OHP- containing PEGylated liposomes at low doses (0.023 μg l-OHP/kg) could trigger a relatively high anti-PEG IgM response. On the other hand, at higher doses of l-OHP- containing PEGylated liposomes (2.3–2300 μg l-OHP/ kg), the l-OHP tended to inhibit the anti-PEG IgM response. Hence, low doses of PEGylated liposomal for- mulations in Phase 1 dose-escalation trials could concei- vably induce anti-PEG IgM and the ABC phenomenon confounding analysis of the Phase I pharmacokinetics. Furthermore, since low total doses of lipids trigger the ABC phenomenon to a greater extent than higher doses, this may also play a role in Suzuki’s observations.
Recent reports have demonstrated that multiple injections of PEGylated liposomes containing topotecan in beagle dogs and Wistar rats could trigger the ABC phe- nomenon [72,73]. Topotecan is a cell cycle-specific drug that exerts its inhibitory action in the S phase of the cell cycle. One can hypothesize that topotecan might not affect the B cell proliferation for B cells in other phases of the cell cycle and still allow them to produce some anti-PEG IgM. Another, more convincing hypothesis is that the rapid release of topotecan from liposomes could result in empty (drug-free) liposomes or drug-depleted liposomes in the blood circulation. The interaction of such drug-free or drug-depleted liposomes with the B cells in spleen could induce the production of anti- PEG IgM, rather than inhibiting its production, and lead to the induction of the ABC phenomenon upon repeated intravenous administration.
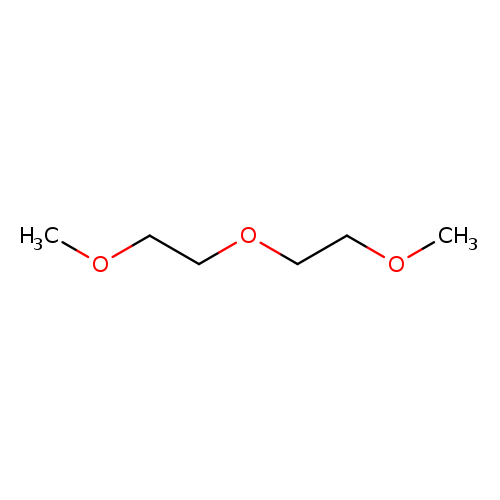
Diethylene glycol dimethyl etherCatalog No.:AA0033AW CAS No.:111-96-6 MDL No.:MFCD00008503 MF:C6H14O3 MW:134.1736 |
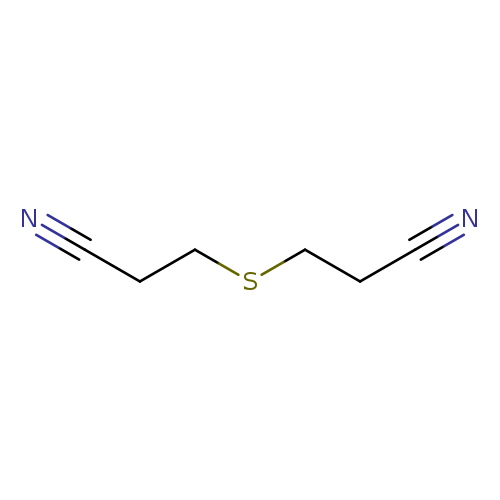
3,3-THIODIPROPIONITRILECatalog No.:AA003IB8 CAS No.:111-97-7 MDL No.:MFCD00013829 MF:C6H8N2S MW:140.2061 |
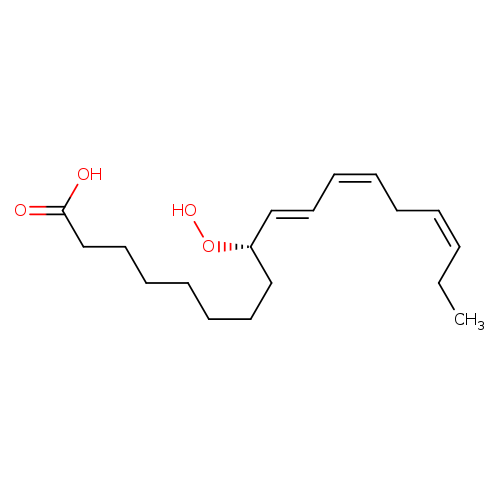
9(S)-HPOTCatalog No.:AA008RAS CAS No.:111004-08-1 MDL No.:MFCD01632746 MF:C18H30O4 MW:310.4284 |
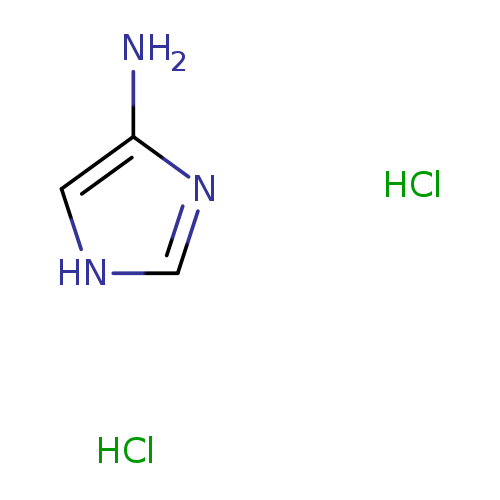
1H-Imidazol-4-amine dihydrochlorideCatalog No.:AA0039FG CAS No.:111005-19-7 MDL No.:MFCD16987731 MF:C3H7Cl2N3 MW:156.0138 |
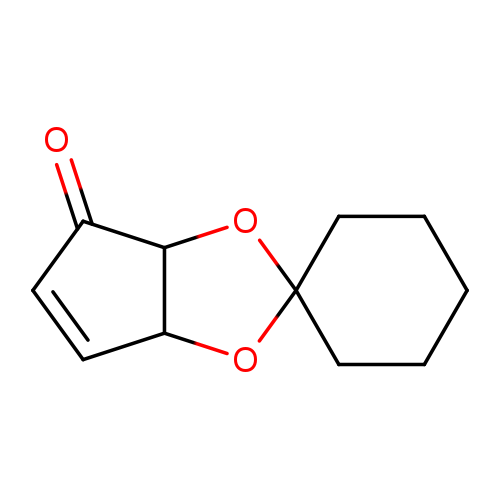
(1R,2R)-1,2-Dihydroxy-3-cyclopropen-5-one 1,2-Cyclohexyl KetalCatalog No.:AA008WYW CAS No.:111005-65-3 MDL No.:MFCD23105261 MF:C11H14O3 MW:194.2271 |
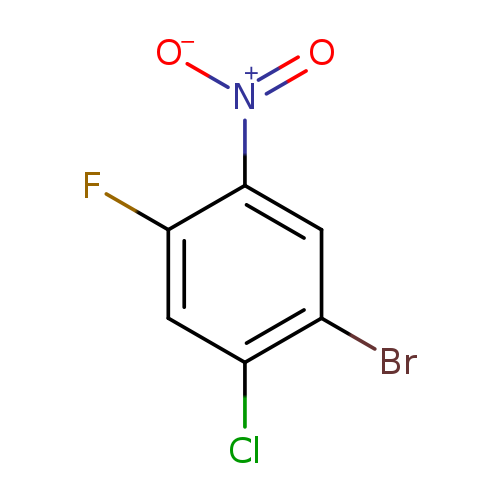
1-Bromo-2-chloro-4-fluoro-5-nitrobenzeneCatalog No.:AA007BMD CAS No.:111010-08-3 MDL No.:MFCD20528320 MF:C6H2BrClFNO2 MW:254.4410 |
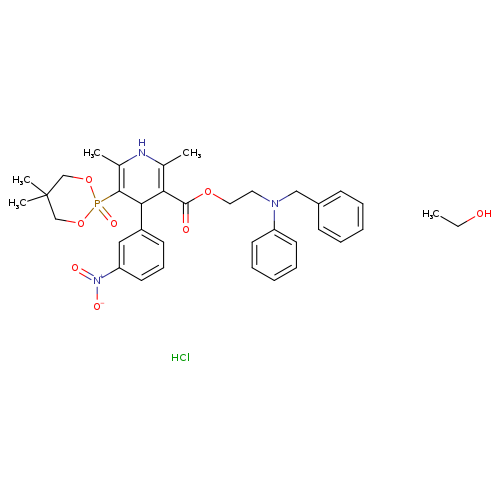
Efonidipine hydrochloride monoethanolateCatalog No.:AA0082WD CAS No.:111011-76-8 MDL No.:MFCD00875717 MF:C36H45ClN3O8P MW:714.1846 |
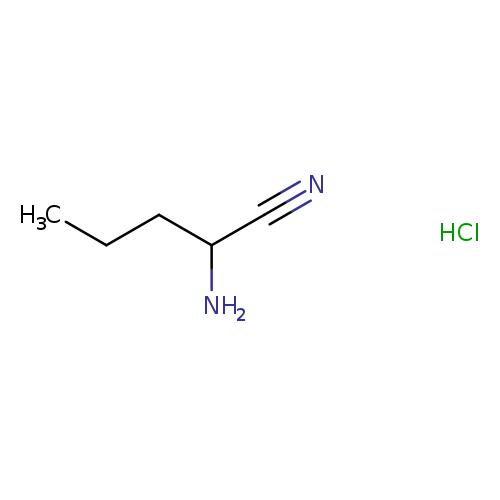
2-Aminopentanenitrile hydrochlorideCatalog No.:AA019N2R CAS No.:111013-52-6 MDL No.:MFCD11505443 MF:C5H11ClN2 MW:134.6072 |
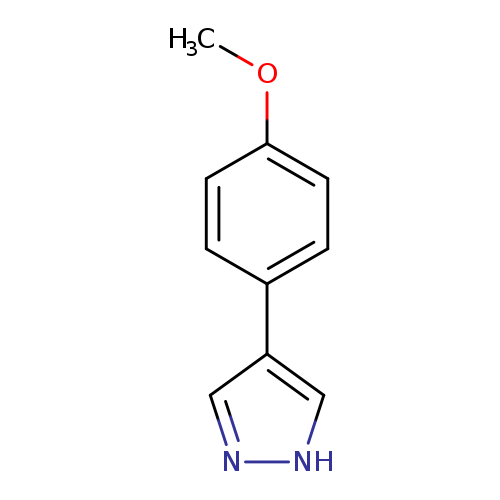
4-(4-Methoxyphenyl)-1H-pyrazoleCatalog No.:AA0099TM CAS No.:111016-45-6 MDL No.:MFCD03646977 MF:C10H10N2O MW:174.1992 |
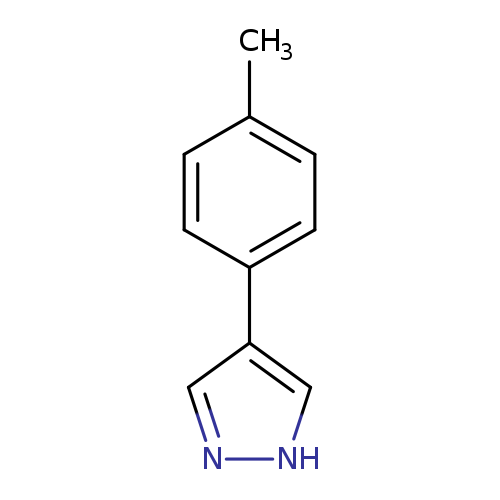
4-(4-Methylphenyl)-1H-pyrazoleCatalog No.:AA00HBPT CAS No.:111016-46-7 MDL No.:MFCD03646991 MF:C10H10N2 MW:158.1998 |
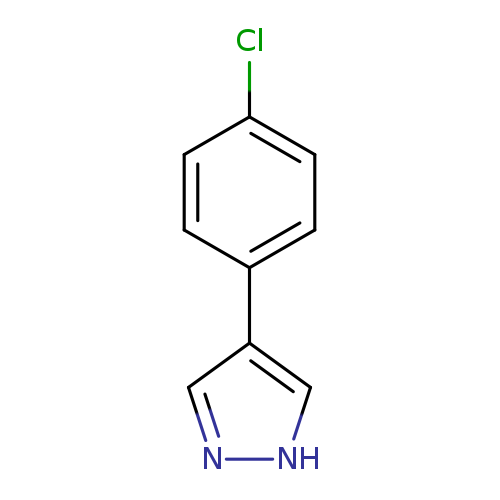
4-(4-Chlorophenyl)-1H-pyrazoleCatalog No.:AA0082WB CAS No.:111016-47-8 MDL No.:MFCD03647018 MF:C9H7ClN2 MW:178.6183 |
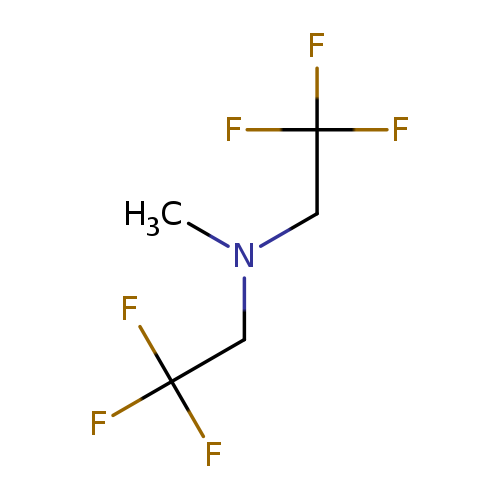
Methyl bis(2,2,2-trifluoroethyl)amineCatalog No.:AA01FCSW CAS No.:111016-65-0 MDL No.:MFCD31654086 MF:C5H7F6N MW:195.1062 |
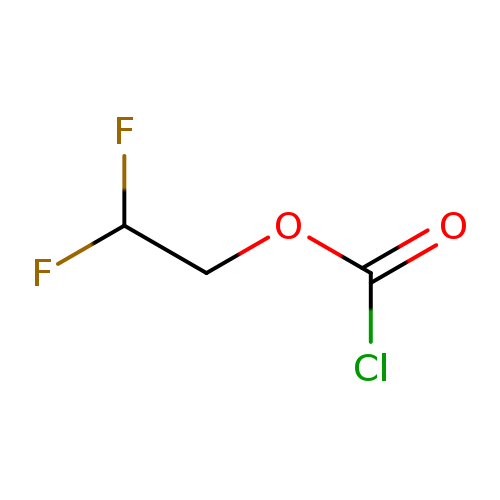
2,2-Difluoroethyl chloroformateCatalog No.:AA00HBPU CAS No.:111022-16-3 MDL No.:MFCD24141311 MF:C3H3ClF2O2 MW:144.5045 |
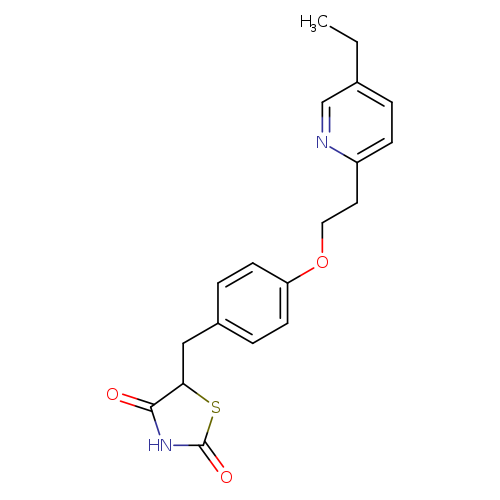
5-({4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}methyl)-1,3-thiazolidine-2,4-dioneCatalog No.:AA00HBPV CAS No.:111025-46-8 MDL No.:MFCD00865504 MF:C19H20N2O3S MW:356.4387 |
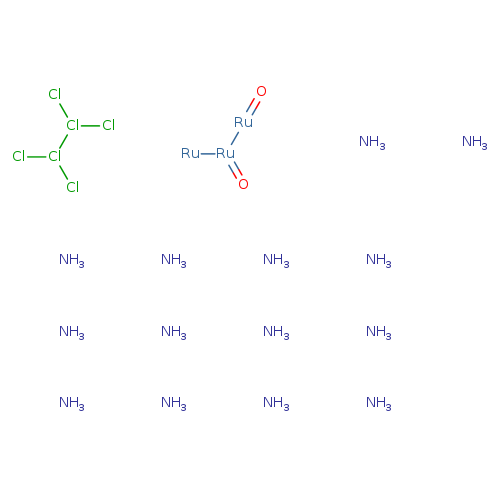
RUTHENIUM REDCatalog No.:AA008R4J CAS No.:11103-72-3 MDL No.:MFCD00011479 MF:Cl6H42N14O2Ru3 MW:786.3541 |
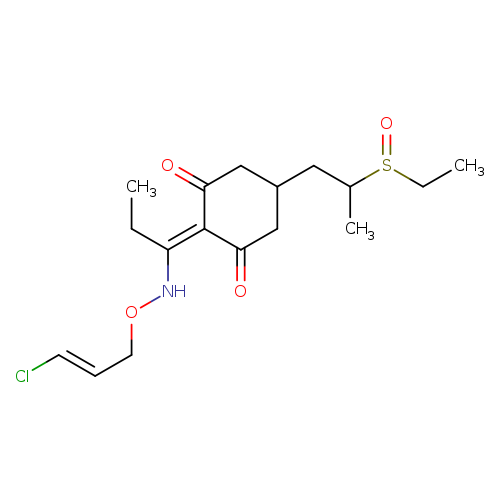
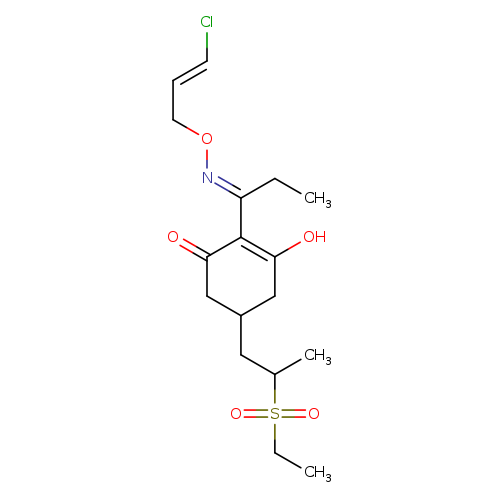
2-(1-(((3-Chloroallyl)oxy)imino)propyl)-5-(2-(ethylsulfinyl)propyl)-3-hydroxycyclohex-2-enoneCatalog No.:AA008VR5 CAS No.:111031-14-2 MDL No.:MFCD21363545 MF:C17H26ClNO4S MW:375.9106 |
ClethodiM SulfoneCatalog No.:AA008X4Z CAS No.:111031-17-5 MDL No.:MFCD21363546 MF:C17H26ClNO5S MW:391.9100 |
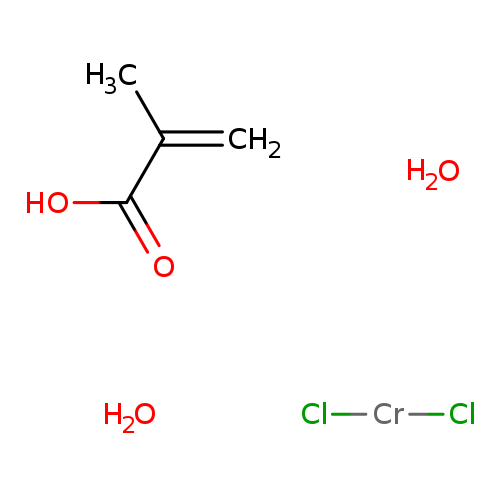
VOLAN(R) BONDING AGENTCatalog No.:AA008UR6 CAS No.:111031-82-4 MDL No.: MF:C4H10Cl2CrO4 MW:245.0219 |
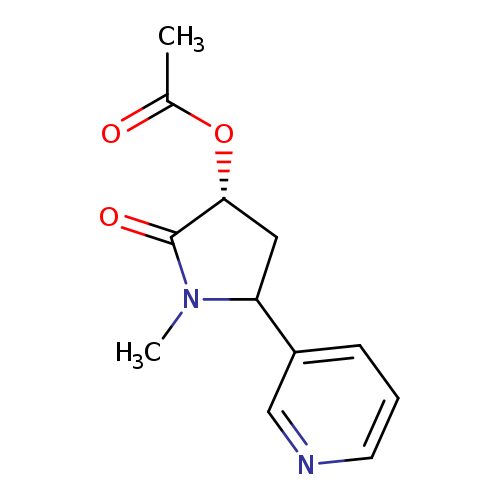
(3'R,5'S)-3'-Hydroxycotinine AcetateCatalog No.:AA007BM5 CAS No.:111034-55-0 MDL No.:MFCD09864794 MF:C12H14N2O3 MW:234.2512 |
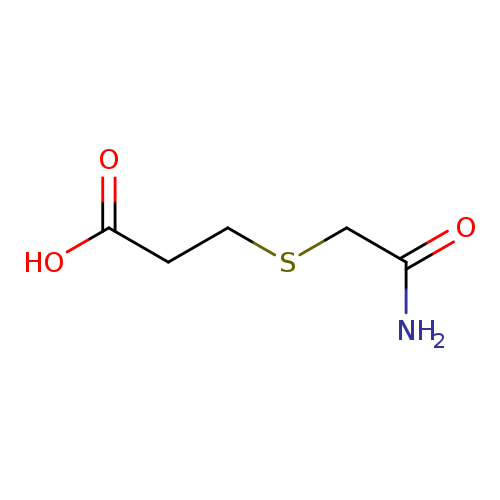
3-[(carbamoylmethyl)sulfanyl]propanoic acidCatalog No.:AA019LC7 CAS No.:111035-51-9 MDL No.:MFCD09971379 MF:C5H9NO3S MW:163.1949 |
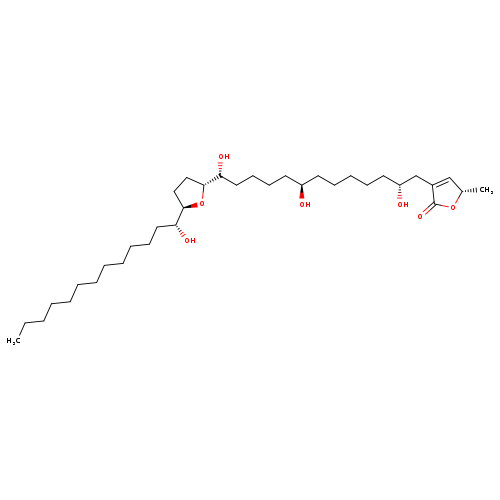
AnnonacinCatalog No.:AA008Y71 CAS No.:111035-65-5 MDL No.:MFCD27664980 MF:C35H64O7 MW:596.8785 |
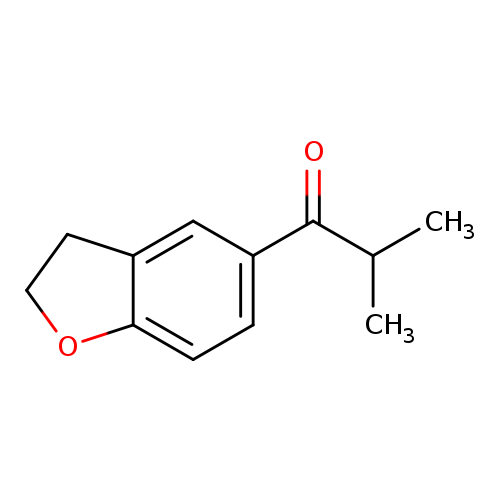
1-(2,3-dihydro-1-benzofuran-5-yl)-2-methylpropan-1-oneCatalog No.:AA01A8AY CAS No.:111038-58-5 MDL No.:MFCD11205116 MF:C12H14O2 MW:190.2384 |
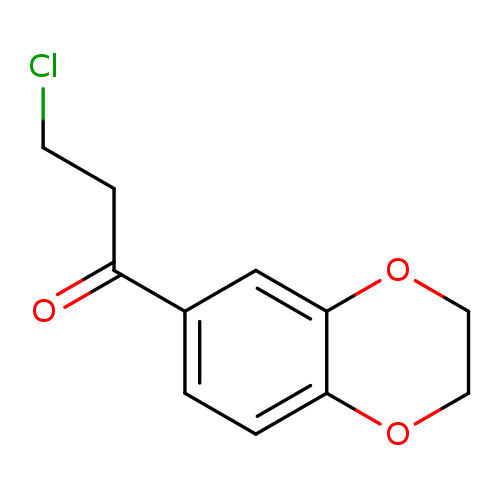
3-chloro-1-(2,3-dihydro-1,4-benzodioxin-6-yl)propan-1-oneCatalog No.:AA01BF4H CAS No.:111038-91-6 MDL No.:MFCD11868925 MF:C11H11ClO3 MW:226.6562 |
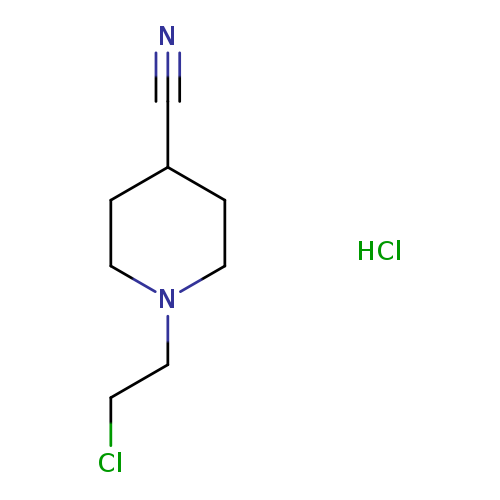
1-(2-Chloroethyl)piperidine-4-carbonitrile hydrochlorideCatalog No.:AA008ZDR CAS No.:111041-03-3 MDL No.:MFCD00203877 MF:C8H14Cl2N2 MW:209.1162 |
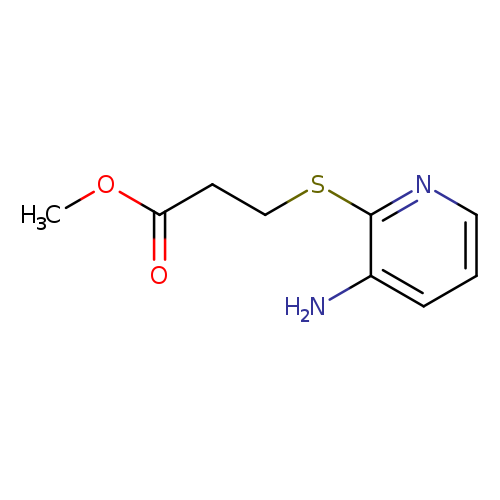
methyl 3-[(3-aminopyridin-2-yl)sulfanyl]propanoateCatalog No.:AA01A6RX CAS No.:111042-83-2 MDL No.:MFCD12092176 MF:C9H12N2O2S MW:212.2688 |
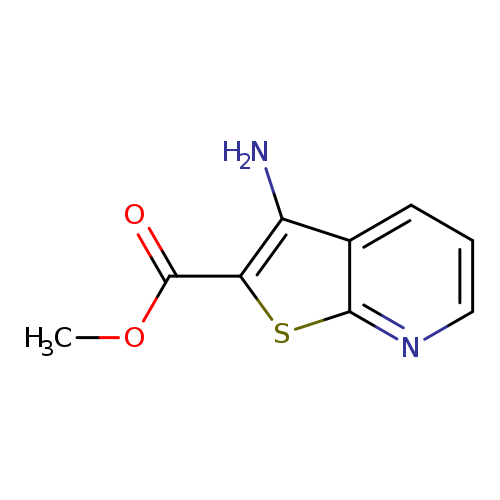
Methyl 3-aminothieno[2,3-b]pyridine-2-carboxylateCatalog No.:AA0082VW CAS No.:111042-89-8 MDL No.:MFCD00174679 MF:C9H8N2O2S MW:208.2370 |
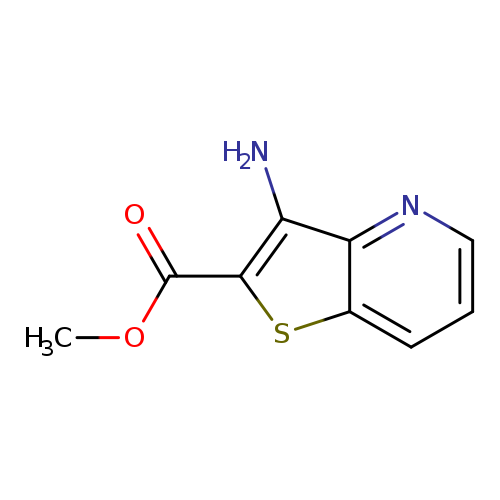
Methyl 3-aminothieno[3,2-b]pyridine-2-carboxylateCatalog No.:AA007BHU CAS No.:111042-90-1 MDL No.:MFCD08448152 MF:C9H8N2O2S MW:208.2370 |
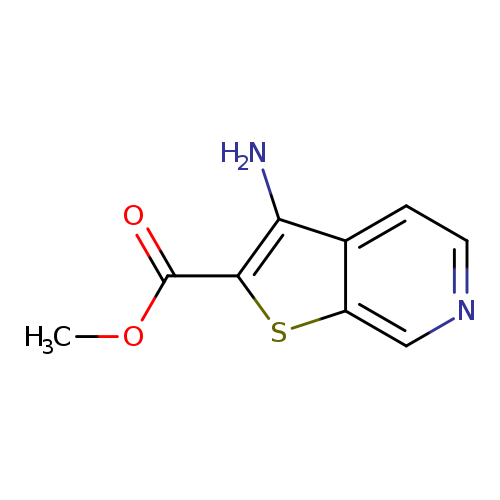
methyl 3-aminothieno[2,3-c]pyridine-2-carboxylateCatalog No.:AA009TWH CAS No.:111042-91-2 MDL No.:MFCD04124001 MF:C9H8N2O2S MW:208.2370 |
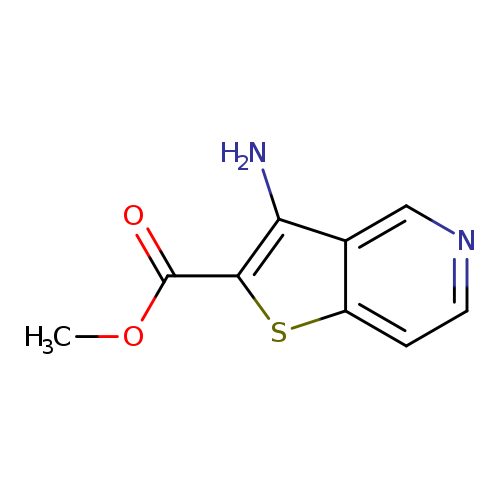
Methyl 3-aminothieno[3,2-c]pyridine-2-carboxylateCatalog No.:AA007TRO CAS No.:111042-92-3 MDL No.:MFCD08234760 MF:C9H8N2O2S MW:208.2370 |
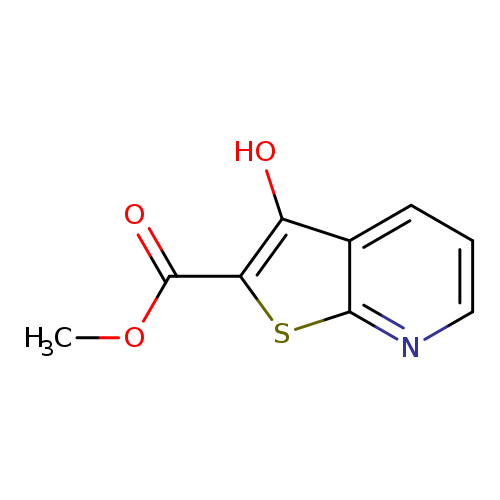
methyl 3-hydroxythieno[2,3-b]pyridine-2-carboxylateCatalog No.:AA00ITQ6 CAS No.:111043-01-7 MDL No.:MFCD00140980 MF:C9H7NO3S MW:209.2218 |
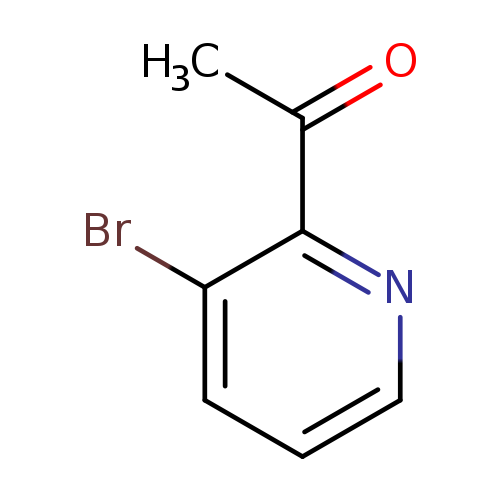
1-(3-Bromopyridin-2-yl)ethanoneCatalog No.:AA0082VV CAS No.:111043-09-5 MDL No.:MFCD18257141 MF:C7H6BrNO MW:200.0326 |
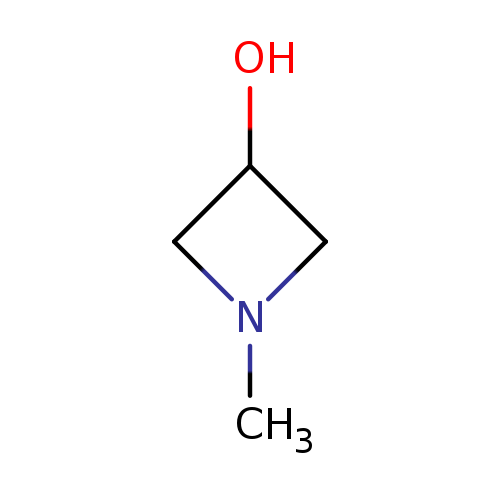
3-Hydroxy-1-methylazetidineCatalog No.:AA003JGB CAS No.:111043-48-2 MDL No.:MFCD10565802 MF:C4H9NO MW:87.1204 |
1-[(4-fluorophenyl)methyl]azetidin-3-olCatalog No.:AA01BHIC CAS No.:111043-49-3 MDL No.:MFCD12406961 MF:C10H12FNO MW:181.2068 |
Ginkgolic Acid C17:1Catalog No.:AA008XZC CAS No.:111047-30-4 MDL No.:MFCD09752804 MF:C24H38O3 MW:374.5567 |
1H-Indole-7-sulfonamide,2,3-dihydro-(9CI)Catalog No.:AA009POX CAS No.:111048-65-8 MDL No.:MFCD18809192 MF:C8H10N2O2S MW:198.2422 |
Methyl 4-amino-5-bromo-2-methoxybenzenecarboxylateCatalog No.:AA0082VN CAS No.:111049-68-4 MDL No.:MFCD02082951 MF:C9H10BrNO3 MW:260.0846 |
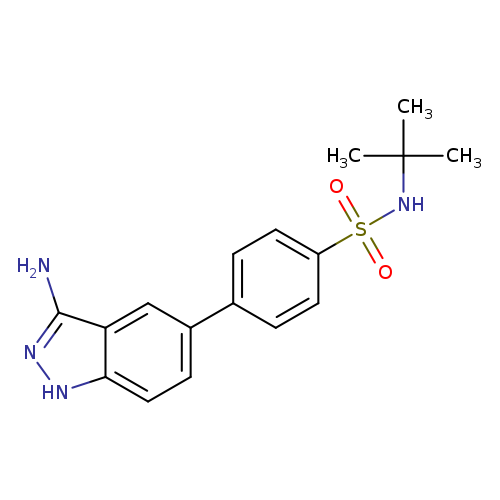
JAK2 Inhibitor IVCatalog No.:AA0097CT CAS No.:1110502-30-1 MDL No.: MF:C17H20N4O2S MW:344.4313 |
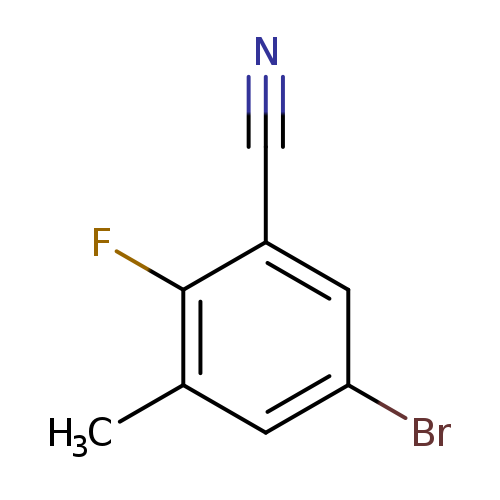
5-Bromo-2-fluoro-3-methylbenzonitrileCatalog No.:AA00HBPZ CAS No.:1110502-49-2 MDL No.:MFCD25955533 MF:C8H5BrFN MW:214.0344 |
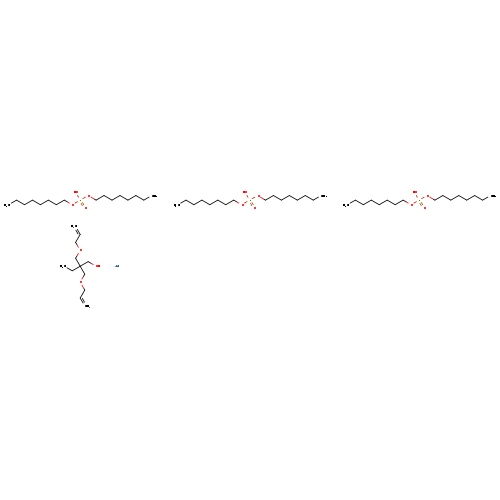
ZIRCONIUM (BIS-2,2-(ALLYLOXYMETHYL)BUTOXIDE)TRIS(DIOCTYLPHOSPHATE)Catalog No.:AA008UQR CAS No.:111053-49-7 MDL No.:MFCD08275480 MF:C60H127O15P3Zr MW:1272.7867 |
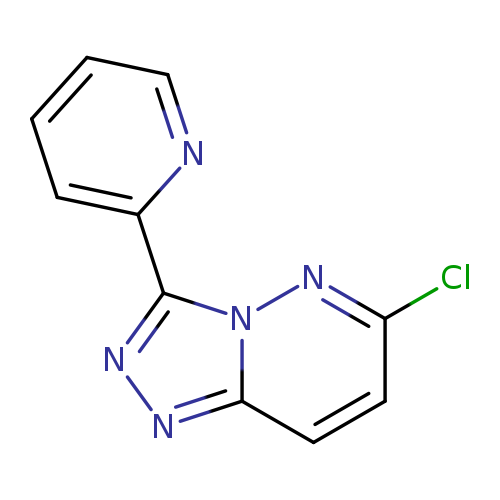
2-{6-chloro-[1,2,4]triazolo[4,3-b]pyridazin-3-yl}pyridineCatalog No.:AA01ARFG CAS No.:1110542-95-4 MDL No.:MFCD11103595 MF:C10H6ClN5 MW:231.6411 |
Alpha-d-thiomannose sodium saltCatalog No.:AA009TX8 CAS No.:111057-34-2 MDL No.:MFCD09952949 MF:C6H11NaO5S MW:218.2033 |
(R)-MethyglycidateCatalog No.:AA003527 CAS No.:111058-32-3 MDL No.:MFCD00274191 MF:C4H6O3 MW:102.0886 |
Trt-l-ser-oh teaCatalog No.:AA008Z1N CAS No.:111061-44-0 MDL No.:MFCD01075008 MF:C28H36N2O3 MW:448.5970 |
Fmoc-lys(trt)-ohCatalog No.:AA008Z2M CAS No.:111061-54-2 MDL No.:MFCD00270545 MF:C40H38N2O4 MW:610.7407 |
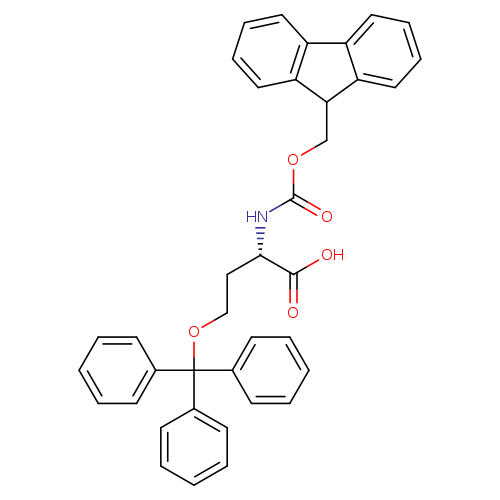
Fmoc-Hse(Trt)-OHCatalog No.:AA0034V4 CAS No.:111061-55-3 MDL No.:MFCD00270544 MF:C38H33NO5 MW:583.6723 |
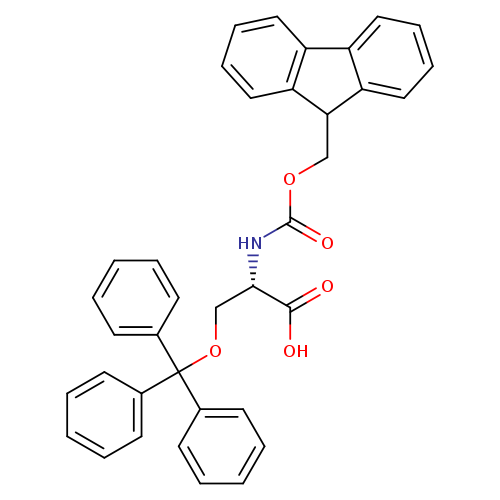
Fmoc-Ser(Trt)-OHCatalog No.:AA003QLC CAS No.:111061-56-4 MDL No.:MFCD00153364 MF:C37H31NO5 MW:569.6457 |
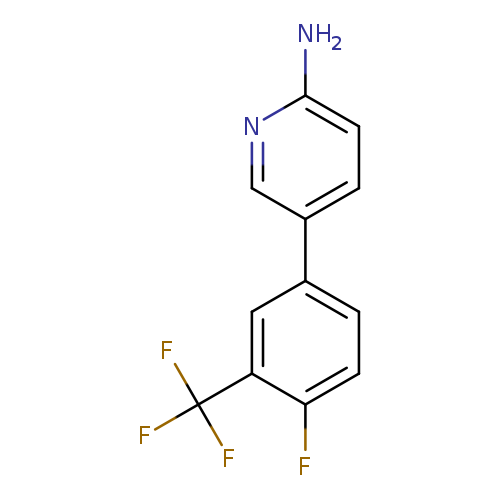
2-Amino-5-[4-fluoro-3-(trifluoromethyl)phenyl]pyridineCatalog No.:AA007TR5 CAS No.:1110656-35-3 MDL No.:MFCD11848376 MF:C12H8F4N2 MW:256.1989 |
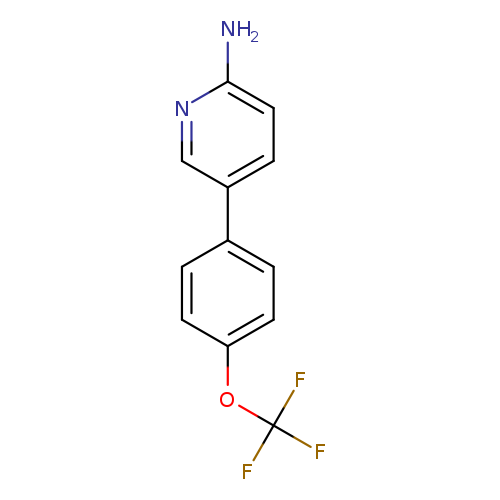
2-Amino-5-(4-trifluoromethoxyphenyl)pyridineCatalog No.:AA00946Y CAS No.:1110656-38-6 MDL No.:MFCD17496170 MF:C12H9F3N2O MW:254.2079 |
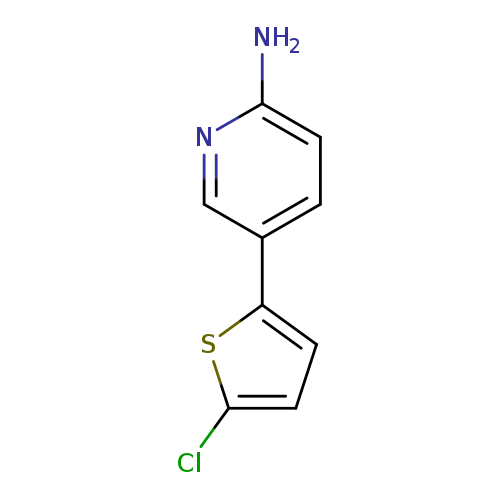
5-(5-chlorothiophen-2-yl)pyridin-2-amineCatalog No.:AA01BG0I CAS No.:1110657-44-7 MDL No.:MFCD23865491 MF:C9H7ClN2S MW:210.6833 |
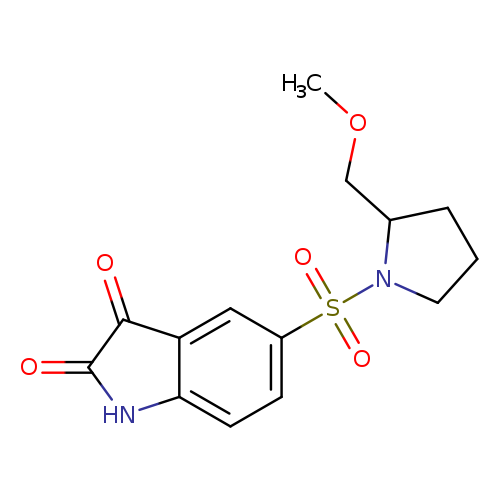
5-[(S)-(-)-2-(METHOXYMETHYL)PYRROLIDINO]SULFONYLISATINCatalog No.:AA008RKH CAS No.:1110670-49-9 MDL No.: MF:C14H16N2O5S MW:324.3522 |
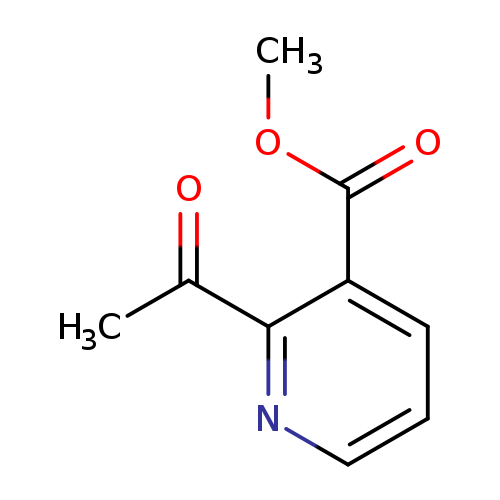
methyl 2-acetylpyridine-3-carboxylateCatalog No.:AA01BVG3 CAS No.:111068-03-2 MDL No.:MFCD19690400 MF:C9H9NO3 MW:179.1727 |
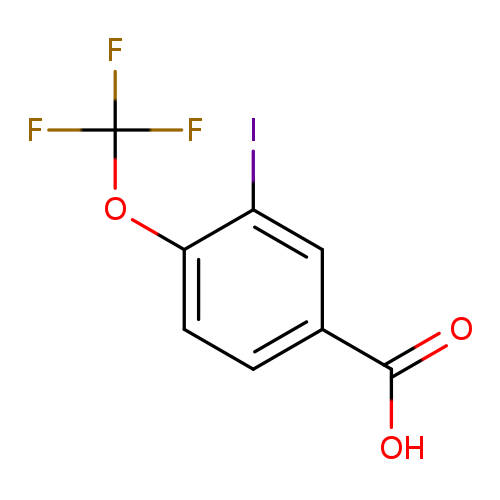
3-Iodo-4-(trifluoromethoxy)benzoic acidCatalog No.:AA008RKN CAS No.:1110709-70-0 MDL No.:MFCD08459287 MF:C8H4F3IO3 MW:332.0152 |
(11bS)-4,4-Dibutyl-4,5-dihydro-2,6-bis[3,5-bis(trifluoroMethyl)phenyl]-3H-dinaphtho[2,1-cCatalog No.:AA008WV2 CAS No.:1110711-01-7 MDL No.:MFCD16875654 MF:C46H38BrF12P MW:929.6525 |
4-[4-(5-chloro-2-methoxybenzenesulfonyl)piperazin-1-yl]-4-oxobutanoic acidCatalog No.:AA01A0MB CAS No.:1110717-62-8 MDL No.:MFCD09959852 MF:C15H19ClN2O6S MW:390.8392 |
3-({2-oxo-1H,2H,3H-imidazo[4,5-b]pyridin-5-yl}carbamoyl)propanoic acidCatalog No.:AA01A0MH CAS No.:1110717-63-9 MDL No.:MFCD09959854 MF:C10H10N4O4 MW:250.2108 |
3-({1,3-dimethyl-2-oxo-1H,2H,3H-imidazo[4,5-b]pyridin-5-yl}carbamoyl)propanoic acidCatalog No.:AA01A0MI CAS No.:1110717-64-0 MDL No.:MFCD09959855 MF:C12H14N4O4 MW:278.2640 |
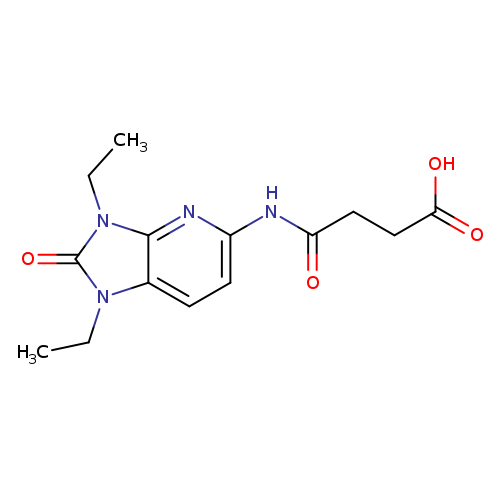
3-({1,3-diethyl-2-oxo-1H,2H,3H-imidazo[4,5-b]pyridin-5-yl}carbamoyl)propanoic acidCatalog No.:AA01A0MJ CAS No.:1110717-65-1 MDL No.:MFCD09959856 MF:C14H18N4O4 MW:306.3171 |
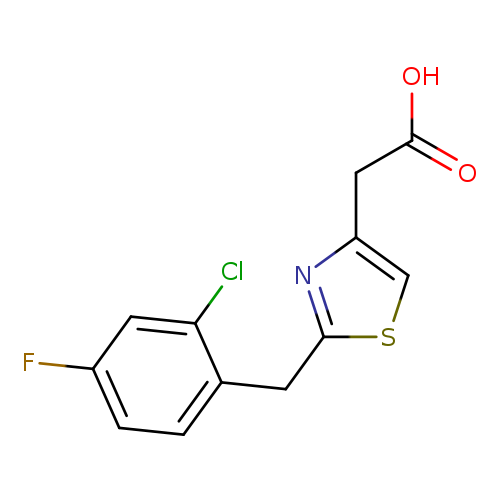
2-{2-[(2-chloro-4-fluorophenyl)methyl]-1,3-thiazol-4-yl}acetic acidCatalog No.:AA01A0QI CAS No.:1110717-81-1 MDL No.:MFCD09959887 MF:C12H9ClFNO2S MW:285.7218 |
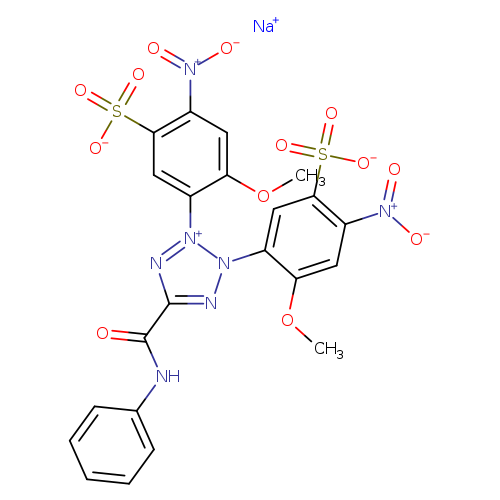
XTT sodium saltCatalog No.:AA00ILIP CAS No.:111072-31-2 MDL No.:MFCD00191206 MF:C22H16N7NaO13S2 MW:673.5213 |
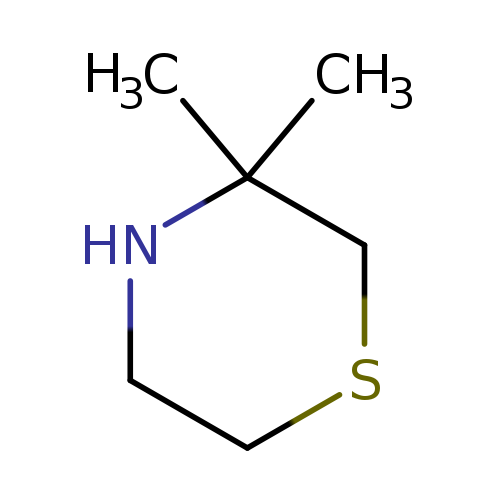
3,3-dimethylthiomorpholineCatalog No.:AA01A2MK CAS No.:111072-94-7 MDL No.:MFCD19220723 MF:C6H13NS MW:131.2391 |
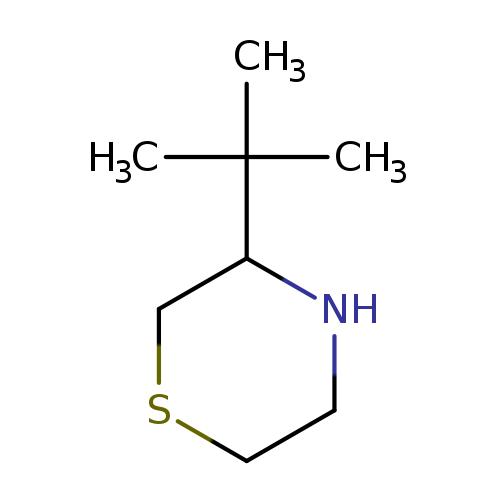
3-tert-butylthiomorpholineCatalog No.:AA01C1XO CAS No.:111073-09-7 MDL No.:MFCD20287214 MF:C8H17NS MW:159.2923 |
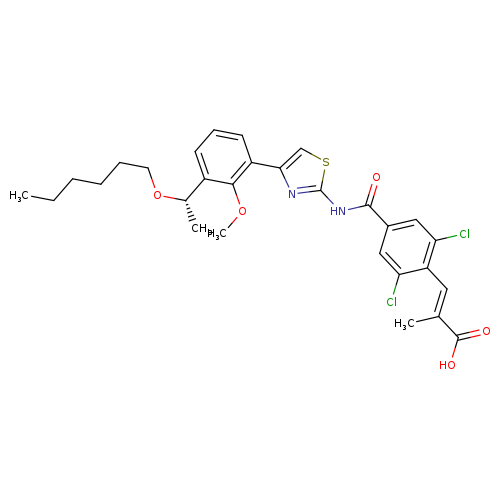
(S,E)-3-(2,6-Dichloro-4-((4-(3-(1-(hexyloxy)ethyl)-2-methoxyphenyl)thiazol-2-yl)carbamoyl)phenyl)-2-methylacrylic acidCatalog No.:AA008ZS5 CAS No.:1110766-97-6 MDL No.:MFCD28502075 MF:C29H32Cl2N2O5S MW:591.5458 |
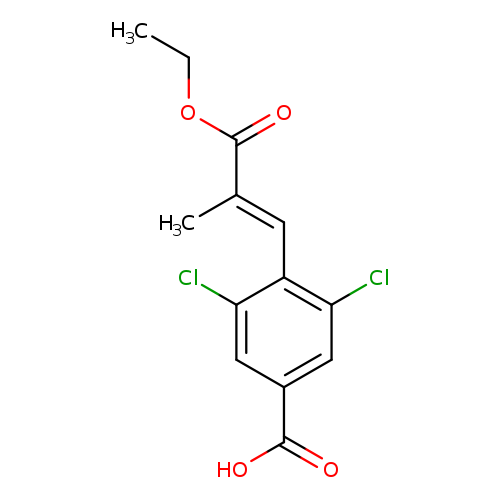
(E)-3,5-Dichloro-4-(3-ethoxy-2-methyl-3-oxoprop-1-en-1-yl)benzoic acidCatalog No.:AA008ZS9 CAS No.:1110767-89-9 MDL No.:MFCD29918463 MF:C13H12Cl2O4 MW:303.1380 |
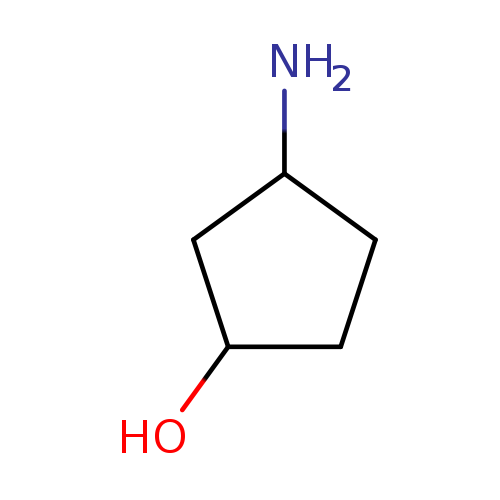
(1R,3S)-3-AminocyclopentanolCatalog No.:AA008RM9 CAS No.:1110772-05-8 MDL No.:MFCD11052498 MF:C5H11NO MW:101.1469 |
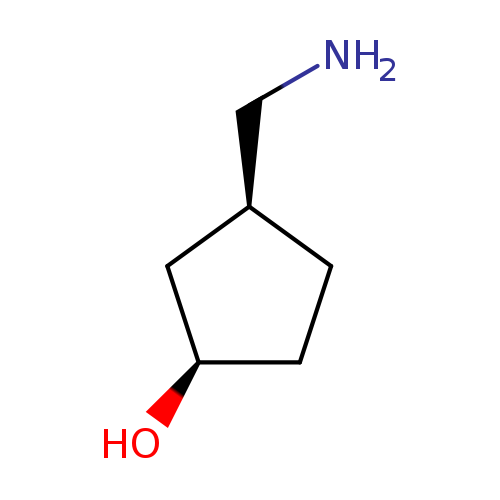
(1R,3S)-3-(Aminomethyl)cyclopentanolCatalog No.:AA008SQQ CAS No.:1110772-09-2 MDL No.:MFCD11052502 MF:C6H13NO MW:115.1735 |
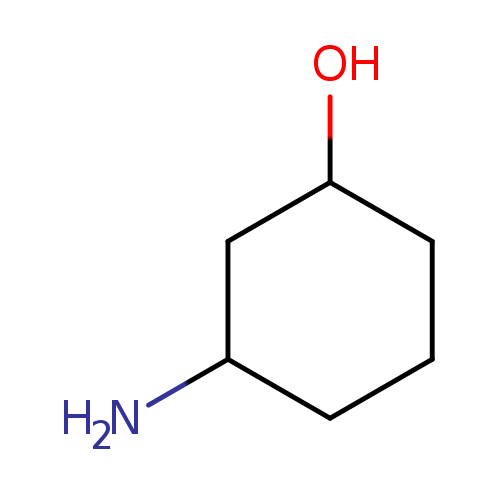
(1R,3S)-3-AminocyclohexanolCatalog No.:AA008U7H CAS No.:1110772-22-9 MDL No.:MFCD19237868 MF:C6H13NO MW:115.1735 |
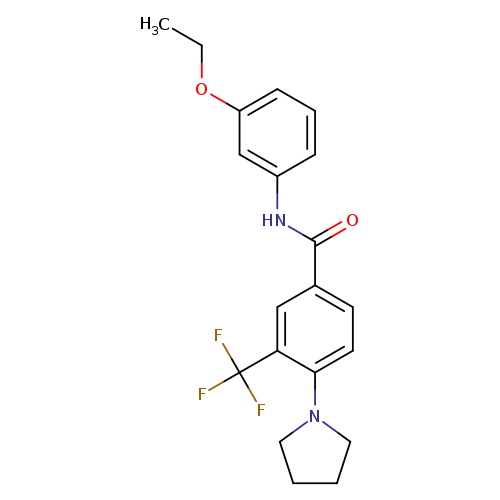
EPPTBCatalog No.:AA01DZJJ CAS No.:1110781-88-8 MDL No.:MFCD21605509 MF:C20H21F3N2O2 MW:378.3881 |
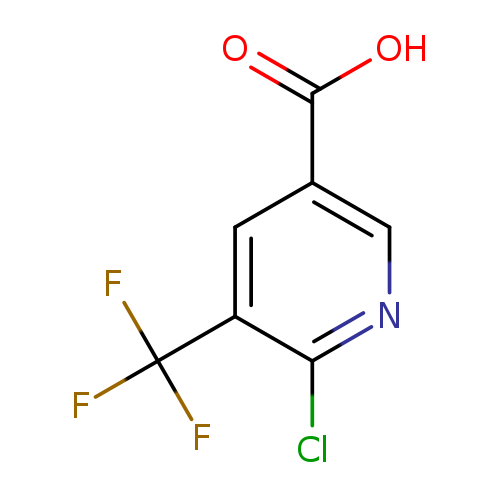
2-Chloro-3-(trifluoromethyl)pyridine-5-carboxylic acidCatalog No.:AA007TQV CAS No.:1110782-41-6 MDL No.:MFCD11848376 MF:C7H3ClF3NO2 MW:225.5524 |
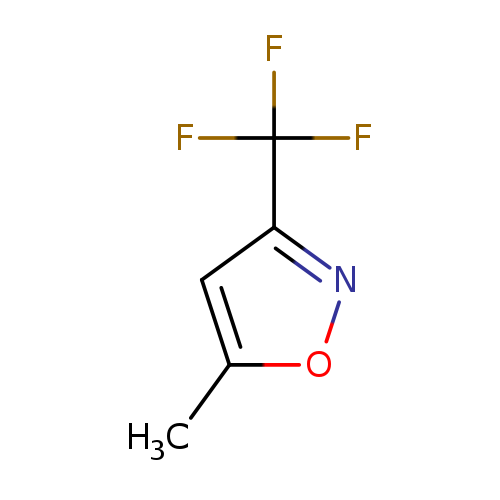
5-Methyl-3-(trifluoromethyl)isoxazoleCatalog No.:AA009QR3 CAS No.:111079-03-9 MDL No.:MFCD02666153 MF:C5H4F3NO MW:151.0866 |
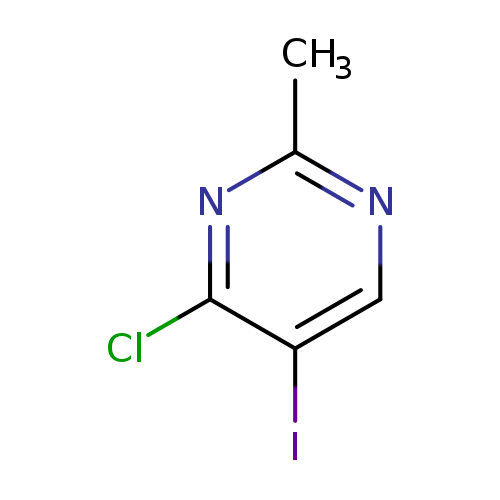
4-Chloro-5-iodo-2-methylpyrimidineCatalog No.:AA009M1E CAS No.:111079-17-5 MDL No.:MFCD09746250 MF:C5H4ClIN2 MW:254.4561 |
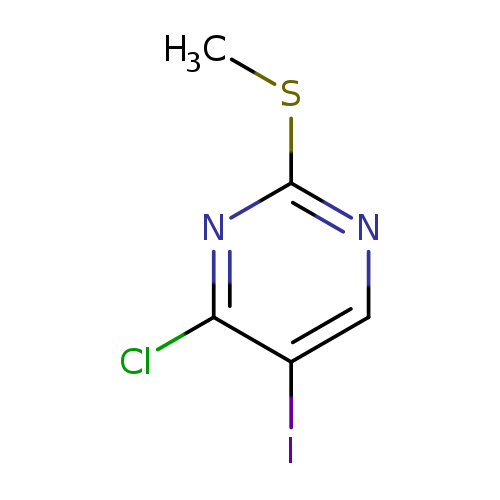
4-Chloro-5-iodo-2-(methylthio)pyrimidineCatalog No.:AA008Z0X CAS No.:111079-19-7 MDL No.:MFCD20492491 MF:C5H4ClIN2S MW:286.5211 |
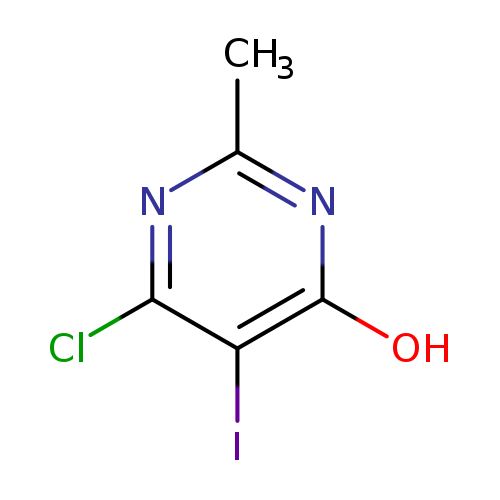
6-Chloro-5-iodo-2-methylpyrimidin-4(1H)-oneCatalog No.:AA00IRJA CAS No.:111079-42-6 MDL No.:MFCD12025846 MF:C5H4ClIN2O MW:270.4555 |
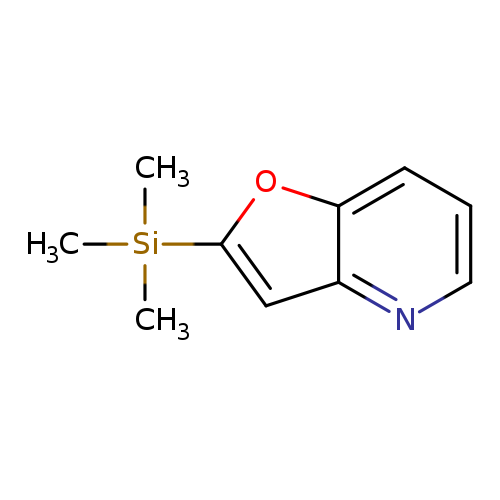
2-(Trimethylsilyl)furo[3,2-b]pyridineCatalog No.:AA003F3E CAS No.:111079-44-8 MDL No.:MFCD18255045 MF:C10H13NOSi MW:191.3018 |
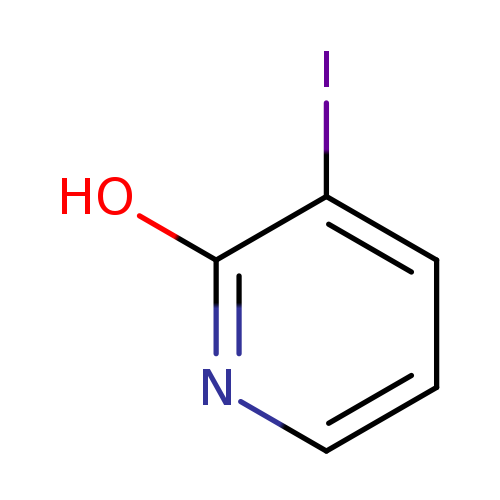
3-Iodopyridin-2-olCatalog No.:AA008Z1J CAS No.:111079-46-0 MDL No.:MFCD09835279 MF:C5H4INO MW:220.9958 |
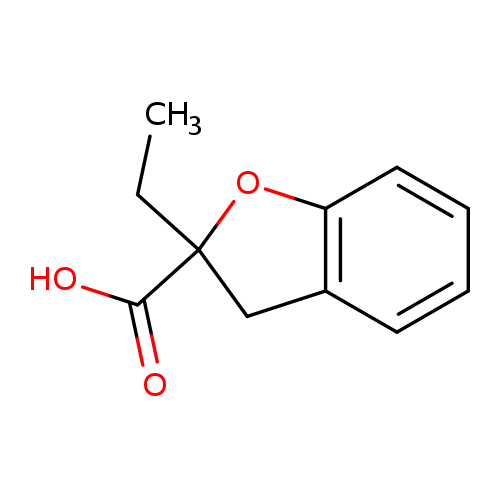
2-Ethyl-2,3-dihydrobenzofuran-2-carboxylic acidCatalog No.:AA007BH2 CAS No.:111080-50-3 MDL No.:MFCD13193696 MF:C11H12O3 MW:192.2112 |
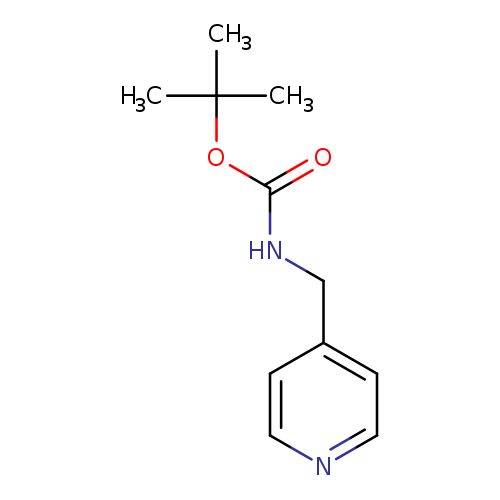
4-(Boc-aminomethyl)pyridineCatalog No.:AA003K3N CAS No.:111080-65-0 MDL No.:MFCD07369734 MF:C11H16N2O2 MW:208.2569 |
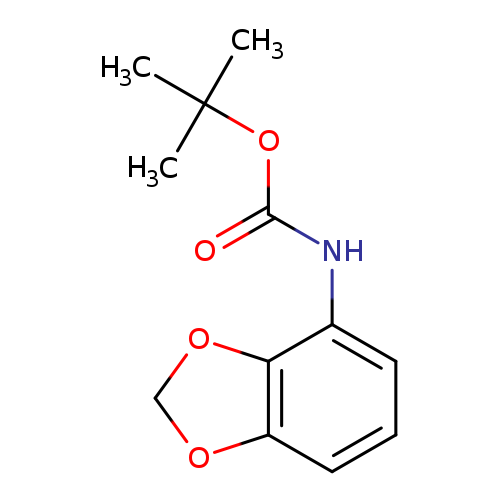
tert-Butyl benzo[d][1,3]dioxol-4-ylcarbamateCatalog No.:AA007TQT CAS No.:111081-10-8 MDL No.:MFCD09038117 MF:C12H15NO4 MW:237.2518 |
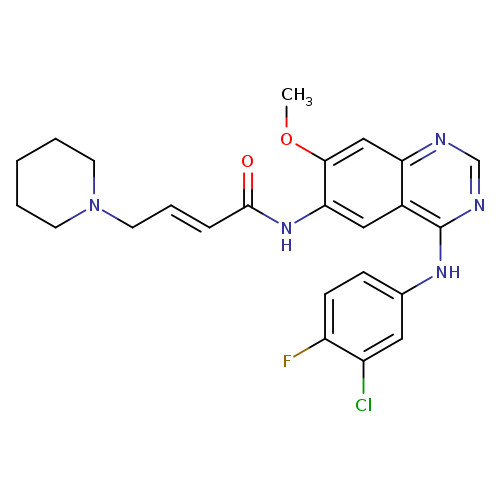
DacomitinibCatalog No.:AA008TCL CAS No.:1110813-31-4 MDL No.:MFCD19443734 MF:C24H25ClFN5O2 MW:469.9390 |
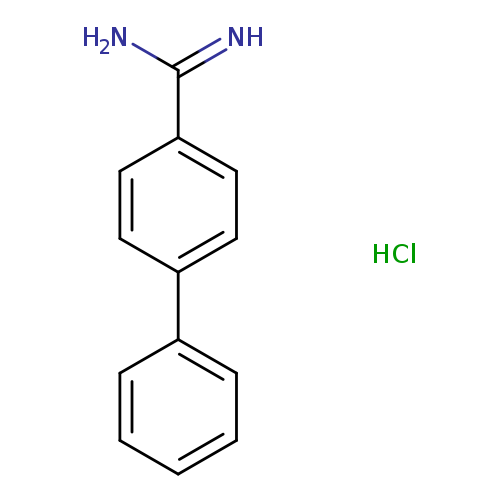
Biphenyl-4-carboxamidine hydrochlorideCatalog No.:AA008SC2 CAS No.:111082-23-6 MDL No.:MFCD04114455 MF:C13H13ClN2 MW:232.7087 |
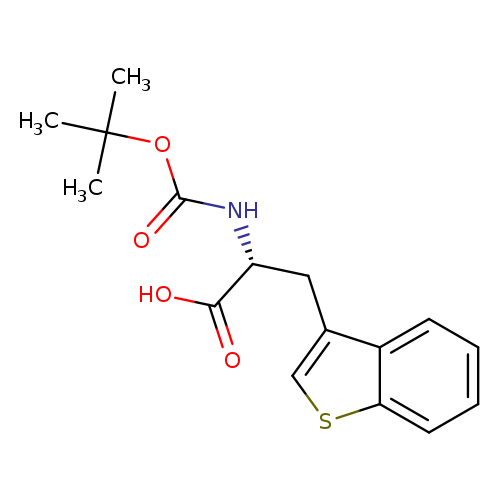
Boc-d-3-benzothienylalanineCatalog No.:AA003OD8 CAS No.:111082-76-9 MDL No.:MFCD00079679 MF:C16H19NO4S MW:321.3914 |
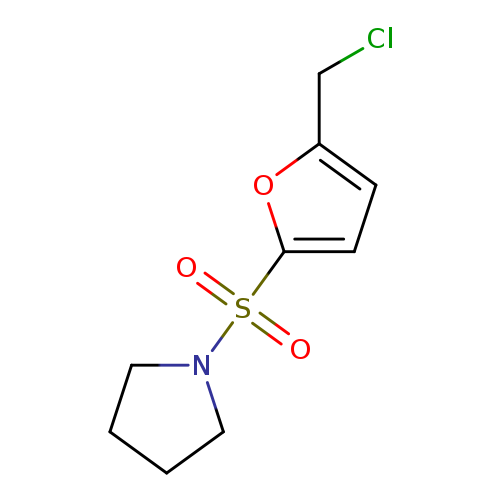
1-{[5-(chloromethyl)furan-2-yl]sulfonyl}pyrrolidineCatalog No.:AA019NE0 CAS No.:1110820-18-2 MDL No.:MFCD11505593 MF:C9H12ClNO3S MW:249.7145 |
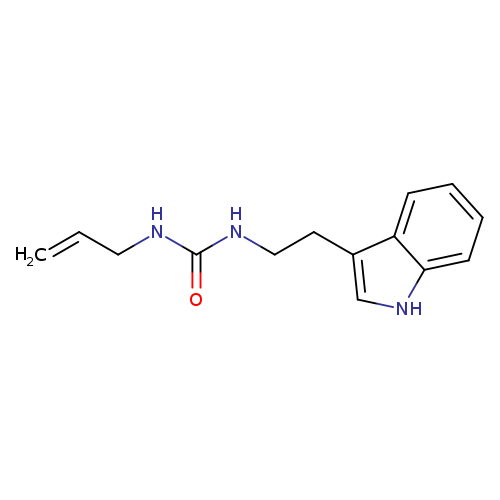
1-[2-(1H-indol-3-yl)ethyl]-3-(prop-2-en-1-yl)ureaCatalog No.:AA01C6HZ CAS No.:1110820-96-6 MDL No.:MFCD11784039 MF:C14H17N3O MW:243.3043 |
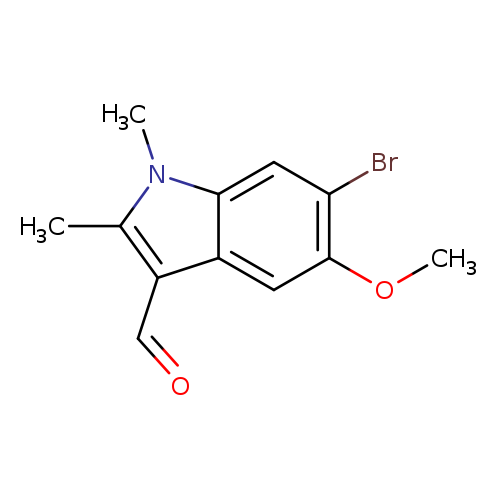
6-Bromo-5-methoxy-1,2-dimethyl-1H-indole-3-carbaldehydeCatalog No.:AA0082TZ CAS No.:111083-32-0 MDL No.:MFCD05864564 MF:C12H12BrNO2 MW:282.1332 |
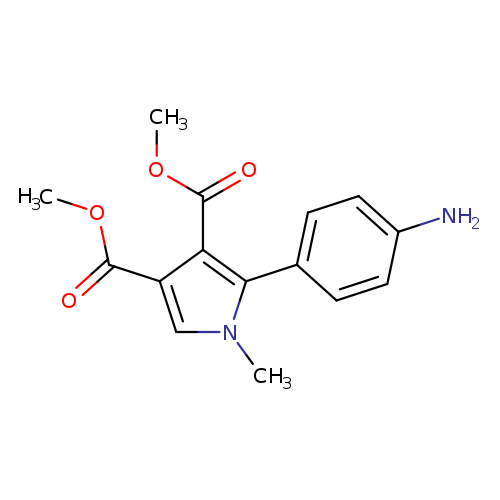
3,4-dimethyl 2-(4-aminophenyl)-1-methyl-1H-pyrrole-3,4-dicarboxylateCatalog No.:AA019NCY CAS No.:1110865-98-9 MDL No.:MFCD11505578 MF:C15H16N2O4 MW:288.2985 |