2019-11-16 15:11:20
Xiao-Yu Liu and Yong Qin*
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Sichuan Engineering Laboratory for
Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy,
Sichuan University, Chengdu 610041, China
The same as spectrum chemistry, there are a large assortment of high quality organic research chemicals including organic synthetic reagents and organic synthetic intermediates in aablocks.
1. INTRODUCTION
Indole alkaloids belong to one of the largest families of secondary metabolites created by nature.1 As a unique category of these molecules, the monoterpene indole alkaloids biogenetically originate from tryptamine and secologanin (Figure 1).2 Condensation of these two fragments via an enzymatic Pictet−Spengler reaction affords strictosidine; diversification of the latter common intermediate results in over 40 structural types and more than 3000 members of monoterpene indole alkaloids.1 Of particular note, many of these compounds exhibit a wide range of bioactivities, and molecules such as reserpine (antihypertensive), vincamine (nootropic), quinine (antimalaria), and vinblastine/vincristine (antitumor) have been used as important drugs.3
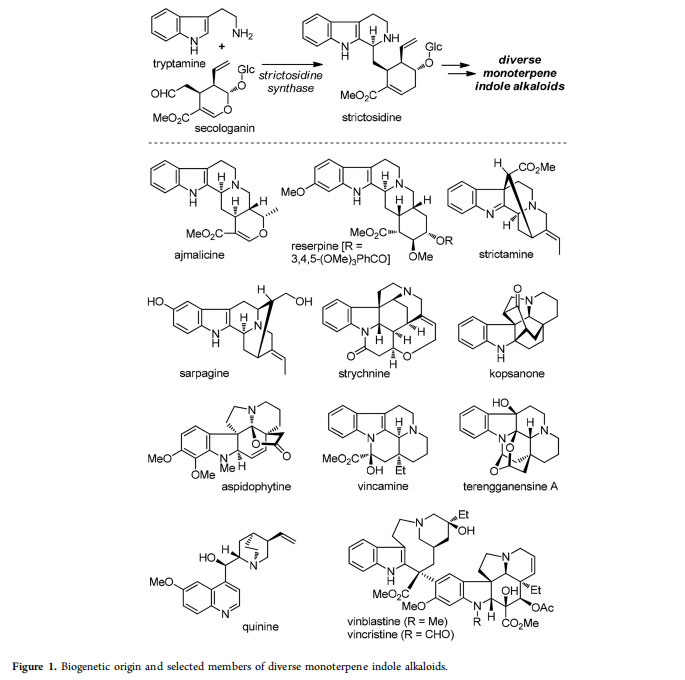
Due to their significant biological profiles and intricate structures, various monoterpene indole alkaloids have long challenged synthetic chemists. Such synthetic campaigns could be traced back to as early as the 1950s, when Woodward and co-workers first conquered strychnine4 and reserpine.5 Since then, the structural diversity and complexity of monoterpene indole alkaloids have made them important testing grounds for synthetic methodologies and tactics.6 Traditionally, many elegant approaches were documented but were only suitable for the synthesis of individual molecules or small groups of molecules from this natural product family. However, efficient access to large collections of complex indole alkaloids is always desirable and would call for a breakthrough in developing innovative synthetic methods and strategies.
Visible-light-mediated photoredox chemistry has been an active research field in the most recent decade and offers chemists new opportunities to form chemical bonds.7 Nevertheless, these single electron transfer (SET) processes have only begun to benefit natural product total synthesis,8,9 and they were mostly employed to realize single transformations. Design of visible light photoinduced radical cascade reactions to facilitate multiple bond-forming events in one pot would allow chemists to assemble the cores of natural products as well as to achieve their total synthesis in highly efficient fashion.

Stemming from a long-standing interest in the synthesis of intriguing indole alkaloids,10,11 our group recently developed a new photoredox catalytic radical cascade strategy that led to the asymmetric total synthesis of a collection of monoterpene indole alkaloids.12−16 As illustrated in Scheme 1, treating the sulfonamide substrate A with base and photocatalyst under the irradiation of blue light-emitting diodes (LEDs) generated a key nitrogen-centered radical B, which could be subsequently involved in further bond-forming events. Specifically, we designed three types of radical cascade pathways [intra-/
intramolecular, intra-/intermolecular, and intra-/inter-/intramolecular] and realized facile construction of the aspidosperma (type I, via C), tetrahydrocarbolinone (type II, via D), and corynanthe (type III, via E) core structures. While some of the obtained compounds already possessed the frameworks of indole alkaloids, the versatile functionalities in these cores could be manipulated to access more alkaloid types. As a result, harnessing the three types of photocatalytic radical cascades as key reactions allowed the asymmetric total synthesis of 42 monoterpene indole alkaloids belonging to 7 structural types.12−16 This Account summarizes the radical cascade methodologies developed in our laboratory and their applications in natural product total synthesis.
2. TYPE I RADICAL CASCADE AND ITS APPLICATIONS IN TOTAL SYNTHESIS
2.1. Development of the Type I Radical Cascade Reaction Bearing in mind the idea of developing a radical cascade approach to indole-alkaloid skeletons, we initiated our studies with the preparation of chiral substrate 4 (Scheme 2) from tertbutyl methyl malonate (1) and o-nitrocinnamic aldehyde (2). The organocatalytic asymmetric Michael addition of commercially available 1 to 2 followed by decarboxylation yielded chiral aldehyde ester 3 (96% ee),17 which was readily
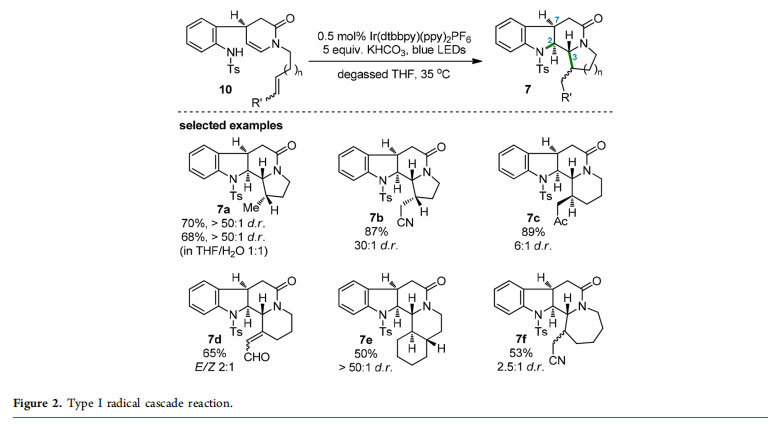
conducted on a 500 g scale in our laboratory. Compound 3 was then converted to 4 in three steps. By subjecting sulfonamide 4 to various non-photoredox conditions, we observed the cyclization product (tetrahydroquinoline 5) or oxidation product (pyridinone 6) or no reaction. Generation of tetrahydroquinoline 5 was, not surprisingly, the result of a C−N bond formation between the aniline nitrogen and the electron-deficient α-carbon of the enamide functionality. To reach the desired indoline product, a C−N bond formation at the β-position of the enamide was required. Classical methods for the generation of nitrogen-centered radicals18 from N−heteroatom bonds or directly from N−H bonds under harsh oxidative conditions were neither economic nor environmentally benign, which also proved to be unsuitable for our substrate. After much experimentation, we were delighted to isolate the tetracyclic product 7a (41% yield) along with tricycles 8 (6% yield) and 9 (35% yield) in the reaction of 4 with Ir(dtbbpy)(ppy)2PF6 and Et3N under the irradiation of blue LEDs.

Obviously, not only an unusual reactivity between two nucleophilic amine and enamine groups (in 4) but also an impressive radical cascade reaction (product 7a) was achieved in the aforementioned photocatalytic transformation. With the initial results, we next screened the reaction conditions and found that the radical cascade of 4 proceeded smoothly to produce 7a in 70% yield (>50:1 dr) using Ir(dtbbpy)-(ppy)2PF6 (0.5 mol %; dtbbpy = 4,4′-bis(di-t-butyl)-2,2′-bipyridine; ppy = 2-phenylpyridine) and KHCO3 in degassed. THF with irradiation of 5 W blue LEDs. In addition to 4, a series of substrates were designed and synthesized to examine the generality of this intra-/intramolecular radical cascade reaction. As depicted in Figure 2, 12 substrates possessing either electron-rich or electron-deficient double/triple bonds tethered to the enamide nitrogen atom were suitable for the cascade reaction, furnishing the corresponding products (e.g., selected examples 7b−e) with fused pyrrolidine or piperidine
rings in moderate to high yields and high to excellent dr values.
Exceptionally, the adduct 7f with a more flexible azepane unit was generated with a lower dr value (2.5:1). Of note, this cascade reaction proved to be robust and worked without loss of yield and diastereoselectivity in aqueous THF (product 7a).
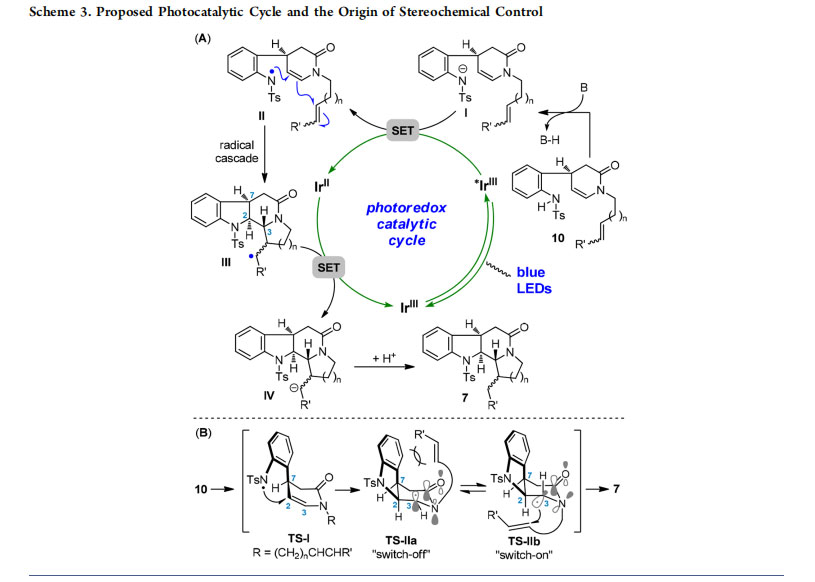
A proposed catalytic cycle for the photoredox radical cascade reaction is shown in Scheme 3A. Initially, deprotonation of the N−H bond in 10 took place in the presence of base to generate anion I. The photoexcited IrIII complex then oxidized I (via SET) and produced nitrogen-centered radical II, triggering subsequent cascade cyclization to yield carbon radical III. After SET reduction of radical III with the
reductive state IrII complex, the resultant anion IV could be converted into product 7 via protonation. Regeneration of the ground-state IrIII photocatalyst completed the catalytic cycle. Concerning the observed stereoselectivity of the radical cascade, formation of a cis relationship between H2 and H7 via transition state TS-I was realized in a substrate-controlled fashion (Scheme 3B).12 The resulting amide nitrogenassociated carbon radical might adopt two transition states, TS-IIa and TS-IIb, which acted as controllable switches for the subsequent reactions. The N-atom in TS-IIa had no contribution to enhancing the nucleophilicity of the adjacent carbon radical due to donation of the nitrogen lone pair electrons to the carbonyl moiety in the characteristic amide functionality. On the other hand, unfavorable steric repulsion between the Ts-protected indoline group and the radical acceptor in TS-IIa also inhibited occurrence of the ensuing reaction. By contrast, the key two-center, three-electron
interaction in TS-IIb greatly contributed to enhancing the radical stability, nucleophilicity, and selectivity.19 First, because of the greater stability, the sufficient lifetime of the carbon radical enabled subsequent bond formations especially in intermolecular manners (type II and III cascades). Second, twisting of the amide bond in TS-IIb restored the electrondonating ability of the N-atom to the carbon radical, thus increasing the radical nucleophilicity. Furthermore, attack of the carbon radical to the intramolecular acceptors (or intermolecular ones in type II and III cascades) exclusively from the bottom face of the piperidinone ring led to complete stereocontrol at C3.
2.2. Total Synthesis of the Eburnane Alkaloids
Inspired by the development of an intra-/intramolecular radical cascade for preparing indole-alkaloid-like skeletons, we sought to investigate its applications in natural product total synthesis. The eburnane indole alkaloids (Figure 3) are a group of structurally diverse molecules featuring a common pentacyclic core (e.g., 11−13) and an all-carbon quaternary center at C20.20,21 Functionalizations at the terminal C18 as well as further cyclizations may result in more variants of these natural products (e.g., 14−17). In this context, the C18 unfunctionalized eburnane alkaloids were envisaged to be synthesized from 18a (R = H), while various C18 functionalized counterparts could be derived from 18b (R = OPG). In turn, the versatile tetracyclic intermediate 18a,b with a crucial C20 quaternary stereogenic center would be accessible by the photocatalytic type I radical cascade of precursor 19a,b.
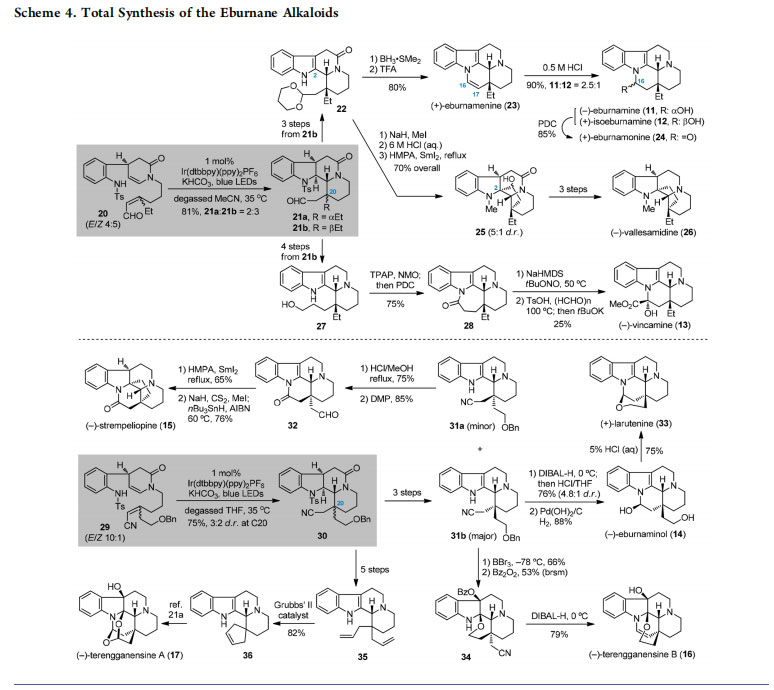
According to the synthetic plan, preparation of the C18 unfunctionalized eburnane alkaloids was examined using sulfonamide 20 as a starting material (Scheme 4).12 To evaluate the potential applications of a new synthetic methodology, especially in natural product total synthesis, an important criterion is whether it could be scaled up without loss of efficacy. Gratifyingly, the designed radical cascade reaction of 20 was easily performed on a decagram scale under photocatalytic conditions,12 where the solvent MeCN was found to be superior to THF, giving the bis-cyclization products 21a and 21b (81% overall yield, 2:3 dr). As most C18 unfunctionalized eburnane alkaloids possess a β-Et substituent at C20, the corresponding intermediate 21b with a β-Et group was used for further manipulations. On one hand, three steps of functional group transformations converted 21b into acetal 22. Reduction of the amide group in 22 to the corresponding amine followed by treatment with TFA afforded (+)-eburnamenine (23) in 80% overall yield. Subsequent hydration of the C16−C17 double bond delivered (−)-eburnamine (11) and (+)-isoeburnamine (12); oxidation of the resultant hydroxyl group with pyridinium dichromate (PDC) gave (+)-eburnamonine (24). The intermediate 22 was also used for the synthesis of (−)-vallesamidine (26). Establishing the remaining five-membered carbocycle in 26 relied on a SmI2-mediated reductive cyclization of the aldehyde to the C2 of indole. On the other hand, subjecting 21b to a four-step sequence produced amino alcohol 27. The latter underwent oxidation/ cyclization/oxidation to generate lactam 28, which was finally converted into (−)-vincamine (13) according to a literature method.22
Employing precursor 29 with a C18-OBn group for the radical cascade reaction under the standard photoredox conditions smoothly furnished the tetracyclic product 30 as a pair of inseparable diastereomers (Scheme 4).14 Both isomers were used for accessing eburnane indole alkaloids with C18 functionalities. Specifically, the diastereomeric mixture of 30 was converted to separable 31a and 31b by three steps.
Treatment of the minor 31a with HCl/MeOH at reflux forged a new lactam ring with simultaneous removal of the benzyl group, which was followed by Dess−Martin periodinane (DMP) oxidation of the resultant primary alcohol, leading to aldehyde 32. A SmI2-mediated radical cyclization of 32 with subsequent Barton deoxygenation generated (−)-strempeliopine (15). Meanwhile, starting from 31b, formation of a hemiaminal with ensuing deprotection of the benzyl group yielded (−)-eburnaminol (14); the latter was treated with HCl to give (+)-larutenine (33). On the other hand, first removal of
the benzyl group in 31b, followed by oxidation and cyclization in the presence of Bz2O2, furnished tetrahydropyran 34.
Exposure of 34 to di-isobutyl aluminum hydride (DIBAL-H) at 0 °C completed the synthesis of (−)-terengganensine B (16). Moreover, the radical cascade product (30) underwent a five-step transformation to provide access to the diallyl product 35. Ring-closing metathesis (RCM) of 35 with Grubbs’ II catalyst delivered 36 (82% yield); the latter was further converted to (−)-terengganensine A (17) based on the
method developed by Zhu and colleagues.21a
3. TYPE II RADICAL CASCADE AND ITS APPLICATIONS IN TOTAL SYNTHESIS
3.1. Development of the Type II Radical Cascade Reaction Having secured the intra-/intramolecular (type I) radical cascade, we next explored an intra-/intermolecular (type II) cascade process using sulfonamide 37 and an external radical acceptor as reaction partners. Pleasingly, reactions of 37 with various acceptors (39−42) took place under the same photocatalytic conditions as those applied to the type I reaction, providing corresponding tetrahydrocarbolinones 38a−l in moderate to high yields (Figure 4).12 Of note, this protocol proved to be useful for generating tetrahydrocarbolinone products with versatile functional groups. For instance, products 38k and 38l bearing a methylene group α and β to C3 were obtained by employing 41 and 42 as the external radical acceptors, respectively. Again, the stereochemical outcomes of the newly formed stereocenters (C2 and C3) were excellently controlled in all of the products.

3.2. Total Synthesis of the Akuammiline Alkaloids (−)-Strictamine and (−)-Rhazinoline
Strictamine and its associated akuammiline alkaloids (43−45, Figure 5) are structurally unique natural products possessing a characteristic methanoquinolizidine motif (CDE rings) as well as differentiated stereochemistries at the C16 position.23 These fascinating molecules have attracted considerable attention of synthetic chemists for decades.24,25 We envisioned that taking advantage of the photoredox type II radical cascade strategy would facilitate the total synthesis of strictamine and related alkaloids. Retrosynthetically, the C ring and E ring in (−)-strictamine and (−)-rhazinoline were expected to be
assembled from advanced intermediates 46a and 46b at a late stage. The critical C7−C16 bond in 46a and 46b would be established from 47 via a Tsuji−Trost allylation.26 The latter could be synthetically traced back to the tricyclic compound 48, which was envisioned to be synthesized through a type II radical cascade reaction between 49 and acrolein (50).

In the forward synthesis, the radical cascade reaction between 49 and acrolein (50) was conducted using Ir-(dtbbpy)(ppy)2PF6 (0.5 mol %) and KHCO3 in degassed THF by irradiation with blue LEDs (Scheme 5).16 Without workup, upon sequentially adding vinylmagnesium bromide (51) and Na/naphthalene to the reaction mixture, the allylic alcohol 52 was obtained as a pair of inseparable diastereomers (1.2:1 dr at C16) in 47% yield in one pot. The readily accessed tetrahydrocarbolinone 52 was converted to enal 53 in 8 steps.
After further functional group manipulations of 53 to yield enoate 54 possessing a vinyl iodide side chain, the stage was set for E ring formation. The palladium-catalyzed reductive Heck cyclization of 54 occurred effectively in the presence of Pd2(dba)3/PPh3/HCO2Na (dba = dibenzylideneacetone), which was followed by mask of the resulting secondary amine as a carbamate (Boc), giving 55 as a single diastereomer (48% yield). The configuration of the key C16 stereocenter Figure 4. Type II radical cascade reaction. was determined via nuclear Overhauser effect (NOE) experiments. Assembly of the remaining C ring (55 to 56) was realized in three additional steps, providing 16-epi-strictramine(56). Finally, conversion of the C16 ester to corresponding aldehyde enabled the first synthesis of (−) rhazinoline[(−)-44]. On the other hand, unsuccessful attempts to directly epimerize the C16 stereocenter in 16-epi-strictramine (56) prompted us to seek an alternative approach to (−)-strictamine [(−)-43].16 In this context, allylic alcohol 57 containing a vinyl iodide chain tethered to N4 was prepared via four steps starting from the advanced intermediate 53. A nickel-mediated cyclization of 57 followed by intramolecular N4-alkylation (with 2-fluoropyridine and Tf2O) allowed the E and C rings to be successively established. Notably, selective hydroboration−oxidation of the resultant exocyclic terminal alkene was achieved by employing 9-BBN followed by treatment with NaBO3·4H2O, which afforded the primary alcohol 58 and secured the anticipated C16 configuration. Further functionality manipulations via three steps provided the target alkaloid (−)-strictamine [(−)-43].

With both C16-epimers of strictamine (56 and 43) in hand, we again investigated the direct interconversion between them, which was experimentally verified to be unsuccessful (Scheme 6). Presumably, H16 is located at the sterically hindered concave face in 56, and thus, deprotonation of H16 (in 56) to yield anion 59 would be hampered, preventing its further transformation to 43 (via 60). On the other hand, deprotonation of the convex H16 in (−)-strictamine [(−)-43] would occur to produce 61. After enolization (61 to 60), selective protonation at the sterically less hindered convex face could regenerate 43. Consequently, these naturally occurring C16 diastereomers in the strictamine akuammiline alkaloid family would be most likely generated during construction of the C7−C16 bond from the corynanthe
intermediates in the biosynthetic processes.2a

4. TYPE III RADICAL CASCADE AND ITS APPLICATIONS IN TOTAL SYNTHESIS
4.1. Development of the Type III Radical Cascade Reaction With the success of the above-mentioned two types of visible light photoredox radical cascades, we were ambitious to further expand the method to an intra-/inter-/intramolecular cascade process that would enable the formation of three bonds and two rings in one pot. To our delight, under the typical photocatalytic reaction conditions, the programmed radical cascade worked smoothly with different external acceptors(40) and enamide substrates (62) containing unsaturated side chains, leading to corresponding tetracyclic products (63) with satisfactory yields and excellent stereochemical outcomes at the newly formed C2, C3, and C20 stereocenters (Figure 6).

When the substrate bearing a simple alkene rather than a Michael acceptor attached at the enamide nitrogen was employed in the radical reaction, the corresponding product(e.g., 63h) was obtained with lower yield and diastereoselectivity. Obviously, an unactivated alkene was relatively inert toward the intramolecular attack of a carbon radical in the third step.

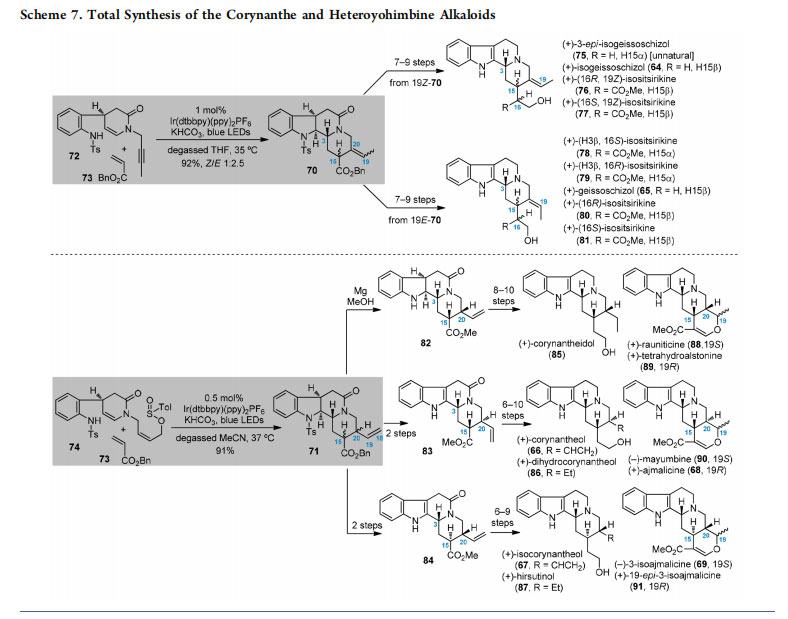
4.2. Total Synthesis of the Corynanthe and Heteroyohimbine Alkaloids
Application of the established type III radical cascade to the total synthesis of indole alkaloids was first demonstrated in the synthesis of corynanthe and heteroyohimbine alkaloids. The naturally occurring compounds in these two subfamilies possess common tetracyclic or pentacyclic skeletons but with varied stereochemistries at different positions (Figure 7).27 For example, molecules of the corynanthe-type include those with internal C19−C20 alkenes (e.g., 64, Z-olefin, 65, E-olefin) or external C18−C19 alkenes (e.g., 66 and 67 with different configurations at C15 and C20).28 In the heteroyohimbinetype alkaloids, compounds 68 and 69 differ in their configurations at C15, C19, and C20.29 By careful design of reaction substrates, our radical cascade approach would be suitable for collective total synthesis of these alkaloids with diverse stereochemistries. Specifically, the heteroyohimbine alkaloids could be synthesized from corynanthes, and the latter was envisaged to be generated from two intermediates, 70 and 71, corresponding to internal and external alkenes, respectively. Disconnection of the tetracyclic 70 and 71 would lead to different reaction partners (72 + 73 and 74 + 73) via the type III radical cascades.
According to our previously developed synthetic route,12 enamide substrates 72 and 74 with different side chains were prepared and ready for the photoinduced reactions. We designed the use of an alkenyl-type external radical acceptor in the cascade reactions, which would provide us the products with both C15 diastereomers and satisfy the stereochemical diversity of the target alkaloids (Scheme 7).12 In this context, subjecting enamide 72 with a 2-butynyl side chain to photoredox catalysis in the presence of benzyl acrylate (73) induced the three-step radical cascade to deliver 70 with an
internal C19−C20 alkene. The Z- and E-alkene products were separable and the former (a mixture of H15α and H15β) was further converted into natural products 64, 76, and 77, together with the unnatural 75 in a few steps. Similarly, starting from the cascade products with a C19−C20 E-alkene moiety, we accomplished the total synthesis of 65 and 78−81. In addition, when the sulfinate-containing enamide 74 was utilized for the cascade reaction, the tetracyclic 71 with an external C18−C19 olefin was generated. The obtained products comprised a mixture of the C15 and C20 diastereomers (91% overall yield), which were separable at different stages in subsequent transformations. Thus, the isolated intermediates 82−84 were employed for the preparation of corynanthe-type alkaloids 66, 67, and 85−87,
respectively. Such intermediates (82−84) with an external C18−C19 olefin also served as important precursors for the synthesis of heteroyohimbine-type alkaloids 68, 69, and 88−91 containing an additional dihydropyran ring as well as varied configurations at C15, C19, and C20.
4.3. Total Synthesis of the Yohimbine Alkaloids
The above success for the total synthesis of corynanthe and heteroyohimbine alkaloids encouraged us to explore different substrate combinations in the type III radical cascade for the total synthesis of more types of indole alkaloids. The yohimbine alkaloids are structurally characterized by a pentacyclic nucleus and possess an additional six-membered carbocycle E ring (Scheme 8) compared to the aforementioned corynanthe molecules. These compounds exhibited intriguing bioactivities and have been attractive targets for total synthesis.30 In our synthetic plan, an array of yohimbine alkaloids could be prepared from a common intermediate 92 through functional group manipulations. The key pentacyclic enone 92 would be accessible by harnessing a type III radical cascade between enal 93 and methyl vinyl ketone (94) followed by an intramolecular aldol condensation.
As expected, the intra-/inter-/intramolecular cascade reaction between 93 and 94 proceeded under the standard photocatalytic conditions (Scheme 8),12 the crude products of which were directly treated with p-TsOH in PhMe to effect an ensuing aldol condensation, affording enone 92a,b along with their C15 diastereomers (not shown) in 56% combined yield and 11:1 dr at C15. The two separable major isomers 92a and 92b were subjected to functional-group transformations, resulting in the total synthesis of the yohimbine alkaloids 95−102.
4.4. Total Synthesis of the Cinchona Alkaloid(+)-Cinchonidine
The power of the type III radical cascade protocol was further showcased in a new synthetic approach to the Cinchona alkaloids. Biogenetically, the Cinchona alkaloids (104−107, Figure 8) were derived from the corynanthe-type molecules(e.g., corynantheal 103) through a sequence of enzymatic processes including N4−C5 bond cleavage, N4−C17 bond formation, and indole to quinoline transformation.2 Therefore, despite the absence of an indole core, the Cinchona alkaloids were biogenetically categorized as indole alkaloids.31,32 We envisioned that construction of the quinuclidine unit as well as
bioinspired “indole to quinoline” transformation33 from 108 would complete the total synthesis of corresponding Cinchona alkaloids. Another key step in this synthesis would be assembly of the tetracyclic 109 from sulfonamide 74 and an alkynyl radical acceptor (40) by means of a type III radical cascade. Cleavage of the amide bond in the photocatalytic product (i.e., 109) could be harnessed to generate aldehyde 108.
According to the above analysis, our total synthesis of(+)-cinchonidine is outlined in Scheme 9. The synthetic efforts commenced with sulfonamide 74 and isopropyl propionate (110). Both starting materials were subjected to slightly modified photoredox conditions (switching of the solvent from THF or MeCN to DME and employing 34 W rather than 5 W blue LEDs as the light source) to efficiently deliver 111 (83% yield, 17:1 dr at C20). Subsequent removal of the N-Ts group as well as reduction of the C14−C15 alkene and the isopropyl ester in 111 gave aldehyde 112, establishing the C15 stereocenter with 20:1 dr. Compound 112 underwent a three-step transformation to yield alcohol 113. The latter was ready for the N4−C5 amide bond cleavage and was fruitfully converted into an advanced intermediate 114 via four steps.
We next investigated the bioinspired “indole to quinoline” rearrangement and observed that oxidation of the indole C2−C7 double bond in 114 with dimethyldioxirane (DMDO) followed by treatment with a THF/AcOH/H2O (10:1:1) mixture promoted the desired transformation, providing 115 in 73% yield. Extensive screening of various reductants indicated that lithium diisobutyl-t-butoxyaluminum hydride (LDBBA) was able to reduce the C2 carbonyl group of 115 in a stereoselective manner to furnish the secondary alcohol 116 (90% yield, 20:1 dr). Construction of the remaining quinuclidine ring in the target molecule was accomplished through exposure of 116 to aqueous NaOH/iPrOH at 50 °C, completing the synthesis of (+)-cinchonidine [(+)-106].
4.5. Total Synthesis of (+)-Strychnine
The Strychnos alkaloid strychnine (117, Figure 9) represents one of the most famous indole alkaloids, found over two centuries ago, which has long been pursued in the synthetic community due to its intricate heptacyclic architecture.34,35 Close inspection of the structures of strychnine and its biosynthetic precursors (e.g., 118 and 119) encouraged us to develop a new asymmetric synthetic approach to this landmark molecule relying on a biomimetic rearrangement and a type III photocatalytic radical cascade reaction as key steps. As outlined in Figure 9, disconnection of strychnine could lead back to the
pentacycle 120. The intermediate 120 was envisaged to be derived from a biogenetically inspired rearrangement36 of compound 121, and the latter could be accessed from 122 through functionality manipulations. In turn, 122 would be generated via a three-step radical cascade reaction from 123 and 39.
The first objective in the total synthesis of strychnine was to secure a crucial C19−C20 E-alkene that is present in the target molecule via the proposed radical cascade (Scheme 10).15 Initial experiments using either 123a and 73 or 123b and 124 as reaction partners under the optimal photocatalytic conditions gave the cyclization products 125 or 126 in good yields but with poor geometrical selectivities of the C19−C20 alkene. Additionally, in hopes of yielding the C19−C20 Ealkene via an intra-/intra-/intramolecular cascade pathway, enamide 127 was prepared containing an acrylate linked at the terminal propargyl hydroxyl group, which failed to give any desired product (i.e., 128) with photoredox catalysis.
Fortunately, after much experimentation, we were able to access the hemiacetal 130 (80% yield) with an exclusive Ealkene by the photoinduced cascade of propargyl alcohol 123b and acrolein (50) under the same reaction conditions. Presumably, an E/Z geometrical mixture of C19−C20 alkene(129a and 129b) might be initially formed, which would undergo isomerization via triplet sensitization under the
photocatalytic conditions.37 Formation of the desired hemiacetal 130 from 129b then served as a driving force for the complete Z to E isomerization. The diastereomeric ratio(2.5:1) at C15 in 130 was determined after elimination of the C16 configuration through subsequent transformations, and the major isomer possessed the desired stereochemistry(15Hβ). Starting from 130, an ensuing 7-step sequence produced the corynanthe-type tetracycle 131, which set the stage for a bioinspired rearrangement. To this end, according to Martin’s protocol,36 subjecting 131 to SnCl4 and tert-butyl
hypochlorite followed by hexamethyldisilazane lithium salt(LiHMDS) yielded the pentacyclic 132. NaBH3CN reduction of the C2−C16 double bond in 132, followed by triisopropylsilyl ether (TIPS) deprotection and conversion of the cyano group to the methyl ester with HCl/MeOH, during which the C16 configuration was inverted as well, delivered the known compound 133. 38 Finally, the synthesis of (+)-strychnine [(+)-117] was completed from 133 based on a literature method.38
5. CONCLUSION
Structurally fascinating indole alkaloids are important targets in total synthesis for testing synthetic methods and tactics. The photoredox catalysis-initiated radical cascades discussed in this Account represent a new supplement to the toolkit for such synthetic pursuits.39 The power of the methodologies has been illustrated by successful asymmetric total synthesis of 42 monoterpene indole alkaloids belonging to 7 different groups. Notable highlights of these visible-light-induced approaches include (1) direct and mild conversion of an inert sulfonamide N−H bond to a nitrogen-centered radical,18d (2) reversion of the conventional reactivity between two nucleophilic amine and enamine groups, and (3) precise control of the reaction pathways and stereochemical outcomes of elusive radical species.
Furthermore, from the reported examples, our
methods exhibited good functional-group compatibility, thereby permitting wide applications in the total synthesis of indole alkaloids. As discussed in this Account, the photoinduced cascade protocols have been demonstrated to greatly benefit natural product synthesis, which is believed to be only a beginning. One can anticipate that design of radical cascades by harnessing visible light photoredox catalysis will further facilitate the expedient assembly of complex and useful molecular architectures in the future.
AUTHOR INFORMATION
Corresponding Author
*E-mail: [email protected].
ORCID
Xiao-Yu Liu: 0000-0003-3773-8554
Yong Qin: 0000-0003-3434-5747
Notes
The authors declare no competing financial interest.
Biographies
Xiao-Yu Liu was born in 1985 in Sichuan, China. He obtained his B.Sc. in 2006 and received a Ph.D. degree in 2012 at Sichuan University. He then carried out postdoctoral studies at Seoul National
University with Professor David Y.-K. Chen. After that, he joined the faculty of Sichuan University in 2014 and has since been focused on the synthesis of complex natural products. Yong Qin received his B.S. degree from Yunnan University in 1989 and his Ph.D. from the Institute of Chemistry, Chinese Academy of Sciences, in 1995. After a short stay at the Chengdu Institute of Organic Chemistry, he moved to the USA, working as a postdoctoral associate (1996 to 2000) at the University of Vermont. He was a research scientist at Triad Therapeutics (San Diego) before he joined the faculty of Sichuan University as a full professor in 2003. His research interest involves the total synthesis of bioactive natural products and medicinal chemistry.
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support from the National Science Foundation of China (21732005) and the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09711003-015).
3-(2-Bromophenyl)-5-(2-chlorophenyl)-1,2,4-oxadiazoleCatalog No.:AA00009S CAS No.:1000339-28-5 MDL No.:MFCD02217449 MF:C14H8BrClN2O MW:335.5831 |
2-Chloro-6-(pyrrolidin-1-yl)pyrazineCatalog No.:AA00009Q CAS No.:1000339-30-9 MDL No.:MFCD09864953 MF:C8H10ClN3 MW:183.6381 |
1-(3-Chloro-4-fluorophenyl)pyrrolidineCatalog No.:AA00009N CAS No.:1000339-33-2 MDL No.:MFCD09878399 MF:C10H11ClFN MW:199.6524 |

2-(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)propan-2-olCatalog No.:AA00009M CAS No.:1000339-34-3 MDL No.:MFCD09878410 MF:C11H12BrN3O MW:282.1365 |
5-Bromo-N-cyclopropyl-2-methoxybenzenesulfonamideCatalog No.:AA00009L CAS No.:1000339-35-4 MDL No.:MFCD08235093 MF:C10H12BrNO3S MW:306.1762 |
N-Ethyl-N-(4-methoxybenzyl)benzenesulfonamideCatalog No.:AA00009K CAS No.:1000339-36-5 MDL No.:MFCD09878415 MF:C16H19NO3S MW:305.3920 |
3-Fluoro-2-nitrobenzoic acidCatalog No.:AA00009I CAS No.:1000339-51-4 MDL No.:MFCD07368342 MF:C7H4FNO4 MW:185.1094 |
3-Fluoro-2-nitrobenzonitrileCatalog No.:AA00009H CAS No.:1000339-52-5 MDL No.:MFCD09864664 MF:C7H3FN2O2 MW:166.1093 |
2-(2-Bromo-3-fluorophenyl)acetonitrileCatalog No.:AA019DS0 CAS No.:1000339-53-6 MDL No.:MFCD09864671 MF:C8H5BrFN MW:214.0344 |
2-Methoxy-3-(trifluoromethyl)benzaldehydeCatalog No.:AA00009G CAS No.:1000339-54-7 MDL No.:MFCD08741399 MF:C9H7F3O2 MW:204.1459 |
3-Methyl-5-(trifluoromethoxy)benzaldehydeCatalog No.:AA00009F CAS No.:1000339-55-8 MDL No.:MFCD08741401 MF:C9H7F3O2 MW:204.1459 |
3-Bromo-5-fluorobenzal chlorideCatalog No.:AA0000AR CAS No.:1000339-58-1 MDL No.:MFCD09258971 MF:C7H4BrCl2F MW:257.9151 |
7-Bromo-6-fluoro-1h-indoleCatalog No.:AA0000AP CAS No.:1000339-62-7 MDL No.:MFCD09749664 MF:C8H5BrFN MW:214.0344 |
2-Methylsulfonyl-5-(trifluoromethyl)benzoic acidCatalog No.:AA0090OU CAS No.:1000339-64-9 MDL No.:MFCD09264536 MF:C9H7F3O4S MW:268.2097 |
2-Chloro-1-methanesulfonyl-4-(trifluoromethyl)benzeneCatalog No.:AA00947D CAS No.:1000339-68-3 MDL No.:MFCD09264544 MF:C8H6ClF3O2S MW:258.6452 |
4-Bromo-2-fluoro-5-methylsulfonyltolueneCatalog No.:AA01F3E3 CAS No.:1000339-74-1 MDL No.:MFCD09264557 MF:C8H8BrFO2S MW:267.1153 |
2-(Piperidin-1-yl)-4-(trifluoromethyl)thiazole-5-carboxylic acidCatalog No.:AA01C0ND CAS No.:1000339-77-4 MDL No.:MFCD09743735 MF:C10H11F3N2O2S MW:280.2667 |
2-Fluoro-3-methylbenzoyl chlorideCatalog No.:AA008XJ6 CAS No.:1000339-85-4 MDL No.:MFCD09743594 MF:C8H6ClFO MW:172.5840 |
Methyl 3-((4-(trifluoromethyl)piperidin-1-yl)methyl)benzoateCatalog No.:AA00ISW2 CAS No.:1000339-86-5 MDL No.:MFCD09864681 MF:C15H18F3NO2 MW:301.3041 |
Methyl 4-(2-(4-(trifluoromethyl)piperidin-1-yl)ethoxy)benzoateCatalog No.:AA00IZO1 CAS No.:1000339-87-6 MDL No.:MFCD09864682 MF:C16H20F3NO3 MW:331.3301 |
2-(4-(Trifluoromethyl)piperidin-1-yl)thiazole-5-carbaldehydeCatalog No.:AA00IWVD CAS No.:1000339-88-7 MDL No.:MFCD09864683 MF:C10H11F3N2OS MW:264.2673 |
3-(2-(4-(Trifluoromethyl)piperidin-1-yl)ethyl)benzaldehydeCatalog No.:AA00IWVC CAS No.:1000339-89-8 MDL No.:MFCD09864684 MF:C15H18F3NO MW:285.3047 |
4-(2-(4-(Trifluoromethyl)piperidin-1-yl)acetyl)-1H-pyrrole-2-carbaldehydeCatalog No.:AA00IUSW CAS No.:1000339-90-1 MDL No.:MFCD09864685 MF:C13H15F3N2O2 MW:288.2656 |
2-Bromo-3-fluorobenzoyl chlorideCatalog No.:AA0096QK CAS No.:1000339-91-2 MDL No.:MFCD09864686 MF:C7H3BrClFO MW:237.4535 |
4-Fluoro-2-(hydroxymethyl)benzonitrileCatalog No.:AA0000AL CAS No.:1000339-93-4 MDL No.:MFCD09864693 MF:C8H6FNO MW:151.1377 |
3-Chloro-4-(trifluoromethoxy)phenolCatalog No.:AA0000AK CAS No.:1000339-94-5 MDL No.:MFCD04972754 MF:C7H4ClF3O2 MW:212.5537 |
1H-Indazole, 4-(trifluoromethyl)-Catalog No.:AA0000AJ CAS No.:1000339-98-9 MDL No.:MFCD09263211 MF:C8H5F3N2 MW:186.1339 |
3,5-Dibromo-2-fluoro-4-methylpyridineCatalog No.:AA0000AI CAS No.:1000340-01-1 MDL No.:MFCD09864703 MF:C6H4Br2FN MW:268.9091 |
2-(2-Chlorophenyl)-2-(pyrrolidin-1-yl)acetamideCatalog No.:AA00H91N CAS No.:1000340-02-2 MDL No.:MFCD09910404 MF:C12H15ClN2O MW:238.7133 |
Ethyl 1-cyclopropyl-4-hydroxy-6-oxo-1,6-dihydropyridine-3-carboxylateCatalog No.:AA00H91O CAS No.:1000340-03-3 MDL No.:MFCD09910405 MF:C11H13NO4 MW:223.2252 |
2-(2-Aminopyrimidin-4-yl)-5-morpholinophenolCatalog No.:AA00H91P CAS No.:1000340-04-4 MDL No.:MFCD09910409 MF:C14H16N4O2 MW:272.3024 |
Methyl 2-(5-cyanobenzo[b]thiophen-2-yl)acetateCatalog No.:AA00H91Q CAS No.:1000340-05-5 MDL No.:MFCD09910410 MF:C12H9NO2S MW:231.2704 |
3-(Cyanomethylamino)-2-methylbenzoic acidCatalog No.:AA00H91R CAS No.:1000340-07-7 MDL No.:MFCD09910415 MF:C10H10N2O2 MW:190.1986 |
Ethyl 5-fluoro-4-hydroxy-6-oxo-1,6-dihydropyridine-3-carboxylateCatalog No.:AA00H91S CAS No.:1000340-08-8 MDL No.:MFCD09910417 MF:C8H8FNO4 MW:201.1518 |
4-(Pyridin-3-yloxy)benzenesulfonic acidCatalog No.:AA00H91T CAS No.:1000340-09-9 MDL No.:MFCD09910428 MF:C11H9NO4S MW:251.2585 |
2-(6-Propoxynaphthalen-1-yl)propane-1,3-diolCatalog No.:AA00H91U CAS No.:1000340-10-2 MDL No.:MFCD09910432 MF:C16H20O3 MW:260.3282 |
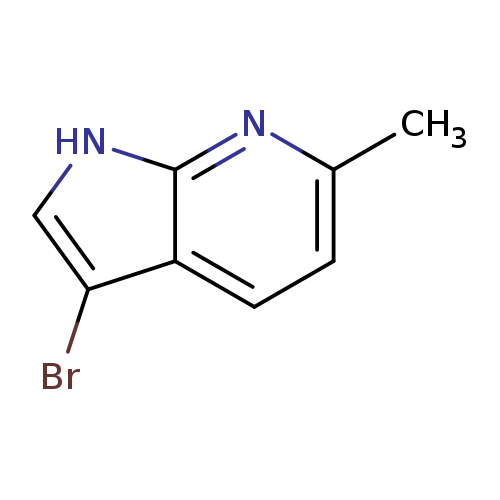
3-Bromo-6-methyl-7-azaindoleCatalog No.:AA0000BE CAS No.:1000340-28-2 MDL No.:MFCD09880116 MF:C8H7BrN2 MW:211.0586 |

3-Iodo-6-methyl-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA0000BD CAS No.:1000340-29-3 MDL No.:MFCD09880117 MF:C8H7IN2 MW:258.0591 |
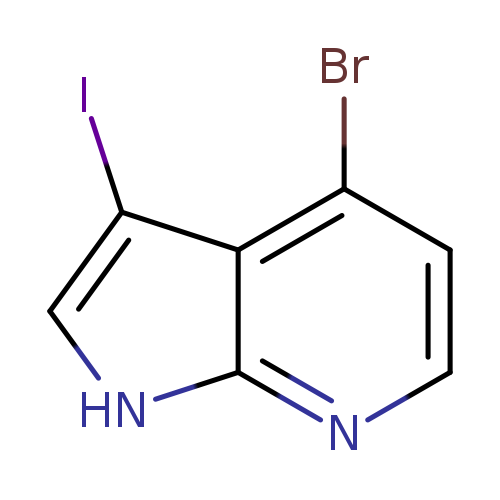
4-Bromo-3-iodo-7-azaindoleCatalog No.:AA0000B8 CAS No.:1000340-34-0 MDL No.:MFCD09880123 MF:C7H4BrIN2 MW:322.9285 |
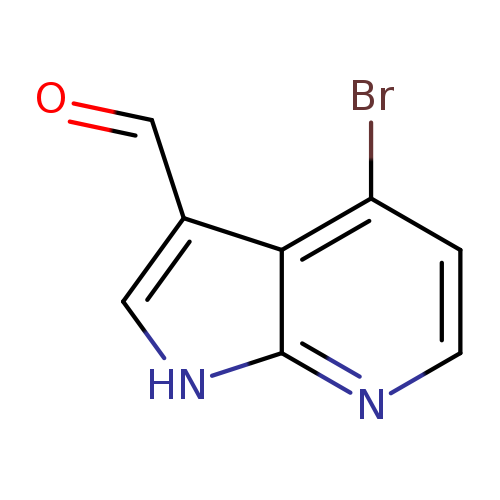
4-Bromo-1h-pyrrolo[2,3-b]pyridine-3-carbaldehydeCatalog No.:AA0000B7 CAS No.:1000340-35-1 MDL No.:MFCD09880126 MF:C8H5BrN2O MW:225.0421 |
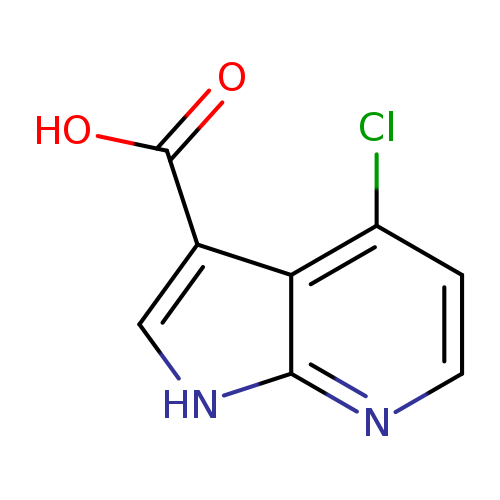
4-Chloro-1h-pyrrolo[2,3-b]pyridine-3-carboxylic acidCatalog No.:AA0000B5 CAS No.:1000340-37-3 MDL No.:MFCD09880128 MF:C8H5ClN2O2 MW:196.5905 |
4-Chloro-1h-pyrrolo[2,3-b]pyridin-3-amineCatalog No.:AA0000B4 CAS No.:1000340-38-4 MDL No.:MFCD09880130 MF:C7H6ClN3 MW:167.5956 |
3-Bromo-4-chloro-1H-pyrrolo[2,3-b]pyridineCatalog No.:AA0000B3 CAS No.:1000340-39-5 MDL No.:MFCD09880132 MF:C7H4BrClN2 MW:231.4771 |
3-Iodo-4-nitro-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA0000B2 CAS No.:1000340-40-8 MDL No.:MFCD09880134 MF:C7H4IN3O2 MW:289.0300 |
7-Methyl-1h-indazole-3-carboxylic acidCatalog No.:AA0000C3 CAS No.:1000340-53-3 MDL No.:MFCD07378884 MF:C9H8N2O2 MW:176.1720 |
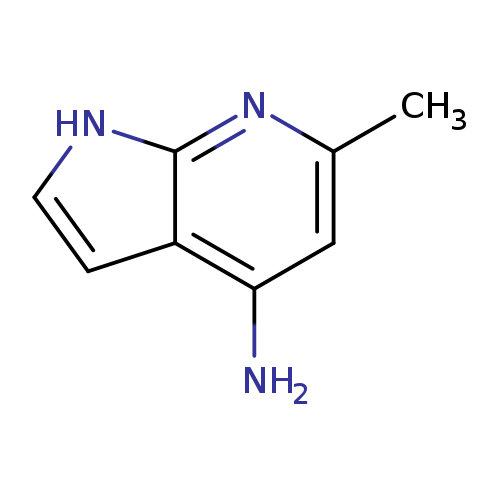
1H-Pyrrolo[2,3-b]pyridin-4-amine, 6-methyl-Catalog No.:AA0000BW CAS No.:1000340-60-2 MDL No.:MFCD09880148 MF:C8H9N3 MW:147.1772 |
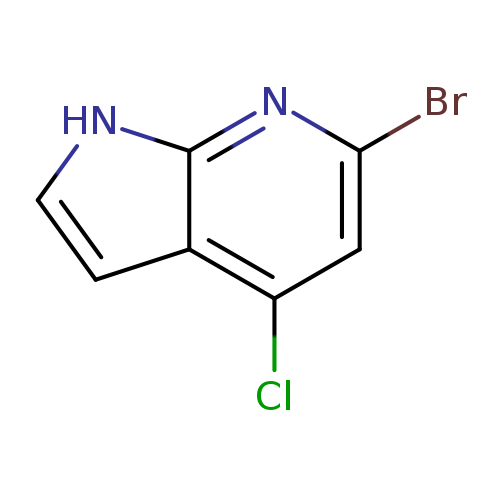
6-Bromo-4-chloro-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA0000BS CAS No.:1000340-64-6 MDL No.:MFCD09880150 MF:C7H4BrClN2 MW:231.4771 |
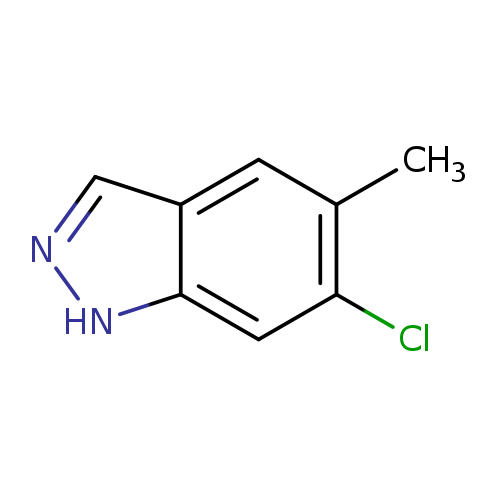
6-Chloro-5-methyl-1h-indazoleCatalog No.:AA0000DJ CAS No.:1000341-02-5 MDL No.:MFCD09880179 MF:C8H7ClN2 MW:166.6076 |
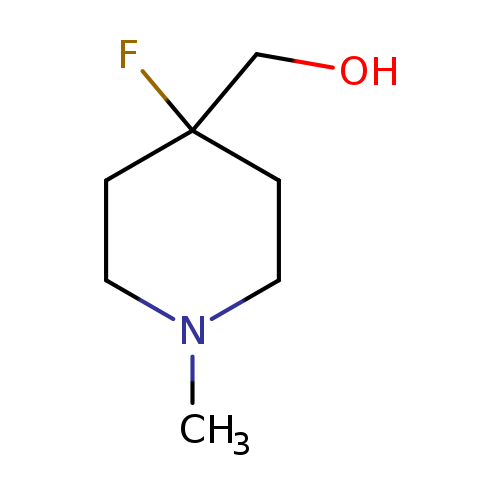
4-Fluoro-1-methyl-4-piperidinemethanolCatalog No.:AA0000DH CAS No.:1000341-04-7 MDL No.:MFCD09880180 MF:C7H14FNO MW:147.1906 |

3-Bromo-5-methoxy-1H-pyrrolo[3,2-b]pyridineCatalog No.:AA0000DC CAS No.:1000341-09-2 MDL No.:MFCD09263206 MF:C8H7BrN2O MW:227.0580 |
3-Amino-5-cyanobenzoic acidCatalog No.:AA0000D3 CAS No.:1000341-18-3 MDL No.:MFCD11049370 MF:C8H6N2O2 MW:162.1454 |
3-Bromo-6-(trifluoromethyl)-1h-indazoleCatalog No.:AA0000D0 CAS No.:1000341-21-8 MDL No.:MFCD09263228 MF:C8H4BrF3N2 MW:265.0300 |
3-Iodo-6-(trifluoromethyl)-1h-indazoleCatalog No.:AA0000E8 CAS No.:1000341-27-4 MDL No.:MFCD09263230 MF:C8H4F3IN2 MW:312.0304 |
3-Chloro-5-tert-butylbenzoic acidCatalog No.:AA0000E3 CAS No.:1000341-32-1 MDL No.:MFCD09880197 MF:C11H13ClO2 MW:212.6727 |
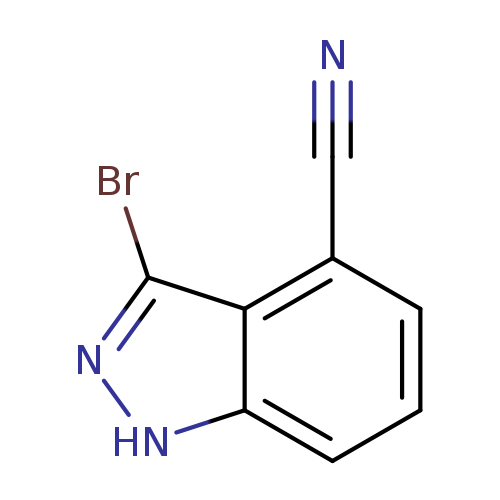
3-Bromo-1H-indazole-4-carbonitrileCatalog No.:AA0000DZ CAS No.:1000341-36-5 MDL No.:MFCD09263234 MF:C8H4BrN3 MW:222.0415 |
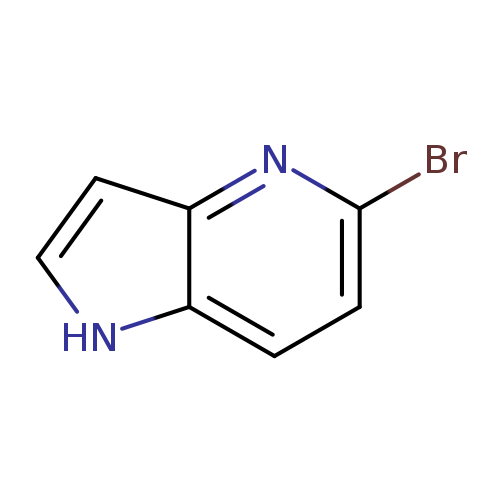
5-Bromo-4-azaindoleCatalog No.:AA0000EY CAS No.:1000341-51-4 MDL No.:MFCD09263244 MF:C7H5BrN2 MW:197.0320 |

6-Chloro-3-iodo-5-azaindoleCatalog No.:AA0000EU CAS No.:1000341-55-8 MDL No.:MFCD09749980 MF:C7H4ClIN2 MW:278.4775 |
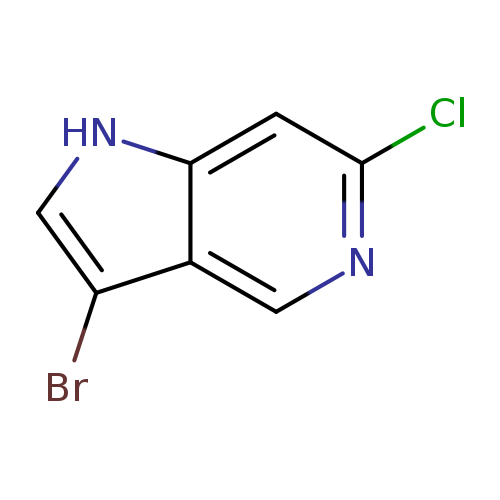
3-Bromo-6-chloro-5-azaindoleCatalog No.:AA0000EP CAS No.:1000341-61-6 MDL No.:MFCD09749982 MF:C7H4BrClN2 MW:231.4771 |
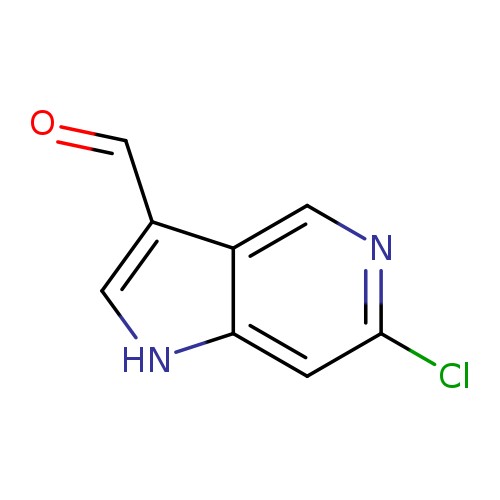
6-Chloro-1h-pyrrolo[3,2-c]pyridine-3-carbaldehydeCatalog No.:AA0000EM CAS No.:1000341-64-9 MDL No.:MFCD09749983 MF:C8H5ClN2O MW:180.5911 |
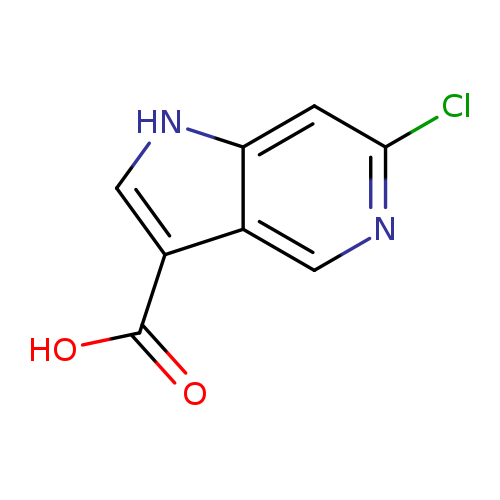
6-Chloro-1h-pyrrolo[3,2-c]pyridine-3-carboxylic acidCatalog No.:AA0000EJ CAS No.:1000341-67-2 MDL No.:MFCD09749984 MF:C8H5ClN2O2 MW:196.5905 |
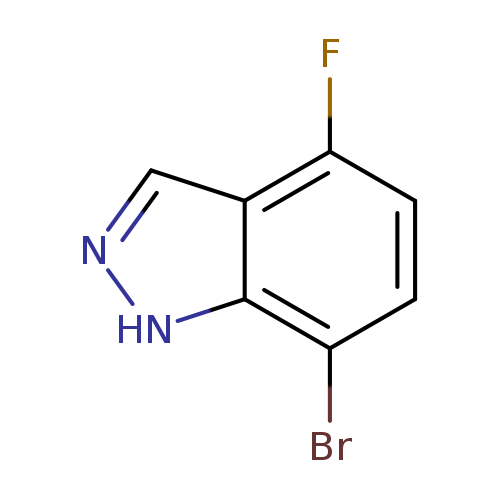
7-Bromo-4-fluoro-1H-indazoleCatalog No.:AA0000EE CAS No.:1000341-72-9 MDL No.:MFCD09749914 MF:C7H4BrFN2 MW:215.0225 |
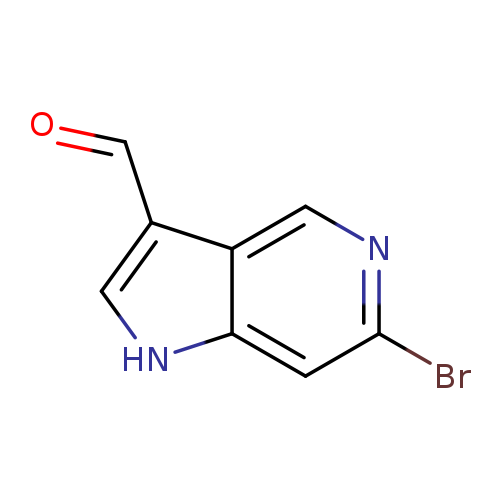
6-Bromo-1h-pyrrolo[3,2-c]pyridine-3-carbaldehydeCatalog No.:AA0000EB CAS No.:1000341-75-2 MDL No.:MFCD09749988 MF:C8H5BrN2O MW:225.0421 |
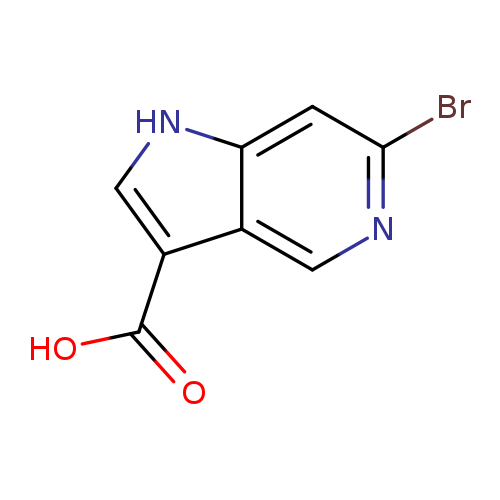
6-Bromo-1h-pyrrolo[3,2-c]pyridine-3-carboxylic acidCatalog No.:AA0000FN CAS No.:1000341-77-4 MDL No.:MFCD09749989 MF:C8H5BrN2O2 MW:241.0415 |
7-Bromo-4-chloro-1H-indazoleCatalog No.:AA0000FC CAS No.:1000341-88-7 MDL No.:MFCD09749923 MF:C7H4BrClN2 MW:231.4771 |
3-Amino-2-methyl-5-bromo benzoic acid methyl esterCatalog No.:AA0000G4 CAS No.:1000342-11-9 MDL No.:MFCD08690071 MF:C9H10BrNO2 MW:244.0852 |
Methyl 5-bromo-1h-indazole-6-carboxylateCatalog No.:AA0000FU CAS No.:1000342-30-2 MDL No.:MFCD09749941 MF:C9H7BrN2O2 MW:255.0681 |
3-Bromo-2-methyl-5-nitroanilineCatalog No.:AA0000FR CAS No.:1000342-34-6 MDL No.:MFCD09880011 MF:C7H7BrN2O2 MW:231.0467 |
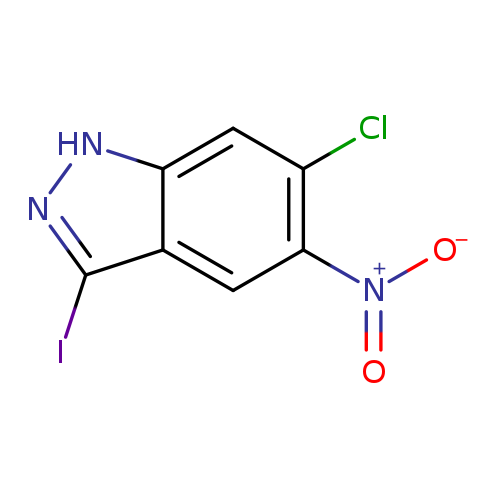
6-Chloro-3-iodo-5-nitro-1h-indazoleCatalog No.:AA0000GU CAS No.:1000342-47-1 MDL No.:MFCD08690112 MF:C7H3ClIN3O2 MW:323.4751 |
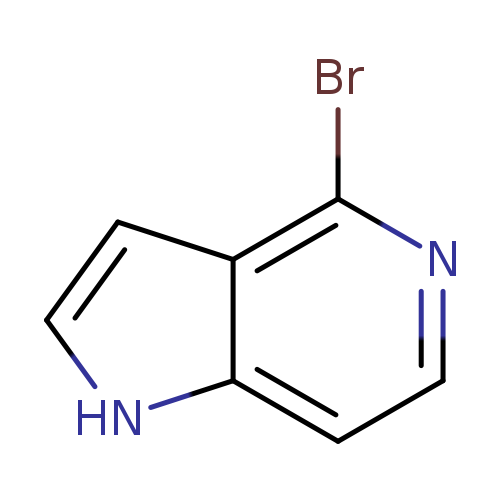
4-Bromo-1h-pyrrolo[3,2-c]pyridineCatalog No.:AA0000HP CAS No.:1000342-68-6 MDL No.:MFCD08690131 MF:C7H5BrN2 MW:197.0320 |
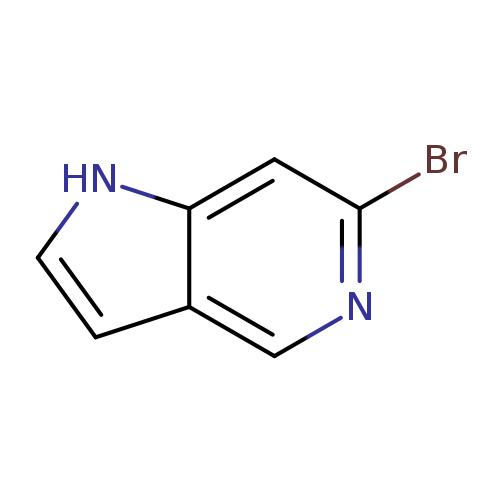
6-Bromo-5-azaindoleCatalog No.:AA0000HM CAS No.:1000342-71-1 MDL No.:MFCD08690134 MF:C7H5BrN2 MW:197.0320 |
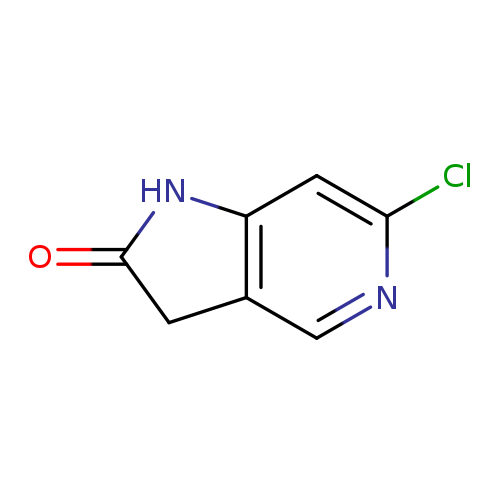
6-Chloro-1,3-dihydro-2h-pyrrolo[3,2-c]pyridin-2-oneCatalog No.:AA0000HF CAS No.:1000342-80-2 MDL No.:MFCD08690138 MF:C7H5ClN2O MW:168.5804 |
2-Amino-3,5-diiodo-6-methylpyridineCatalog No.:AA0000HA CAS No.:1000342-88-0 MDL No.:MFCD09880040 MF:C6H6I2N2 MW:359.9342 |
4-Bromo-6-(trifluoromethyl)-1h-indoleCatalog No.:AA0000H5 CAS No.:1000342-93-7 MDL No.:MFCD09026955 MF:C9H5BrF3N MW:264.0419 |
4-Bromo-6-(trifluoromethyl)-1h-indazoleCatalog No.:AA0000H3 CAS No.:1000342-95-9 MDL No.:MFCD09026956 MF:C8H4BrF3N2 MW:265.0300 |
3-Fluoro-2-methyl-4-nitroanilineCatalog No.:AA00H91V CAS No.:1000342-98-2 MDL No.:MFCD09026961 MF:C7H7FN2O2 MW:170.1411 |
5-Bromo-6-iodo-1h-indoleCatalog No.:AA0000IB CAS No.:1000343-06-5 MDL No.:MFCD09880052 MF:C8H5BrIN MW:321.9405 |
5-Bromo-6-methyl-1H-indoleCatalog No.:AA0000I6 CAS No.:1000343-13-4 MDL No.:MFCD09026969 MF:C9H8BrN MW:210.0705 |
5-Fluoro-6-methyl-1h-indoleCatalog No.:AA0000I4 CAS No.:1000343-16-7 MDL No.:MFCD09026971 MF:C9H8FN MW:149.1649 |
5-Cyano-6-methyl indoleCatalog No.:AA0000I0 CAS No.:1000343-22-5 MDL No.:MFCD09026973 MF:C10H8N2 MW:156.1839 |
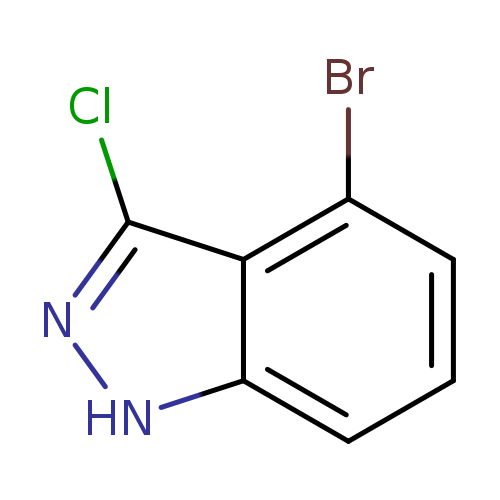
4-Bromo-3-chloro-1h-indazoleCatalog No.:AA0000IZ CAS No.:1000343-46-3 MDL No.:MFCD09026985 MF:C7H4BrClN2 MW:231.4771 |
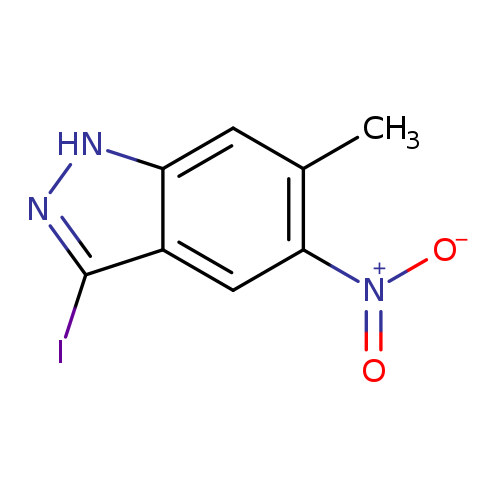
3-Iodo-6-methyl-5-nitro-1h-indazoleCatalog No.:AA0000IT CAS No.:1000343-55-4 MDL No.:MFCD09026988 MF:C8H6IN3O2 MW:303.0566 |
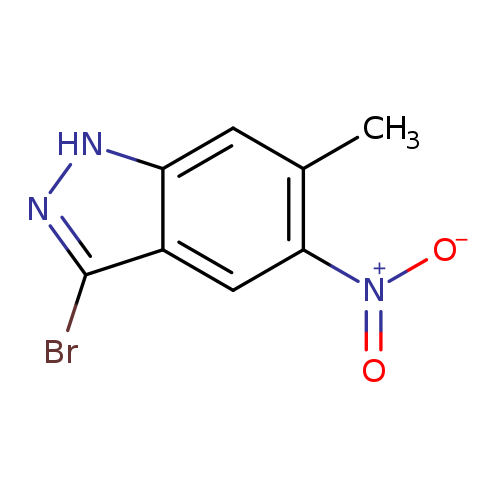
3-Bromo-6-methyl-5-nitro-1H-indazoleCatalog No.:AA0000IR CAS No.:1000343-58-7 MDL No.:MFCD09026989 MF:C8H6BrN3O2 MW:256.0561 |
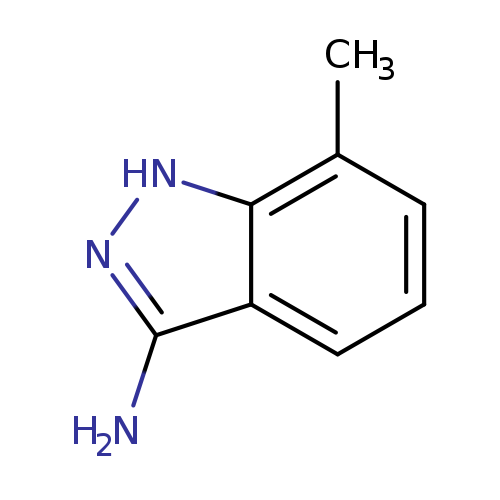
3-Amino-7-methyl (1h)indazoleCatalog No.:AA0000IQ CAS No.:1000343-59-8 MDL No.:MFCD09880079 MF:C8H9N3 MW:147.1772 |
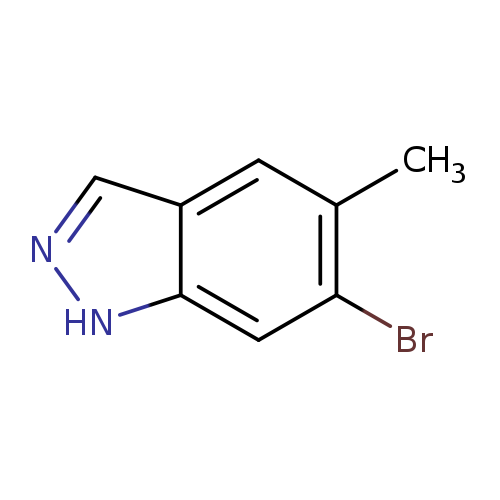
6-Bromo-5-methyl-1H-indazoleCatalog No.:AA0000IK CAS No.:1000343-69-0 MDL No.:MFCD09026997 MF:C8H7BrN2 MW:211.0586 |
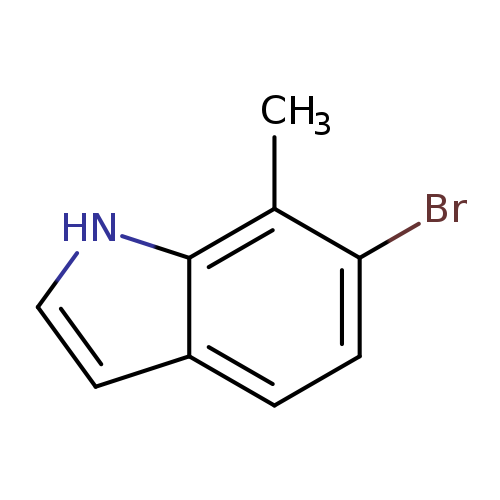
6-Bromo-7-methyl-1H-indoleCatalog No.:AA0000JE CAS No.:1000343-89-4 MDL No.:MFCD09027015 MF:C9H8BrN MW:210.0705 |
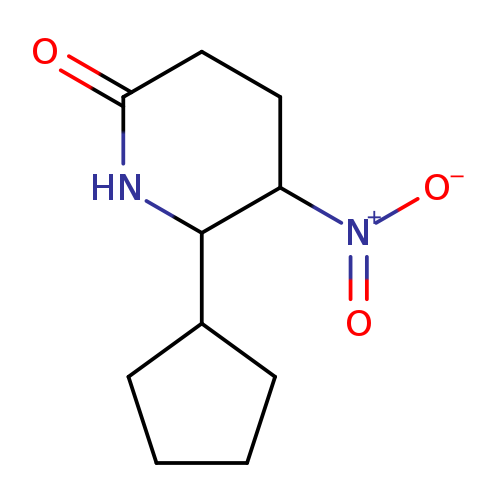
6-cyclopentyl-5-nitropiperidin-2-oneCatalog No.:AA01B9WE CAS No.:1000345-72-1 MDL No.:MFCD11050969 MF:C10H16N2O3 MW:212.2456 |
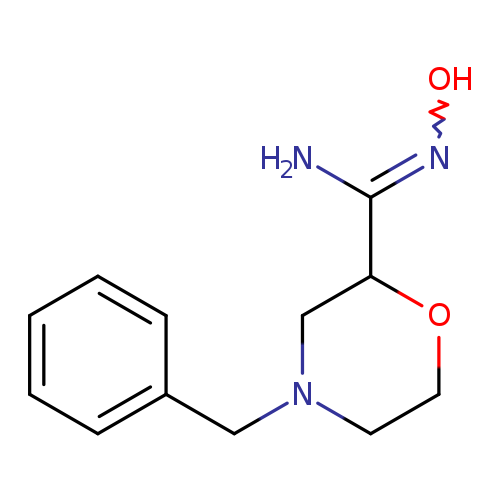
4-Benzyl-N'-hydroxymorpholine-2-carboximidamideCatalog No.:AA01A5OC CAS No.:1000349-55-2 MDL No.:MFCD09953991 MF:C12H17N3O2 MW:235.2823 |
6-(tert-Butyl)-2-hydroxypyrimidine-4-carboxylic acidCatalog No.:AA00J2I9 CAS No.:1000353-37-6 MDL No.:MFCD11049200 MF:C9H15ClN2O4 MW:250.6794 |
3'-Trifluoromethylbiphenyl-4-carbaldehydeCatalog No.:AA0000JT CAS No.:100036-64-4 MDL No.:MFCD03424645 MF:C14H9F3O MW:250.2159 |
Sodium 1,1'-(piperazine-1,4-diyl)diethanesulfonate 1-(4-(1-sulfoethyl)piperazin-1-yl)ethanesulfonate(3:2)Catalog No.:AA0000JO CAS No.:100037-69-2 MDL No.:MFCD00066393 MF:C16H33N4Na3O12S4 MW:670.6821 |
4-[(3-Fluorophenyl)methoxy]-3-[(3-fluorophenyl)methyl]benzaldehydeCatalog No.:AA0000K1 CAS No.:1000370-24-0 MDL No.:MFCD28502380 MF:C21H16F2O2 MW:338.3473 |
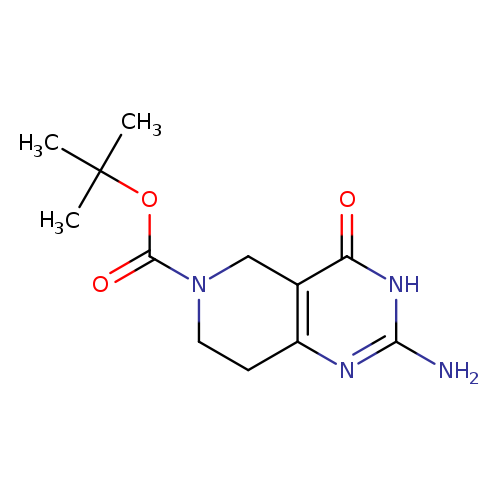
2-Amino-4-oxo-1,5,7,8-tetrahydro-4h-pyrido[4,3-d]pyrimidine-6-carboxylic acid tert-butyl esterCatalog No.:AA0000JX CAS No.:1000386-01-5 MDL No.:MFCD09999172 MF:C12H18N4O3 MW:266.2963 |
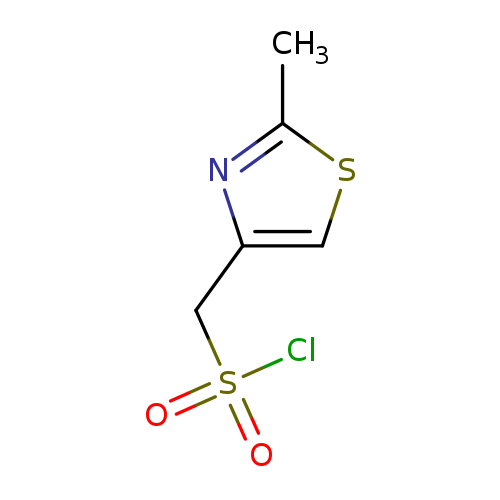
(2-methyl-1,3-thiazol-4-yl)methanesulfonyl chlorideCatalog No.:AA01AF8M CAS No.:1000394-87-5 MDL No.:MFCD16671829 MF:C5H6ClNO2S2 MW:211.6896 |
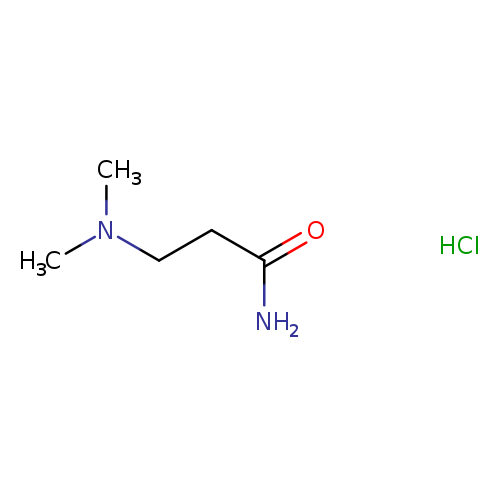
3-(Dimethylamino)propanamide hydrochlorideCatalog No.:AA00H91X CAS No.:1000395-57-2 MDL No.:MFCD29090668 MF:C5H13ClN2O MW:152.6225 |

HymexazolCatalog No.:AA0000KE CAS No.:10004-44-1 MDL No.:MFCD00144468 MF:C4H5NO2 MW:99.0880 |
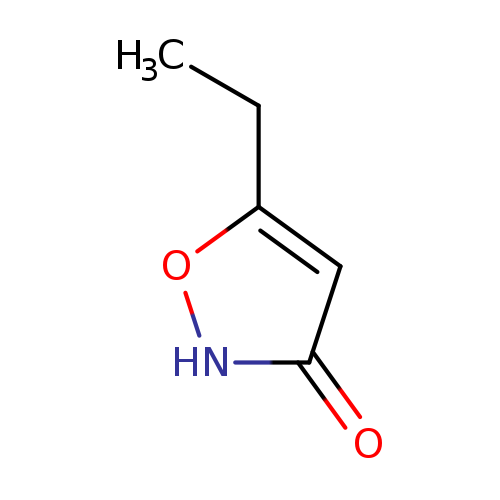
5-Ethylisoxazol-3-olCatalog No.:AA0000KD CAS No.:10004-45-2 MDL No.:MFCD15474929 MF:C5H7NO2 MW:113.1146 |
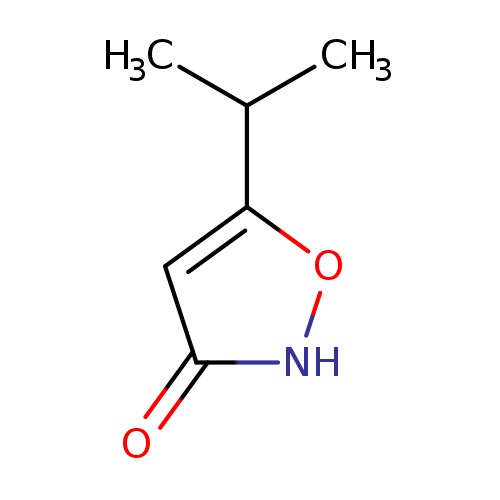
3(2H)-Isoxazolone, 5-(1-Methylethyl)-Catalog No.:AA0093RD CAS No.:10004-47-4 MDL No.:MFCD20486309 MF:C6H9NO2 MW:127.1412 |
gastric inhibitory polypeptide (human)-Asn-Ile-Thr-Gln-OHCatalog No.:AA01EAXE CAS No.:100040-31-1 MDL No.:MFCD00081634 MF:C226H338N60O66S MW:4983.5293 |
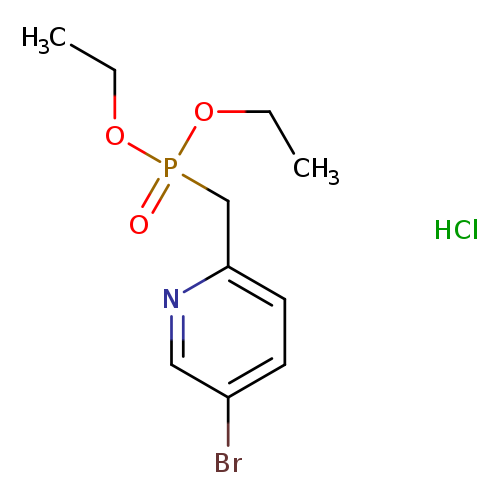
Diethyl [(5-bromopyridin-2-yl)methyl]phosphonate hydrochlorideCatalog No.:AA00IXIB CAS No.:1000400-92-9 MDL No.:MFCD22383959 MF:C10H16BrClNO3P MW:344.5697 |
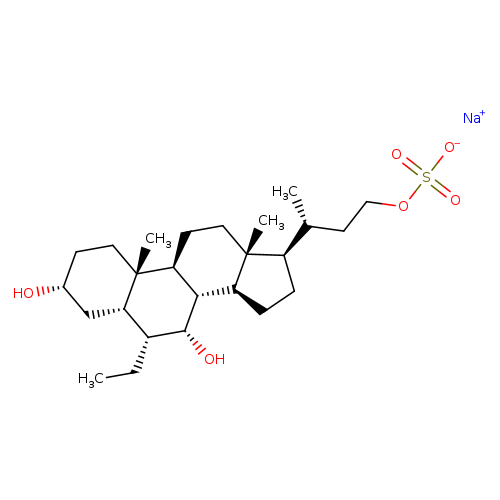
Int-767Catalog No.:AA008TJG CAS No.:1000403-03-1 MDL No.:MFCD28386211 MF:C25H43NaO6S MW:494.6601 |
Resorufin b-D-cellobiosideCatalog No.:AA01EB02 CAS No.:1000404-48-7 MDL No.:MFCD09954381 MF:C24H27NO13 MW:537.4701 |
2-(4-bromophenyl)-2-(piperazin-1-yl)acetonitrileCatalog No.:AA01AAJM CAS No.:1000406-20-1 MDL No.:MFCD09816843 MF:C12H14BrN3 MW:280.1637 |
methyl 3-amino-4-bromo-5-phenylthiophene-2-carboxylateCatalog No.:AA01BIN2 CAS No.:1000409-69-7 MDL No.:MFCD28137934 MF:C12H10BrNO2S MW:312.1823 |
[(3S)-6-({3-[4-(3-methanesulfonylpropoxy)-2,6-dimethylphenyl]phenyl}methoxy)-2,3-dihydro-1-benzofuran-3-yl]acetic acidCatalog No.:AA0000JU CAS No.:1000413-72-8 MDL No.:MFCD18251445 MF:C29H32O7S MW:524.6252 |
3-Bromo-4-phenoxybenzaldehydeCatalog No.:AA0000KL CAS No.:1000414-11-8 MDL No.:MFCD27937159 MF:C13H9BrO2 MW:277.1134 |
3-Methoxy-N-methyl-4-nitroanilineCatalog No.:AA00IXEC CAS No.:1017782-97-6 MDL No.:MFCD09972263 MF:C8H10N2O3 MW:182.1766 |

Methyl 5-bromo-2-morpholinonicotinateCatalog No.:AA0005GO CAS No.:1017782-99-8 MDL No.:MFCD09972266 MF:C11H13BrN2O3 MW:301.1365 |
Methyl 5-bromo-2-piperidinonicotinateCatalog No.:AA00IVE8 CAS No.:1017783-01-5 MDL No.:MFCD09972267 MF:C12H15BrN2O2 MW:299.1637 |
1-Methyl-2-(1-methyl-1H-pyrazol-4-yl)-ethylamineCatalog No.:AA0092P0 CAS No.:1017783-02-6 MDL No.:MFCD08453571 MF:C7H13N3 MW:139.1982 |
5-Bromo-2-morpholinonicotinic acidCatalog No.:AA0005GN CAS No.:1017783-03-7 MDL No.:MFCD09972268 MF:C10H11BrN2O3 MW:287.1099 |
N-Benzyl-6-chloro-2-methylpyrimidin-4-amineCatalog No.:AA00ITJX CAS No.:1017783-05-9 MDL No.:MFCD09972270 MF:C12H12ClN3 MW:233.6968 |
methyl 4-(1,3-thiazolidin-2-yl)benzoateCatalog No.:AA00ITJY CAS No.:1017783-07-1 MDL No.:MFCD09972272 MF:C11H13NO2S MW:223.2914 |
6-Bromo-1,4-dihydro-2h-3,1-benzoxazin-2-oneCatalog No.:AA0005GM CAS No.:1017783-09-3 MDL No.:MFCD09972273 MF:C8H6BrNO2 MW:228.0427 |
2-Methyl-2-(thiophen-2-yl)butanoic acidCatalog No.:AA0090VF CAS No.:1017783-11-7 MDL No.:MFCD09865028 MF:C9H12O2S MW:184.2554 |
(4-Chloro-1-methyl-1H-pyrazol-3-yl)methanolCatalog No.:AA00JG3G CAS No.:1017783-29-7 MDL No.:MFCD06202887 MF:C5H7ClN2O MW:146.5749 |
Methyl 4-chloro-1-methylpyrazole-3-carboxylateCatalog No.:AA00H9JV CAS No.:1017784-04-1 MDL No.:MFCD06203977 MF:C6H7ClN2O2 MW:174.5850 |
(4-chloro-1-methyl-1H-pyrazol-3-yl)methanamineCatalog No.:AA01AODK CAS No.:1017785-44-2 MDL No.:MFCD04967128 MF:C5H8ClN3 MW:145.5901 |
(4-(1H-Pyrazol-5-yl)phenyl)methanamineCatalog No.:AA0005GJ CAS No.:1017785-80-6 MDL No.:MFCD11200994 MF:C10H11N3 MW:173.2144 |
2-(1-phenyl-1H-pyrazol-5-yl)ethanamineCatalog No.:AA01BHYH CAS No.:1017787-52-8 MDL No.:MFCD08448959 MF:C11H13N3 MW:187.2410 |
1-Cbz-2-cyanopiperidineCatalog No.:AA0005GH CAS No.:1017788-63-4 MDL No.:MFCD08704536 MF:C14H16N2O2 MW:244.2890 |
4-Chloro-7-hydroxy-quinoline-3-carbonitrileCatalog No.:AA0005GG CAS No.:1017788-66-7 MDL No.:MFCD12962824 MF:C10H5ClN2O MW:204.6125 |
2-(4-Bromo-1-methyl-1h-pyrazol-5-yl)ethanamineCatalog No.:AA008UNN CAS No.:1017788-72-5 MDL No.:MFCD08449281 MF:C6H10BrN3 MW:204.0677 |
2-(5-Bromo-pyrimidin-2-yloxy)-benzonitrileCatalog No.:AA008S66 CAS No.:1017789-04-6 MDL No.:MFCD09991555 MF:C11H6BrN3O MW:276.0888 |
5-Bromo-2-(pyridin-4-yloxy)-pyrimidineCatalog No.:AA0005GE CAS No.:1017789-07-9 MDL No.:MFCD09991557 MF:C9H6BrN3O MW:252.0674 |
3-Amino-2-cyano-5-ethylthiopheneCatalog No.:AA0005GB CAS No.:1017789-16-0 MDL No.:MFCD09991576 MF:C7H8N2S MW:152.2168 |
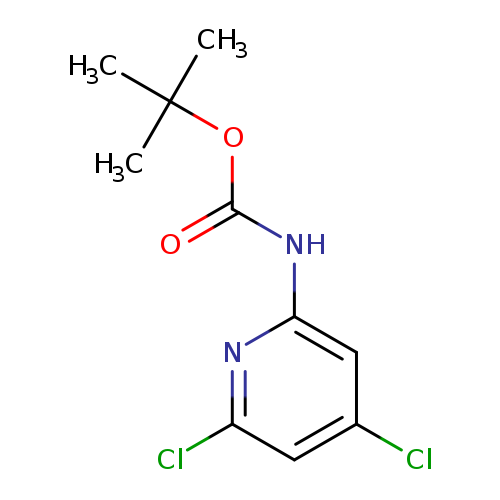
2-Boc-amino-4,6-dichloropyridineCatalog No.:AA0005HH CAS No.:1017789-38-6 MDL No.:MFCD09991634 MF:C10H12Cl2N2O2 MW:263.1205 |
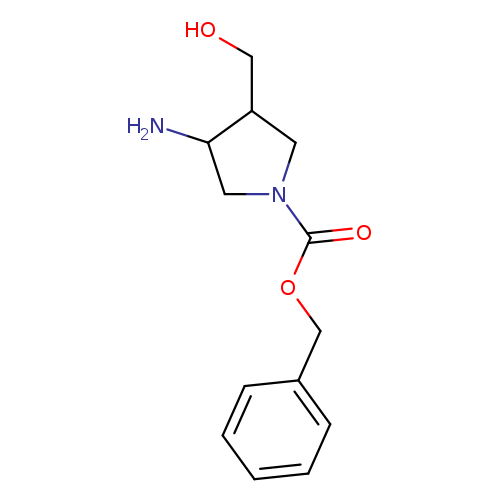
1-Cbz-3-amino-4-hydroxymethylpyrrolidineCatalog No.:AA0005HF CAS No.:1017789-40-0 MDL No.:MFCD09991641 MF:C13H18N2O3 MW:250.2936 |

Cyclopropanecarboxamide, N-(methoxymethyl)-1-(trifluoromethyl)-Catalog No.:AA0005H5 CAS No.:1017789-65-9 MDL No.:MFCD09991723 MF:C7H10F3NO2 MW:197.1550 |
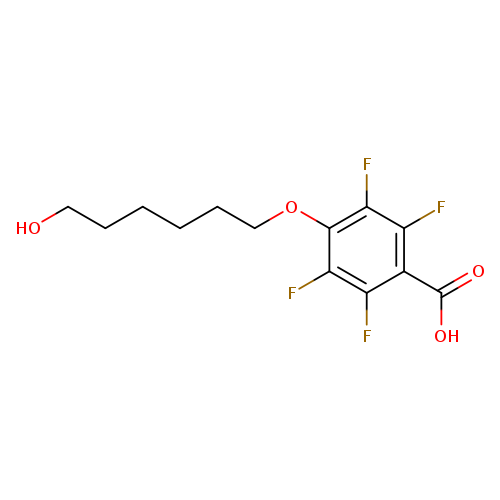
2,3,5,6-Tetrafluoro-4-(6-hydroxyhexyloxy)benzoic acidCatalog No.:AA0005H2 CAS No.:1017789-70-6 MDL No.:MFCD09991743 MF:C13H14F4O4 MW:310.2415 |
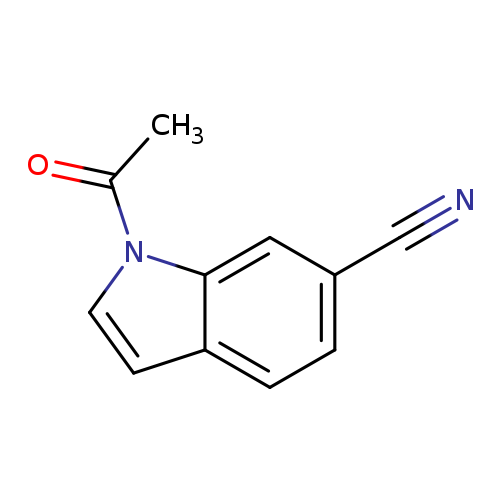
1-Acetyl-1h-indole-6-carbonitrileCatalog No.:AA008VC5 CAS No.:1017791-09-1 MDL No.:MFCD09972002 MF:C11H8N2O MW:184.1940 |
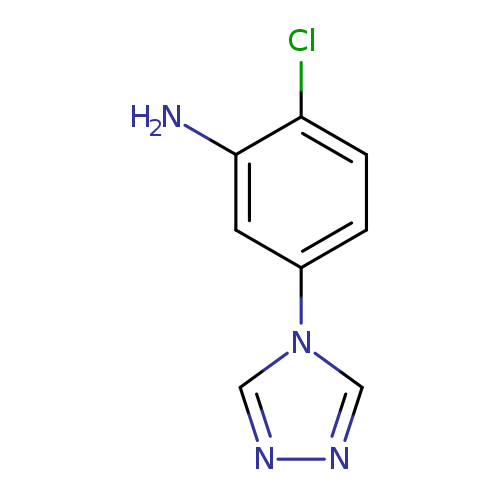
2-Chloro-5-(4H-1,2Catalog No.:AA01FOAF CAS No.:1017791-27-3 MDL No.:MFCD09972035 MF:C8H7ClN4 MW:194.6210 |
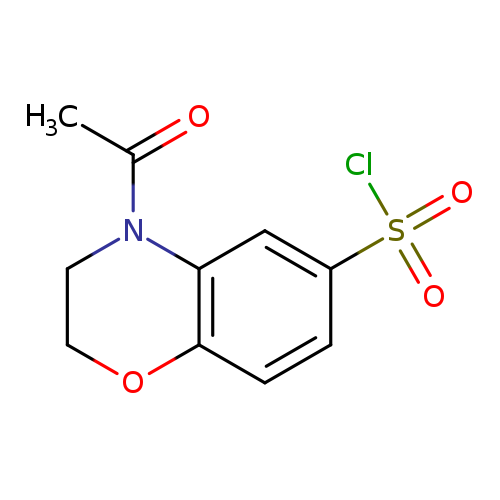
4-Acetyl-3,4-dihydro-2h-1,4-benzoxazine-6-sulfonyl chlorideCatalog No.:AA0005GZ CAS No.:1017791-37-5 MDL No.:MFCD09972061 MF:C10H10ClNO4S MW:275.7087 |
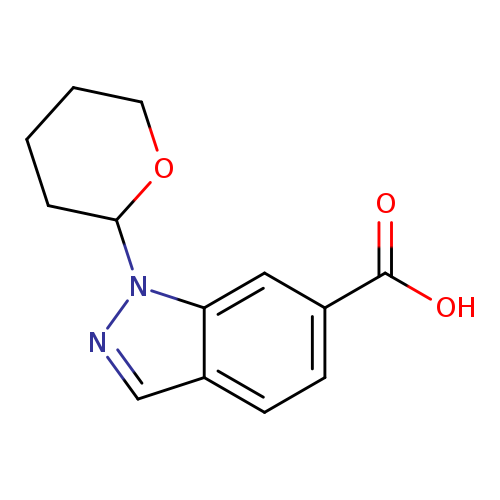
1-(Tetrahydro-pyran-2-yl)-1h-indazole-6-carboxylic acidCatalog No.:AA008TR9 CAS No.:1017792-97-0 MDL No.:MFCD09909303 MF:C13H14N2O3 MW:246.2619 |
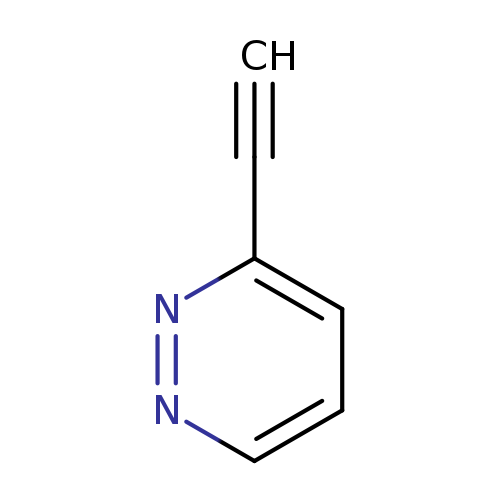
3-EthynylpyridazineCatalog No.:AA0005GY CAS No.:1017793-08-6 MDL No.:MFCD16988473 MF:C6H4N2 MW:104.1094 |
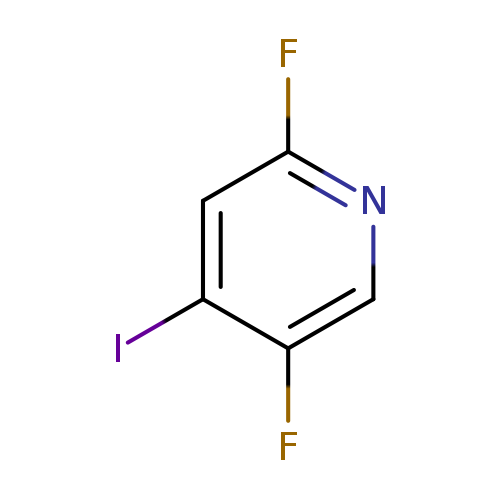
2,5-Difluoro-4-iodopyridineCatalog No.:AA0005HR CAS No.:1017793-20-2 MDL No.:MFCD11040606 MF:C5H2F2IN MW:240.9774 |
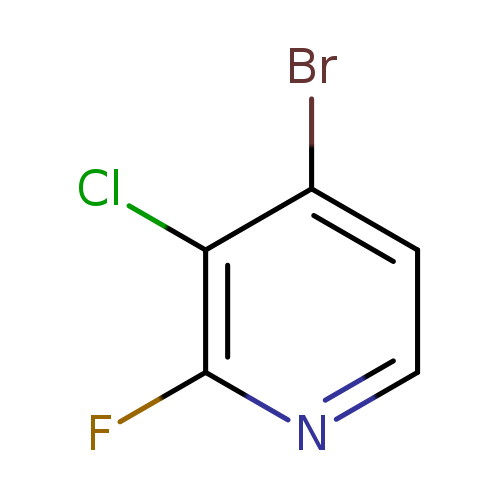
4-Bromo-3-chloro-2-fluoropyridineCatalog No.:AA0005HQ CAS No.:1017793-21-3 MDL No.:MFCD11040607 MF:C5H2BrClFN MW:210.4315 |
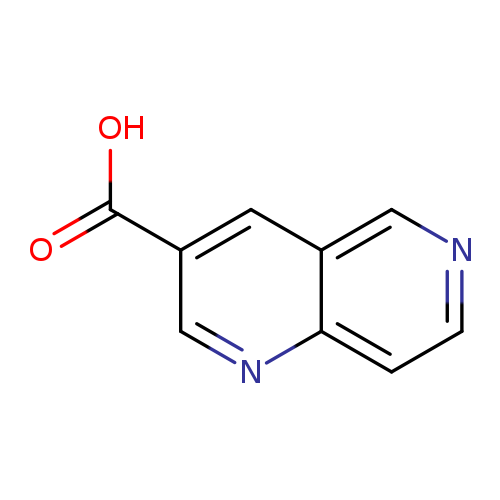
1,6-Naphthyridine-3-carboxylic acidCatalog No.:AA0005HP CAS No.:1017793-59-7 MDL No.:MFCD07366468 MF:C9H6N2O2 MW:174.1561 |
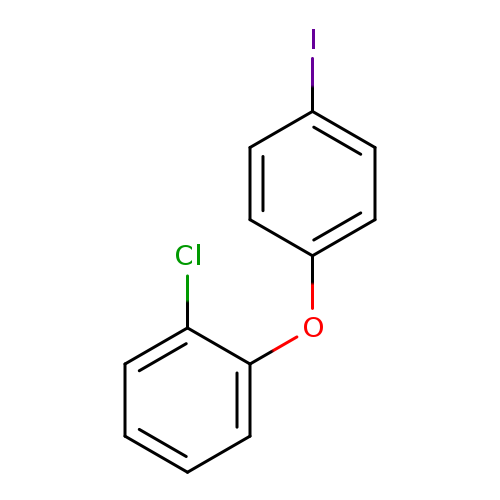
1-Chloro-2-(4-iodophenoxy)benzeneCatalog No.:AA019SXS CAS No.:1017793-89-3 MDL No.:MFCD06796297 MF:C12H8ClIO MW:330.5488 |
4-(4-Methylphenoxy)iodobenzeneCatalog No.:AA00H9JZ CAS No.:1017793-91-7 MDL No.:MFCD06796301 MF:C13H11IO MW:310.1303 |
3-Amino-5-(4-morpholinylmethyl-d2)-2-Oxazolidinone-4,4,5-d3Catalog No.:AA008T0G CAS No.:1017793-94-0 MDL No.:MFCD04973439 MF:C8H10D5N3O3 MW:206.2538 |
3-(1H-Pyrazol-4-yl)benzaldehydeCatalog No.:AA008RZ9 CAS No.:1017794-46-5 MDL No.:MFCD08056281 MF:C10H8N2O MW:172.1833 |
4-(1H-Pyrazol-4-yl)benzoic acidCatalog No.:AA008RZD CAS No.:1017794-47-6 MDL No.:MFCD06803014 MF:C10H8N2O2 MW:188.1827 |
4-AMINO-N-(3-METHOXYPROPYL)BENZAMIDECatalog No.:AA00982F CAS No.:1017795-08-2 MDL No.:MFCD09878551 MF:C11H16N2O2 MW:208.2569 |
tert-Butyl 3-sulfanylpiperidine-1-carboxylateCatalog No.:AA0005HN CAS No.:1017798-34-3 MDL No.:MFCD10566038 MF:C10H19NO2S MW:217.3284 |
7-Chloro-2,2-dimethyl-2,3-dihydrobenzofuranCatalog No.:AA0005I9 CAS No.:10178-55-9 MDL No.:MFCD00276976 MF:C10H11ClO MW:182.6467 |
7-broMo-2,2-diMethyl-2,3-dihydro-1-benzofuranCatalog No.:AA0092RV CAS No.:10178-57-1 MDL No.:MFCD11858106 MF:C10H11BrO MW:227.0977 |
2-(dibenzylamino)-2-methylpropan-1-olCatalog No.:AA01BBTR CAS No.:101781-44-6 MDL No.:MFCD28714636 MF:C18H23NO MW:269.3813 |
Ethyl 1-cyclohexyl-5-hydroxy-2-methyl-1h-indole-3-carboxylateCatalog No.:AA0005HZ CAS No.:101782-20-1 MDL No.:MFCD00408072 MF:C18H23NO3 MW:301.3801 |
1-[bis(4-chlorophenyl)methyl]-4-methyl-piperazineCatalog No.:AA01EPDK CAS No.:101784-44-5 MDL No.: MF:C18H20Cl2N2 MW:335.2708 |
2H-Indol-2-one, 5-bromo-6-fluoro-1,3-dihydro-3,3-dimethyl-Catalog No.:AA0091Q7 CAS No.:1017860-04-6 MDL No.:MFCD13192270 MF:C10H9BrFNO MW:258.0870 |
MLS-573151Catalog No.:AA0097CZ CAS No.:10179-57-4 MDL No.:MFCD00358662 MF:C21H19N3O2S MW:377.4595 |
3H-1,2,3-Triazolo[4,5-d]pyrimidin-5-amineCatalog No.:AA0005I5 CAS No.:10179-84-7 MDL No.:MFCD18806992 MF:C4H4N6 MW:136.1148 |
7-Hydroxyquinoline-4-carboxylic acidCatalog No.:AA0005ID CAS No.:1017969-32-2 MDL No.:MFCD11847889 MF:C10H7NO3 MW:189.1675 |
4-(4-Chlorophenylsulfanyl)piperidine, HClCatalog No.:AA0005II CAS No.:101798-64-5 MDL No.:MFCD03840148 MF:C11H15Cl2NS MW:264.2145 |
4-(Phenylthio)piperidine hydrochlorideCatalog No.:AA0005IH CAS No.:101798-66-7 MDL No.:MFCD03840144 MF:C11H16ClNS MW:229.7694 |
4-(4-Fluorophenylsulfanyl)piperidine, HClCatalog No.:AA0005IF CAS No.:101798-76-9 MDL No.:MFCD04117760 MF:C11H15ClFNS MW:247.7599 |
6-(Chloromethyl)benzo[de]isochromen-1(3H)-oneCatalog No.:AA0005IW CAS No.:1018-47-9 MDL No.:MFCD00683109 MF:C13H9ClO2 MW:232.6624 |
1,4-Dihydroxynaphthalene-2,3-dicarbonitrileCatalog No.:AA0005IS CAS No.:1018-79-7 MDL No.:MFCD00192049 MF:C12H6N2O2 MW:210.1882 |
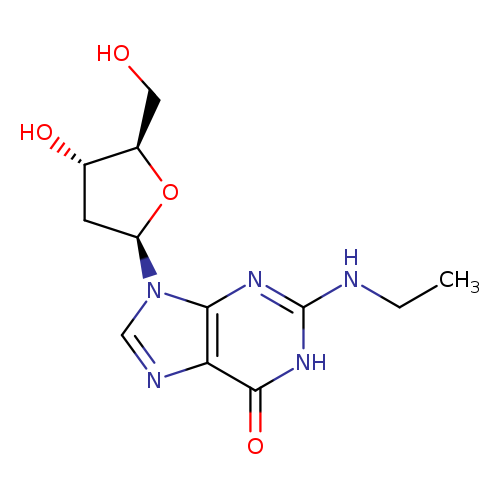
Guanosine, 2'-deoxy-N-ethyl-Catalog No.:AA0005JF CAS No.:101803-03-6 MDL No.:MFCD01631004 MF:C12H17N5O4 MW:295.2945 |
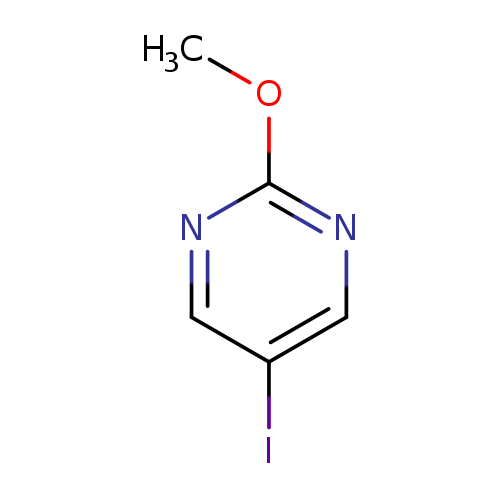
5-Iodo-2-methoxypyrimidineCatalog No.:AA0005JE CAS No.:101803-06-9 MDL No.:MFCD18412677 MF:C5H5IN2O MW:236.0105 |
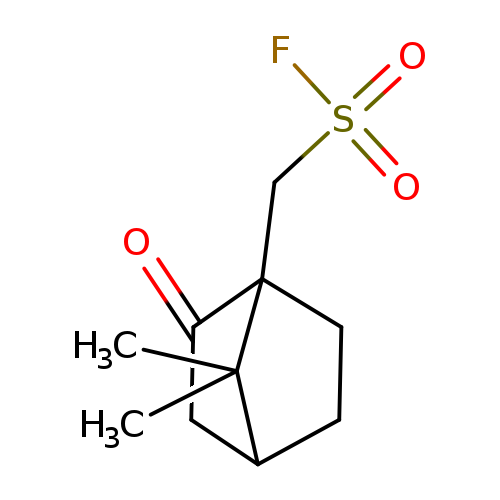
{7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl}methanesulfonyl fluorideCatalog No.:AA019VZL CAS No.:101803-61-6 MDL No.:MFCD12913926 MF:C10H15FO3S MW:234.2877 |
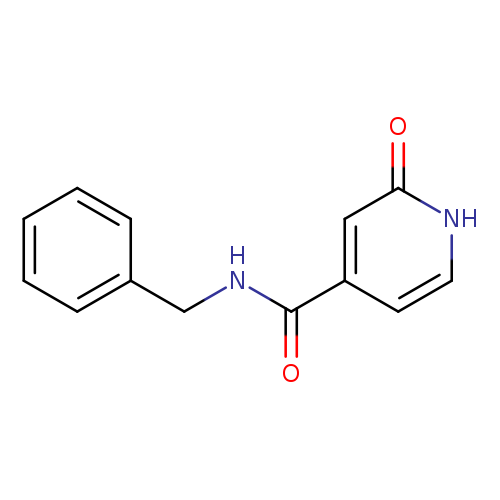
N-Benzyl-2-oxo-1,2-dihydropyridine-4-carboxamideCatalog No.:AA01ABSJ CAS No.:1018046-16-6 MDL No.:MFCD24854204 MF:C13H12N2O2 MW:228.2466 |