2019-11-20 15:37:35
Indranil Dutta, Abir Sarbajna, Pragati Pandey, S. M. Wahidur Rahaman, Kuldeep Singh, and Jitendra K. Bera*
Department of Chemistry and Center for Environmental Sciences and Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, India
INTRODUCTION
The prospect of cooperative participation of metal ions renders the bimetallic complexes as potential catalysts for organic transformations.1 Metal−metal bonded complexes are particularly interesting because of enforced proximity between the metals and the ability of the dimetal core to attain valence delocalization.2 Elementary oxidative addition and reductive elimination processes are more favored on a bimetallic platform than on a single-metal entity.3 Metal−metal singly bonded dirhodium(II,II) systems are the most prominent catalysts for a wide variety of organic reactions.4 Although the reactions almost exclusively take place at one of the axial sites, the second metal plays a significant role. A 3c/4e bonding manifold has been proposed to explain the greater density of electronic states and consequently diverse reactivity.5 Well-defined stoichiometric reactions of small organic and inorganic molecules across the metal−metal bonds have been widely reported.6 Catalytic transformations on a bimetal platform utilizing equatorial sites have been relatively less explored.7 Suitable ligands capable of holding two metals in close proximity and accommodating structural and electronic changes during the catalytic cycle are vital to exploit the benefit of bimetallic cooperativity.
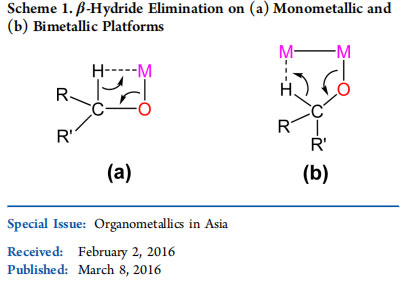
Acceptorless dehydrogenation (AD) is essentially a reaction that removes one hydrogen molecule from ubiquitous yet considerably less reactive alcohols to form carbonyls a more potent synthon. Hydrogen is liberated without the use of stoichiometric acceptor/oxidant, making AD a green and environmentally benign synthetic methodology.8 Several catalysts based on N-heterocyclic carbene and phosphine ligands have been reported for AD reactions.9 The use of a metal−ligand cooperation strategy in the ligand design has led to considerable improvement in the reaction conditions and selectivity.10
However, these studies have focused primarily on a single metal center. A key step in the alcohol AD is β-hydride elimination of a metal−alkoxide intermediate that proceeds via a four-membered agostic species (Scheme 1a).11 A metal−metal bond provides an interesting possibility of an alternative pathway on a bimetallic platform (Scheme 1b). To assess this proposal, we have designed a diruthenium(II,II) acetate-bridged complex incorporating a crescent-shaped naphthyridine-diimine ligand and evaluated its catalytic utility for the AD of alcohols. The catalyst is highly efficient for a range of alcohols and particularly effective for primary alcohols, affording solely the corresponding aldehydes. The AD methodology has been extended to the catalytic olefination of alcohols. Mechanistic studies and DFT
calculations suggest a bimetallic pathway during the catalytic cycle.

RESULTS AND DISCUSSION
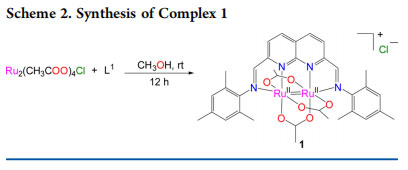
Syntheses and Structures. Synthesis of the naphthyridinediimine ligand 2,7-bis(N-mesitylmethylimino)-1,8-naphthyridine (L1) was achieved by condensation of 1,8-napththyridine-2,7-dicarbaldehyde with mesitylamine (Scheme S1 in the Supporting Information). Treatment of L1 with Ru2(OAc)4Cl in a 1:1 molar ratio in MeOH afforded [Ru2(L1)(OAc)3]Cl (1) as a dark green solid in 79% yield (Scheme 2). A single-electron reduction of Ru25+ to Ru24+ in methanol is consistent with the literature reports.
The molecular structure of 1 was confirmed by X-ray crystallography, which revealed two very similar molecules in the asymmetric unit. The salient features of only one molecule are discussed here. The diruthenium(II,II) core is spanned by the crescent-shaped L1, and three additional acetate ligands bridge between the metal centers (Figure 1). Two imine nitrogen atoms of L1 occupy sites trans to the Ru−Ru double bond. The tetradentate ligand L1 ensures that two acetate units are disposed opposite to each other, whereas the third acetate is trans to the naphthyridine unit. The Ru1−Ru2 distance is 2.2953(8) Å and is consistent with those in similar diruthenium(II,II) complexes.13
The imine nitrogens (axial) make longer Ru1−N3 (2.297(6) Å) and Ru2−N4 (2.278(6) Å) bond distances in comparison to naphthyridine nitrogens at the equatorial sites (Ru1−N1 =2.019(6) Å and Ru2−N2 = 2.023(6) Å).

ESI-MS exhibits a signal at m/z 800.0799, which is assigned to [1 − Cl]+ (Figure 2). 1H NMR signals of 1 are broad and featureless because of the paramagnetic nature of the complex.12c The UV−vis spectrum of 1 shows intense absorption at 331 nm(ϵ = 12000 M−1 cm−1, assigned to intraligand transitions) with the appearance of a shoulder at 425 nm possibly due to transitions from [Ru-Ru] dπ−dπ to ligand acceptor orbitals(Figure S3 in the Supporting Information). Two additional absorptions at 675 nm (ϵ = 6700 M−1 cm−1) and at 740 nm are attributed to metal to ligand transitions. When a dichloromethane solution of 1 is excited at 331 nm, blue emissions are observed at 406 and 428 nm (Figure S4 in the Supporting Information). The cyclic voltammogram of 1 in 0.1 M TBAP/acetonitrile shows a reversible one-electron, metal-centered oxidation at 0.98 V vs Ag/AgCl (Figure S5 in the Supporting Information). The oxidation observed for 1 represents the formation of a formally mixed valence [Ru2]5+ species stable on the cyclic voltammetry time scale.12a,c,14 The high potential of the metal-based oxidation indicates a greater stability of the [Ru2] 4+relative to that of the [Ru2] 5+ core in the presence of L1. Free L1 shows a single reversible two-electron reduction at −1.29 V, which is split into two reversible one-electron reductions in 1 at−0.32 and −1.16 V, indicating significant contribution from the metal d orbitals. The effective magnetic moment of powdered 1 at 295 K is 2.81 μB, corresponding to two unpaired electrons per molecule and is consistent with a σ2 π4 δ2 δ*2 π*2 electronic configuration.12a,14 Catalytic Studies. A few reports of alcohol dehydrogenation reactions on a diruthenium platform using O2 as oxidant have appeared in the literature. Naota et al. reported that the aerobic oxidation of alcohols in water could be performed efficiently in the presence of a catalytic amount of Ru2(μ-OAc)3(μ-CO3) under 1 atm of O2. 15 The Tokii group synthesized phosphinatobridged diruthenium complexes and tested their catalytic efficiency to oxidize cinnamyl alcohol under 1 atm of O2. 16
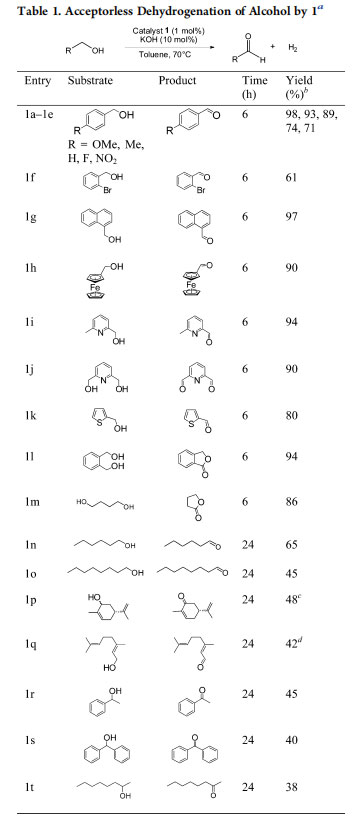
The oxidation of alcohol was also achieved when iodide-bridged diruthenium complexes were employed in the presence of Ag2O.17 These studies, however, use oxidants, are limited in substrate scope, and do not reflect on the mechanistic implications. Complex 1 was tested for the acceptorless dehydrogenation (AD) of benzyl alcohol at 1 mol % catalyst loading in the presence of 10 mol % of KOH, which afforded benzaldehyde in 89% yield (Table 1). Optimization studies showed that KOH was the best choice among a variety of bases(Table S2, entry 2, in the Supporting Information). The reaction was not efficient at lower temperatures, and the best results were obtained in toluene at 70 °C. Increasing the catalyst loading or temperature did not affect the progress of the reaction.

The substrate scope was then examined under the optimized conditions. Electron-rich p-methoxybenzyl alcohol and pmethylbenzyl alcohol gave excellent yields (93−98%; entries 1a,b) in comparison to benzyl alcohol (89%; Table 1, entry 1c). However, electron-withdrawing groups attached to benzyl alcohol reduced the yield of the corresponding aldehyde (61−74%; entries 1d−f). The substrate scope was then extended to polyaromatic and heterocyclic functionalized alcohols, and they showed appreciable yields (80−97%; entries 1g−k). Diols afforded corresponding lactones (86−94%, entries 1l,m) in good yields. The reaction was extended to aliphatic alcohols such as n-hexanol and n-octanol and showed moderate yields (45−65%; entries 1n,o) after extending the reaction time to 24 h.
Natural products such as carveol and geraniol were also tested under AD conditions, and they gave 42−48% yields of the corresponding aldehyde (entries 1p,q). A significant amount of hydrogenated products (15−20%) were also observed, which could be accounted for on the basis of evolved hydrogen during the reaction.
Unlike primary alcohols, secondary alcohols were poorly dehydrogenated (38−45%; Table 1, entries 1r−t) to the corresponding ketones. This is in contrast to reports where secondary alcohols were dehydrogenated easily owing to their low redox potentials.18 Furthermore, a review of literature reports reveals that AD of primary alcohols invariably produces esters as major products by a hemiacetalyzation followed by dehydrogenation or by a Tischenko reaction.19 No such side products were observed for the diruthenium catalyst. Catalyst 1 is clearly a superior alternative for the AD of primary alcohols.

AD of alcohol to aldehyde is accompanied by the concomitant release of one molecule of hydrogen (Scheme 3). A volumetric quantitative analysis of benzyl alcohol dehydrogenation using a gas buret revealed near-quantitative formation of hydrogen(∼92% of theoretical yield; Figure S6 in the Supporting Information). The evolved hydrogen was identified by matching the retention time with an authentic sample using a thermal detector in GC. In another experiment, the AD reaction was conducted in a flask that was connected through a rubber tube to a second flask in which styrene and a catalytic amount of RhCl(PPh3)3 in benzene were placed. After the reaction was completed, ethylbenzene was produced in 76% yield in the second flask, demonstrating that the hydrogen gas generated in the AD reaction is responsible for styrene reduction (Scheme S2 and Figure S7 in the Supporting Information). This dual reaction authenticated that hydrogen is produced during the course of the reaction.10c When the AD reaction was done using benzyl alcohol-α,α-d2 (PhCD2OH), GC-MS analysis of the product showed a single peak at m/z 107, indicating monodeuterated ethylbenzene formed by the in situ generated HD gas (Scheme S3 and Figure S8 in the Supporting Information).
Alcohols are common starting materials for many chemical reactions, although they are largely unreactive. A convenient approach toward alcohol activation/utilization is AD to a more reactive carbonyl group.8 Acceptorless dehydrogenative coupling (ADHC) reactions offer environmentally benign synthetic routes for the preparation of a plethora of useful products such as esters, amides, imines, and heterocycles by the direct reaction of alcohol with an appropriate coupling reagent.20 Direct reaction of an ylide/Wittig reagent with an alcohol to selectively form an olefin, with the liberation of hydrogen gas and avoidance of the use of oxidants, is a useful carbon−carbon bond forming reaction (Scheme 3). Alkanes were obtained as major products when iridium or ruthenium catalysts were used for similar
reactions.21 The product formation was explained on the basis of hydrogenation of generated alkenes with the concomitantly evolved hydrogen. The Milstein group has recently reported the
olefination of alcohols with Wittig salt precursors using an Ru(II)-PNN pincer catalyst in an open system that allowed the escape of hydrogen for the selective synthesis of alkenes.22
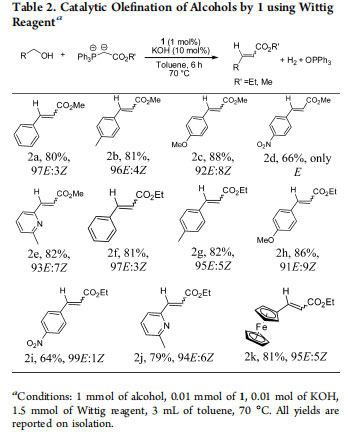
Catalyst 1 performs the same task with equal efficiency but at a significantly lower temperature. A mixture of benzyl alcohol, 1 mol % of 1, 10 mol % of KOH, and triphenylphosphonium methoxycarbonylmethylide (Wittig reagent, 1.5 equiv) was placed in a single vessel and heated to 70°C in toluene for 6 h. Isolated yields were 80% (Table 2, entry 2a). NMR analysis showed predominantly (E)-methyl cinnamate, and only trace amounts of the Z isomer were present. The substrate scope of the reaction was examined. Electron-rich pmethoxybenzyl alcohol and p-methylbenzyl alcohol showed
better yields (81−88%; entries 2b,c), but in the presence of an electron-withdrawing group such as p-nitrobenzyl alcohol, a lesser yield was obtained (66%; entry 2d). However, the selectivity of the reaction improved, yielding E products exclusively. The reaction was expanded to another Wittig reagent(triphenylphosphonium ethoxycarbonylmethylide), and similar trends were obtained (entries 2f−i). Heterocyclic functionalized alcohols such as 2-methyl-6-pyridinemethanol and ferrocenylmethyl alcohol were also notably tolerated under the reaction conditions (entries 2e,j,k) to provide the corresponding (E)- alkenes. Importantly, catalyst 1 exhibited higher E selectivity in comparison with other catalysts.22
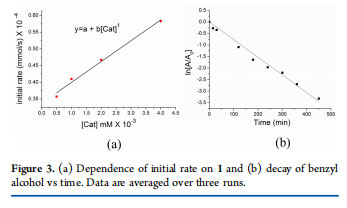
An AD reaction with Ru2(OAc)4Cl, having an accessible axial site, yielded only 30% of benzaldehyde under identical reaction conditions. The presence of a vacant axial site does not necessarily lead to product formation. Rather, a suitably designed framework renders trans ligands labile, and consequently those equatorial sites can be accessed. We propose a mechanism that involves both metals, and the reaction proceeds on the equatorial platform (Scheme 4). Initially, the alkoxide moiety replaces the acetate group trans to the naphthyridine. A bimetallic β-hydride elimination generates a Ru-hydride intermediate with the concurrent formation of aldehyde. The aldehyde is extruded, and an alcohol molecule binds to the metal. The catalytic cycle is closed via a dehydrogenation step that involves an intramolecular proton transfer from alcohol to the metal-bound hydride. Kinetic Studies. To gain support for the proposed mechanism, kinetic studies were performed. The initial rate of reaction was monitored to determine the order with respect to catalyst 1. Reactions were performed with varying concentrations of 1 and equimolar amounts of benzyl alcohol and dodecane(internal standard). The initial rate varied linearly with the catalyst concentration, and the reaction was found to be first order with respect to 1 (Figure 3a). Furthermore, equimolar amounts of benzyl alcohol and dodecane (nalcohol = ndodecane) were mixed with 1 mol % of catalyst 1 in 3 mL of toluene. Aliquots of 0.2 mL were taken out at regular time intervals, and the amount of unreacted alcohol was measured using GC-MS against
dodecane. According to the integrated rate law for a reaction of the type A → B with the restriction [A] = 1 and [B] = 0 at t = 0, the ln[A] vs time plot fitted well to a first-order kinetics (Figure 3b). Both of these experiments suggest the involvement of catalyst 1 and alcohol, one molecule each, in the rate-determining step. As one molecule of the catalyst 1 consists of two ruthenium centers, it is reasonable to assume that the reaction takes place on the bimetallic assembly.23 Close proximity between the metals aided by the ligand architecture allows the second metal to participate in the β-hydride elimination step.
Deuteration Studies. To garner further support in favor of the proposed mechanism, isotope scrambling studies were carried out with deuterated alcohol. A model AD reaction in toluene-d8 did not afford deuterated product, thus ruling out the possibility of isotope scrambling from the solvent. Reaction of PhCD2OH showed deuterated benzaldehyde as the major product (92/8 D/H observed by GC-MS analysis; Figure S9 in the Supporting Information). An AD mechanism for a monometal catalyst typically involves a RuII-dihydride species generated by a sequence of elementary β-hydride elimination/
reductive elimination reactions (Scheme S4 in the Supporting Information).24 Such a process necessarily leads to hydrogen scrambling in the product. For example, for a Ru(II) catalyst bearing an N-heterocyclic carbene based ligand, 42% hydrogen incorporation was observed in deuterated imine products.25 The absence of significant isotope scrambling for catalyst 1 strongly suggests the intermediacy of a Ru-monohydride intermediate, offering support to the proposed mechanism.26
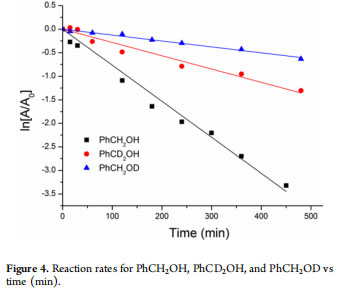
Kinetic Isotope Effects. The involvement of C−H bond breaking in the rate-determining step of the catalysis is indicated by the intermolecular kinetic isotope effect (KIE).27 A direct comparison of two reactions, (a) PhCH2OH in toluene and (b) PhCD2OH in toluene-d8, showed kC−H/kC−D = 2.71 ± 0.04(Figure 4). This proved that the C−H bond breaking is one of the slower steps of the reaction. The rate of the reaction was 4.94± 0.02 times slower when PhCH2OD was used as a substrate instead of PhCH2OH (Figure 4). The high kO−H/kO−D value suggests that hydrogen elimination during the final stage of the catalytic cycle is likely to be the rate-limiting step.
DFT Studies. DFT calculations at the M06 level of theory were carried out to gain insight into the reaction pathway. All DFT optimized structures of the intermediates and transition states along with the energy profile of the reaction (kcal/mol) are presented in Figures 5 and 6, respectively. A simplified system was chosen where the mesityl group was replaced by methyl and bridging acetates were replaced by formates. Methanol was considered as the substrate to reduce the computational cost.

Replacement of one of the bridging formates trans to the naphthyridine by alkoxide produces intermediate A. The optimized structure B was subsequently computed where one of the H atoms of the alkoxide is engaged in an agostic interaction with the second ruthenium center.28 The computed Ru2···H1 distance is 2.04 Å, comparable to metal−hydrogen distances in agostic complexes.29 Subsequent β-hydride elimination leads to the metal−hydride intermediate C, which proceeds via the transition state TSBC (ΔG⧧ = 12.42 kcal/mol, Figure 6). The TSBC has a single imaginary frequency of 359icm−1 and involves movement of H1 toward Ru2, resulting in a decrease in Ru2···H1(1.67 Å) and a simultaneous increase in C1−H1 (1.75 Å). An alternate route involving a single metal center has also been considered where β-hydride elimination occurs on Ru1, affording F (Figure S10 in the Supporting Information). The energy of F is 18.63 kcal/mol higher than that of C. Clearly, a bimetallic β-hydride elimination is a more energy efficient route than a pathway involving a single metal. The next step is the liberation of aldehyde from C followed by coordination of alcohol, a highly downhill process to form the intermediate D. Proton transfer from the alcohol to the metal-bound hydride gives the dihydrogen-bound species E via the transition state TSDE(ΔG⧧ = 14.64 kcal/mol, Figure 6). The endothermic nature of the dehydrogenation step was validated by DFT calculations, which revealed kC−H/kC−D = 2.68 and kO−H/kO−D = 3.73. These results are in agreement with the experimental KIE values. The final step is H2 liberation from E to regenerate A.
CONCLUSION
A diruthenium(II,II) complex incorporating a naphthyridine−diimine ligand was synthesized. The ligand architecture offers accessible sites trans to the naphthyridine unit. The title compound is an excellent catalyst for AD of alcohols to the corresponding carbonyl compounds. This diruthenium assembly is remarkably effective for the clean conversion of primary alcohols to the corresponding aldehydes without esters as side products. A possible explanation is that the generated aldehyde is rapidly extruded from the [RuRu] core and hence the hemiacetalyzation is hindered. The same catalyst was further
exploited for catalytic olefination of alcohols using ylides to react with the in situ produced aldehyde. Kinetic experiments, isotope labeling studies, and DFT calculations point to a bimetallic cooperative mechanism that operates on the equatorial platform. A low-energy bimetallic β-hydride elimination makes dehydrogenation process the rate-limiting step. This study underlines the general utility of bimetallic catalysts in AD and ADHC reactions.
EXPERIMENTAL SECTION
General Procedures. All reactions were carried out under a nitrogen atmosphere with the use of standard Schlenk-line techniques unless stated otherwise. Glassware was flame-dried under vacuum prior
to use. 1 H and 13C NMR spectra were obtained on JEOL JNM-LA 500 MHz and JEOL JNM-LA 400 MHz spectrometers. Chemical shift values were referenced to the residual signals of the deuterated solvents. ESIMS were recorded on a Waters Micro mass Quattro Micro triplequadrupole mass spectrometer. Infrared spectra were recorded on a Bruker Vertex 70 FTIR spectrophotometer in the range 400−4000cm−1. Elemental analyses were performed on a Thermoquest EA1110 CHNS/O analyzer. The crystallized compound was washed several times with dry diethyl ether, powdered, and dried under vacuum for at least 48 h prior to elemental analyses. GC-MS experiments were performed on an Agilent 7890A GC and 5975C MS system.
Cyclic voltammetric studies were performed on a BAS Epsilon electrochemical workstation in acetonitrile with 0.1 M tetra-nbutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte. The working electrode was a BAS Pt-disk electrode, the reference electrode was Ag/AgCl, and the auxiliary electrode was a Pt wire. The ferrocene/ferrocenium couple occurs at E1/2 = +0.51(70) V versus Ag/AgCl under the same experimental conditions. The potentials are reported in volts (V); the ΔE (Ep,a − Ep,c) values are in millivolts(mV) at a scan rate of 100 mV s−1. UV−visible spectra were recorded using a JASCO V-670 UV/vis absorption spectrophotometer. Emission spectra were recorded using a Fluorolog FL3-21 (Horiba Jobin Yvon) spectrofluorometer equipped with a xenon flash lamp and also using a PTI QuantaMaster Model QM-4 scanning spectrofluorometer equipped with a 75 W xenon lamp, emission and excitation monochromators, an excitation correction unit, and a PMT detector for both visible and NIR regions. Materials. Solvents were dried by conventional methods, distilled under nitrogen, and deoxygenated prior to use. RuCl3·xH2O (39% Ru) was purchased from Arora Matthey (India). The compounds [Ru2(OAc)4Cl],30 1,8-naphthyridine-2,7-dicarboxaldehyde,31 and PhCH2OD32 were synthesized following literature procedures.
X-ray Data Collection and Refinement. Single-crystal X-ray structural studies were performed on a CCD Bruker SMART APEX diffractometer equipped with an Oxford Instruments low-temperature attachment. Data were collected at 100(2) K using graphitemonochromated Mo Kα radiation (λα = 0.71073 Å). The frames were indexed, integrated, and scaled using the SMART and SAINT software
package,33 and the data were corrected for absorption using the SADABS program.34 The structure was solved and refined using the SHELX suite of programs. All hydrogen atoms were included in the final
stages of the refinement and were refined with a typical riding model. All non-hydrogen atoms were refined with anisotropic thermal parameters.
The “SQUEEZE” option in the PLATON program was used to remove a disordered solvent molecule from the overall intensity data.35 Crystallographic data and pertinent refinement parameters for
compound 1 are summarized in Table S1 in the Supporting Information. The crystallographic figures used in this paper have been generated using Diamond 3.1e software.36 CCDC 1447839 contains supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Synthesis of L1. 1,8-Naphthyridine-2,7-dicarboxaldehyde (250 mg, 1.34 mmol) was dissolved in 50 mL of methanol and placed in a 100 mL round-bottom flask equipped with a stir bar. To this suspension was introduced mesitylamine (370 mg, 2.71 mmol), and within a few minutes a yellow precipitate appeared. This mixture was stirred overnight. The yellow compound was collected by filtration and washed
with methanol followed by diethyl ether: yield 470 mg (84%); 1 H NMR(400 MHz, CDCl3) δ 8.60 (s, 1H), 8.56 (d, J = 8.24 Hz, 1H), 8.37 (d, J =8.68 Hz, 1H), 6.93 (s, 2H), 2.19 (s, 9H); 13C NMR (100 MHz, CDCl3)
δ 163.4, 158.2, 147.5, 137.6, 134.2, 129.4, 129.2, 129.1, 127.1, 120.1, 20.9, 18.4 ppm; IR (KBr) ν 2949, 2914, 2856, 1636, 1597, 1505, 1479,1373, 1206, 864, 852, 732 cm−1; ESI-MS (CH2Cl2) m/z 421.2453 [M +
H]+. Anal. Calcd for C28H28N4: C, 79.96; H, 6.72; N, 13.33. Found: C,79.81; H, 6.53; N, 13.18.
Synthesis of 1. [Ru2(OAc)4(Cl)] (60 mg, 0.126 mmol) was placed in a flame-dried Schlenk flask, and 10 mL of dry methanol was added to form a brown suspension. Addition of L1 (53 mg, 0.12 mmol) resulted in a deep green solution. The reaction mixture was stirred at room temperature for 12 h. The solution was evaporated completely under reduced pressure, and the residue obtained was redissolved in 0.5 mL of dichloromethane. Diethyl ether was added with stirring to induce precipitation. The solution was discarded by cannula filtration, and the precipitate was further washed with diethyl ether (3 × 10 mL). Finally, the precipitate was dried under vacuum to afford 1 as a green powder. Yield: 73 mg (79%). Needle-shaped green crystals suitable for X-ray diffraction were grown by layering hexane over a concentrated dichloromethane solution of 1 inside an 8 mm o.d. vacuum−sealed glass tube: IR (KBr) ν 2963, 1532, 1441, 1262, 1198, 1096, 1021, 800, 690 cm−1; MS (ESI; CH3CN) m/z 800.0799 [M − Cl]+. Anal. Calcd for C34H37N4O6Ru2: C, 50.93; H, 4.65; N, 6.99. Found: C, 50.79; H, 4.45; N, 6.83. General Procedure for AD of Alcohols. A mixture of alcohol (1 mmol), 1 (0.01 mmol), potassium hydroxide (0.1 mmol), and dodecane(1 mmol) in 3 mL of toluene was placed in an oven-dried reaction vessel.
The reaction mixture was heated to 70 °C with stirring for 6−24 h. The reaction mixture was cooled, diluted with EtOAc, and passed through a short column of silica for GC-MS analysis. Volumetric Estimation of Evolved Hydrogen. Alcohol (1 mmol), 1 (0.01 mmol), and potassium hydroxide (0.1 mmol) in 3 mL of toluene was placed in an oven-dried reaction vessel, and the reaction mixture was heated to 70 °C. The headspace of the reaction vessel was connected to a gas buret. The reaction was continued until evolution of gas ceased. The experiment was repeated three times to get consistent readings, and the number of moles of hydrogen evolved was calculated by taking into account the vapor pressure of water at 293 K = 17.5424 Torr: volume of water displaced 22.6 mL, atmospheric pressure 761.3126 Torr, R = 62.3635 L Torr K−1 mol−1, n(H2) = [(Patm − Pwater) V]/RT = 0.00092 mol, expected value 0.001 mol. Dual Reactions Involving Hydrogenation of Styrene. The catalysis reaction using the catalyst 1 was conducted in a flask that was connected through a rubber tube to another flask in which styrene (1 mmol) and a catalytic amount of RhCl(PPh3)3 (0.05 mmol) in benzene were placed. Ethylbenzene was produced in the latter flask (76%). Deuteration Studies with Styrene. A similar procedure was followed using PhCD2OH as substrate. GC-MS analysis of the product showed a signal for monodeuterated styrene (Scheme S3 in the Supporting Information).
General Procedure for Catalytic Olefination of Alcohols using Wittig Reagent. Alcohol (1 mmol), 1 (0.01 mmol), potassium hydroxide (0.1 mmol), and Wittig reagent (1.5 mmol) were sequentially added to 3 mL of toluene placed in an oven-dried reaction vessel. The reaction mixture was heated to 70 °C with stirring for 6 h. After the completion of the reactions, the products were purified by chromatography on a silica gel column using hexane/EtOAc (9/1 v/v) as eluent. The isolated E products were characterized by 1H and 13C NMR spectra.
Experimental Procedure for Kinetics Studies. A mixture of alcohol (1 mmol), 1 (0.01 mmol), potassium hydroxide (0.1 mmol), and dodecane (1 mmol) in 3 mL of toluene was placed in an oven-dried reaction vessel. The reaction mixture was heated to 70 °C. After stipulated time intervals, small aliquots (0.2 mL) were taken out from the reaction mixture, diluted with EtOAc, and passed through a short column of silica for GC-MS analysis. The experiments were repeated in triplicate with varying catalyst concentrations.
Experimental Procedure for Deuteration Studies and KIE. Deuterated alcohol (1 mmol), 1 (0.01 mmol), NaOD (0.1 mmol), and dodecane (1 mmol) in 3 mL of d8-toluene were placed in an oven-dried
reaction vessel. The reaction mixture was heated to 70 °C. After the stipulated time intervals, small aliquots of 0.2 mL were taken out and passed through silica column for GC-MS analysis. Computational Details. Full geometry optimizations, without any symmetry constraints, were carried out using the hybrid density functional theory (DFT) method M0637 as implemented in the program suite Gaussian 09.38 The Stuttgart−Dresden effective core potential MWB28 and the corresponding basis set were invoked for Ru.39 The ligand atoms H, N, C, and O were described using the 6-31+G(d,p) basis sets.40 All structures were subjected to normal-mode vibrational analysis calculated at the same level of theory as the corresponding geometry optimization. All stationary points on the potential energy surface are
either local minima with no imaginary vibrational frequency or transition states with one imaginary frequency. Solvent effects were accounted for with the SMD model.41 Gas phase optimized structures
were taken as the initial geometries for optimization in solution.42,43 The solvation energies were calculated in toluene (ε = 2.38). The reported energies are Gibbs free energies in toluene using the M06 functional.
ASSOCIATED CONTENT
*S Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.organomet.6b00085. Experimental details and supporting figures (PDF) Crystallographic data (CIF) Cartesian coordinates of all computed molecules (XYZ)
AUTHOR INFORMATION
Corresponding Author
*E-mail for J.K.B.: [email protected].
Notes
The authors declare no competing financial interest ■
ACKNOWLEDGMENTS
Financial support from the Department of Science and Technology (DST) of India and the Department of Atomic Energy (DAE) is gratefully appreciated. J.K.B. thanks the DAE for an SRC-OI fellowship. I.D. and P.P. thank the CSIR of India for fellowships. A.S. and K.S. thank the UGC of India for fellowships. I.D. thanks Mr. Vijay Kumar B. for providing one of the Wittig reagents, Mr. Arunava Sengupta for magnetic
measurements, and Mr. Sooraj Kunnikuruvan for help in KIE calculations.
3-Amino-N-cyclopropyl-4-methylbenzenesulfonamideCatalog No.:AA00946L CAS No.:1017431-08-1 MDL No.:MFCD09900776 MF:C10H14N2O2S MW:226.2954 |
2-(4-bromophenyl)-2-(4-methylpiperazin-1-yl)acetonitrileCatalog No.:AA01ELAU CAS No.:1017433-02-1 MDL No.:MFCD10005275 MF:C13H16BrN3 MW:294.1902 |
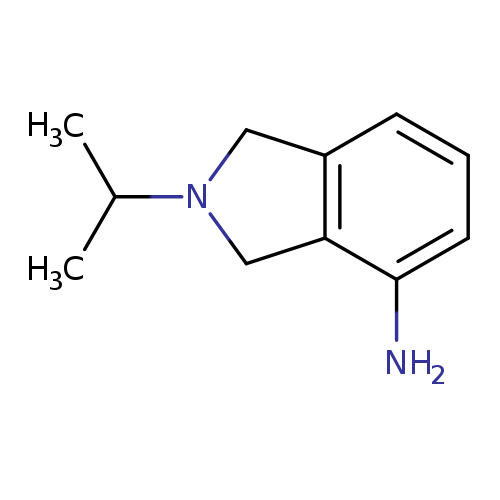
2-(Propan-2-yl)-2,3-dihydro-1h-isoindol-4-amineCatalog No.:AA01A4GA CAS No.:1017433-32-7 MDL No.:MFCD10007253 MF:C11H16N2 MW:176.2581 |
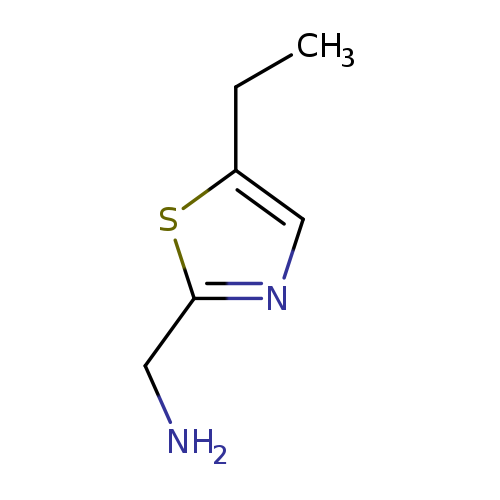
(5-ethyl-1,3-thiazol-2-yl)methanamineCatalog No.:AA019SBL CAS No.:1017435-71-0 MDL No.:MFCD16668972 MF:C6H10N2S MW:142.2220 |
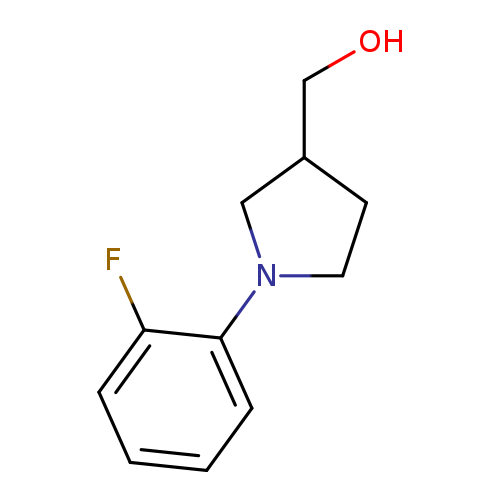
[1-(2-fluorophenyl)pyrrolidin-3-yl]methanolCatalog No.:AA01BAOF CAS No.:1017444-90-4 MDL No.:MFCD10003934 MF:C11H14FNO MW:195.2334 |
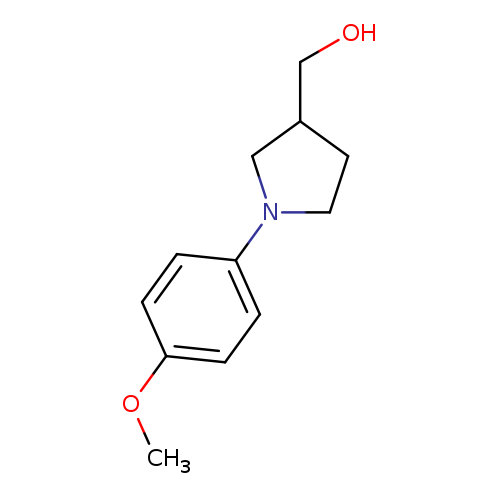
[1-(4-methoxyphenyl)pyrrolidin-3-yl]methanolCatalog No.:AA01C3BZ CAS No.:1017445-06-5 MDL No.:MFCD10003950 MF:C12H17NO2 MW:207.2689 |
3-Amino-4-fluorobenzenesulfonamideCatalog No.:AA00H9I6 CAS No.:1017448-36-0 MDL No.:MFCD09900531 MF:C6H7FN2O2S MW:190.1954 |
3-amino-5-methylbenzene-1-sulfonamideCatalog No.:AA01B2T3 CAS No.:1017448-52-0 MDL No.:MFCD09900545 MF:C7H10N2O2S MW:186.2315 |
3-amino-4-fluoro-N,N-dimethylbenzene-1-sulfonamideCatalog No.:AA01AKAH CAS No.:1017448-64-4 MDL No.:MFCD09900555 MF:C8H11FN2O2S MW:218.2485 |
4-methyl-2-(2-phenylpropan-2-yl)-1,3-thiazole-5-carboxylic acidCatalog No.:AA01A43A CAS No.:1017449-27-2 MDL No.:MFCD09903627 MF:C14H15NO2S MW:261.3394 |
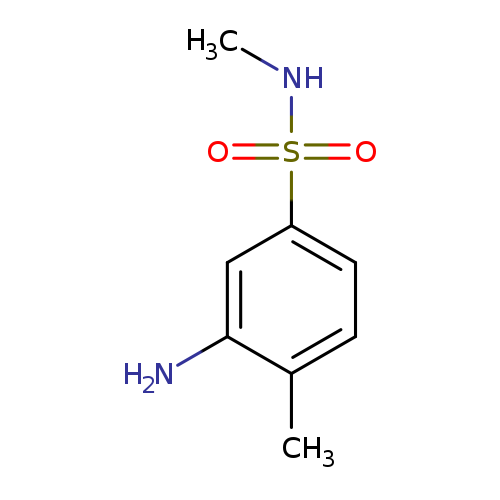
3-Amino-N,4-dimethylbenzenesulfonamideCatalog No.:AA00H9I7 CAS No.:1017450-04-2 MDL No.:MFCD09881074 MF:C8H12N2O2S MW:200.2581 |
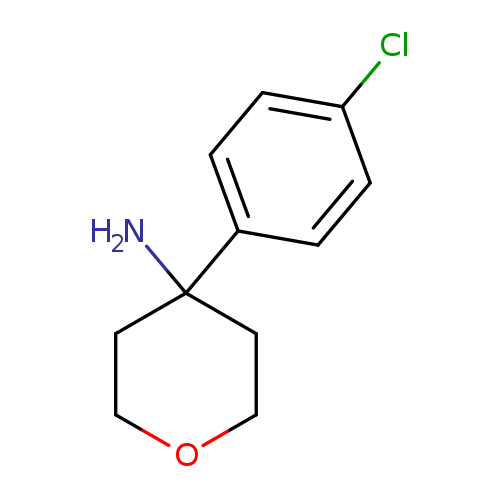
4-(4-Chlorophenyl)tetrahydro-2H-pyran-4-amineCatalog No.:AA01A8QK CAS No.:1017450-84-8 MDL No.:MFCD09904027 MF:C11H14ClNO MW:211.6880 |
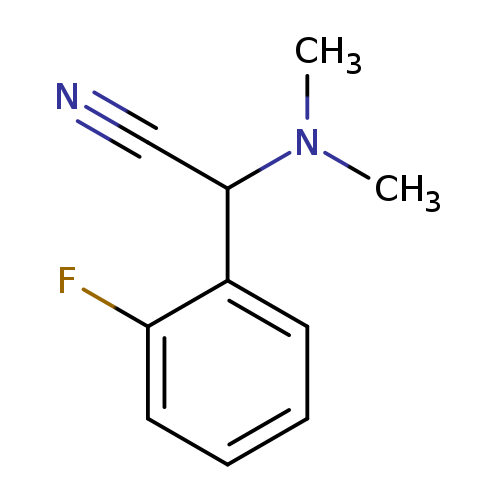
2-(dimethylamino)-2-(2-fluorophenyl)acetonitrileCatalog No.:AA019MDG CAS No.:1017450-89-3 MDL No.:MFCD10005115 MF:C10H11FN2 MW:178.2061 |
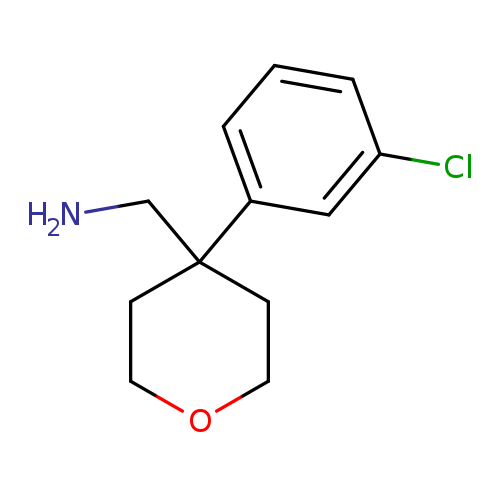
(4-(3-Chlorophenyl)tetrahydro-2H-pyran-4-yl)methanamineCatalog No.:AA019WY0 CAS No.:1017450-92-8 MDL No.:MFCD09904047 MF:C12H16ClNO MW:225.7145 |
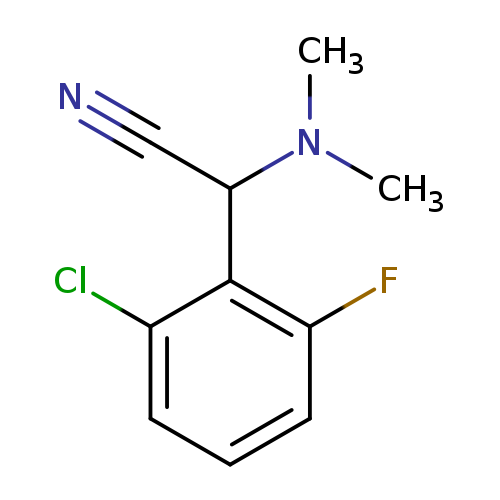
(2-Chloro-6-fluorophenyl)(dimethylamino)acetonitrileCatalog No.:AA01ARCK CAS No.:1017451-92-1 MDL No.:MFCD10005212 MF:C10H10ClFN2 MW:212.6512 |
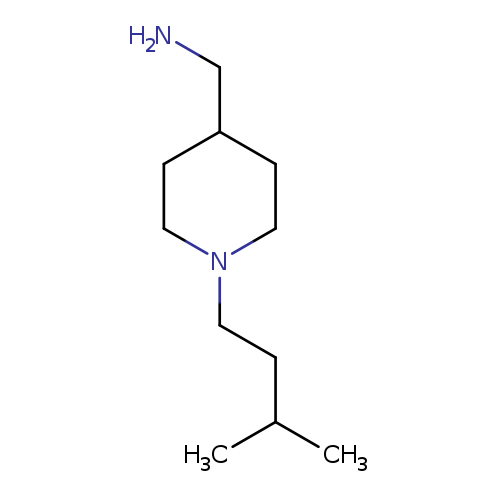
[1-(3-methylbutyl)piperidin-4-yl]methanamineCatalog No.:AA019YGR CAS No.:1017452-47-9 MDL No.:MFCD10003422 MF:C11H24N2 MW:184.3217 |
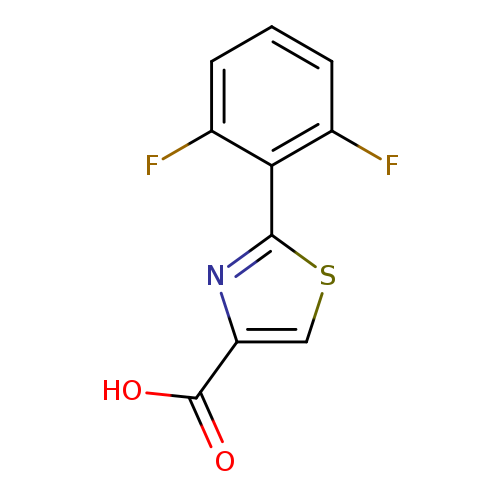
2-(2,6-Difluorophenyl)thiazole-4-carboxylic acidCatalog No.:AA0005AZ CAS No.:1017452-64-0 MDL No.:MFCD07376377 MF:C10H5F2NO2S MW:241.2140 |
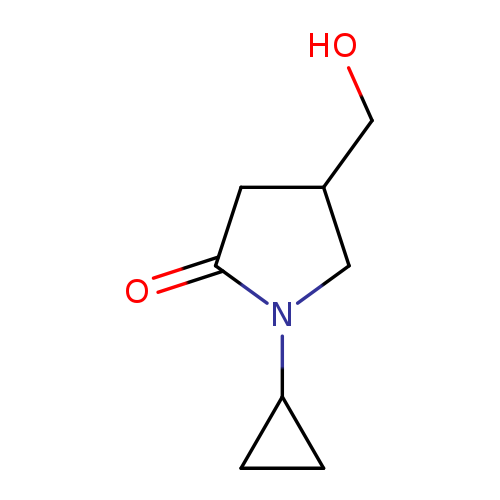
1-cyclopropyl-4-(hydroxymethyl)pyrrolidin-2-oneCatalog No.:AA018GPS CAS No.:1017456-88-0 MDL No.:MFCD10003979 MF:C8H13NO2 MW:155.1943 |
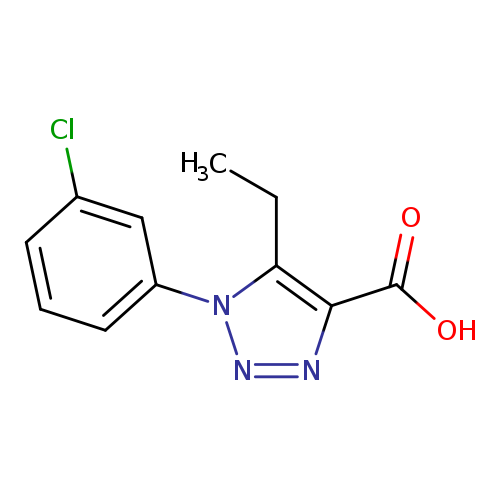
1-(3-chlorophenyl)-5-ethyl-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01AGX9 CAS No.:1017457-41-8 MDL No.:MFCD10006694 MF:C11H10ClN3O2 MW:251.6690 |
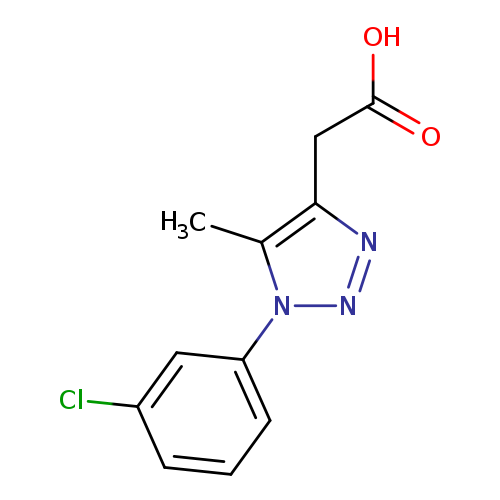
2-[1-(3-Chlorophenyl)-5-methyl-1h-1,2,3-triazol-4-yl]acetic acidCatalog No.:AA019WCL CAS No.:1017457-61-2 MDL No.:MFCD10006709 MF:C11H10ClN3O2 MW:251.6690 |
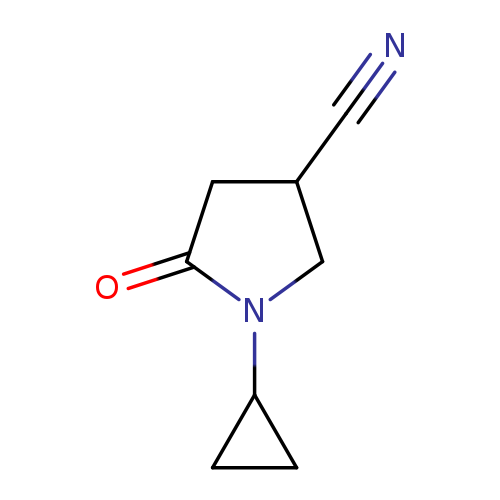
1-cyclopropyl-5-oxopyrrolidine-3-carbonitrileCatalog No.:AA00VSJ7 CAS No.:1017457-67-8 MDL No.:MFCD10004075 MF:C8H10N2O MW:150.1778 |
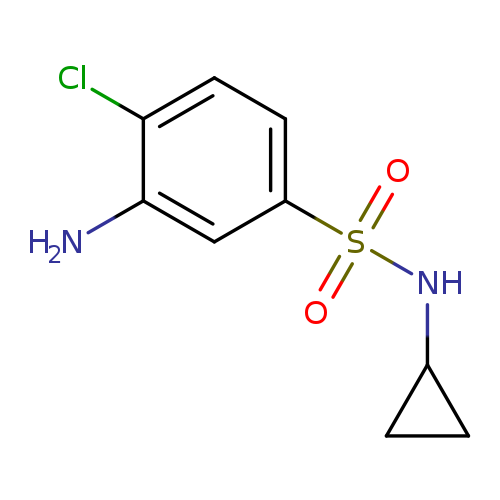
3-Amino-4-chloro-n-cyclopropylbenzene-1-sulfonamideCatalog No.:AA01A7GD CAS No.:1017458-09-1 MDL No.:MFCD09900778 MF:C9H11ClN2O2S MW:246.7138 |
1-(4-bromophenyl)-5-oxopyrrolidine-3-carbonitrileCatalog No.:AA01ABM8 CAS No.:1017458-55-7 MDL No.:MFCD10004143 MF:C11H9BrN2O MW:265.1060 |
5-amino-N-tert-butyl-2-methylbenzene-1-sulfonamideCatalog No.:AA01AK6F CAS No.:1017459-30-1 MDL No.:MFCD11133822 MF:C11H18N2O2S MW:242.3378 |
[1-(4-Fluorophenyl)cyclobutyl]methanamineCatalog No.:AA0096QJ CAS No.:1017462-08-6 MDL No.:MFCD09904317 MF:C11H14FN MW:179.2340 |
2-(2-methylpropyl)-2,3-dihydro-1H-isoindol-4-amineCatalog No.:AA01A1K6 CAS No.:1017462-39-3 MDL No.:MFCD10007255 MF:C12H18N2 MW:190.2847 |
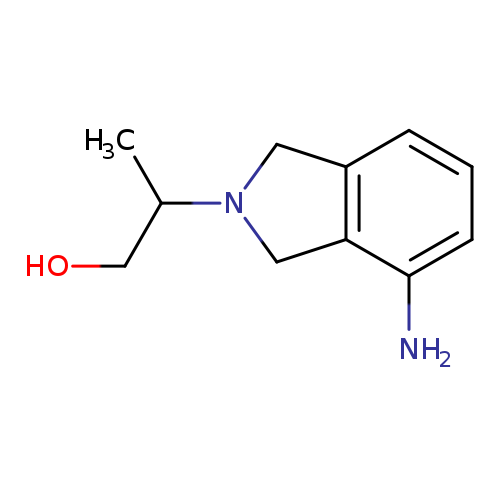
2-(4-amino-2,3-dihydro-1H-isoindol-2-yl)propan-1-olCatalog No.:AA01A47N CAS No.:1017462-47-3 MDL No.:MFCD10007261 MF:C11H16N2O MW:192.2575 |
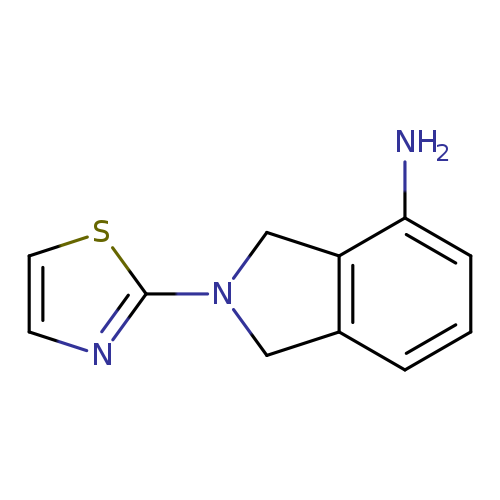
2-(1,3-thiazol-2-yl)-2,3-dihydro-1H-isoindol-4-amineCatalog No.:AA01A2VF CAS No.:1017462-59-7 MDL No.:MFCD10007269 MF:C11H11N3S MW:217.2901 |
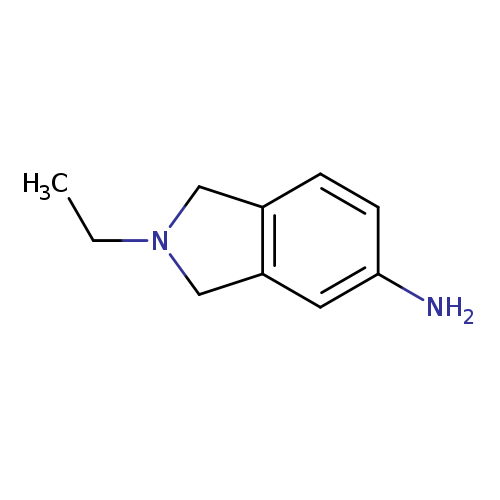
2-ethyl-2,3-dihydro-1H-isoindol-5-amineCatalog No.:AA01BEMH CAS No.:1017463-79-4 MDL No.:MFCD10007345 MF:C10H14N2 MW:162.2316 |
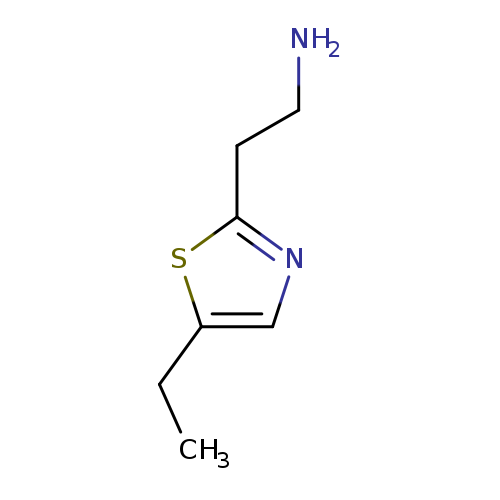
2-(5-Ethyl-1,3-thiazol-2-yl)ethan-1-amineCatalog No.:AA019SBJ CAS No.:1017463-86-3 MDL No.:MFCD09904509 MF:C7H12N2S MW:156.2486 |
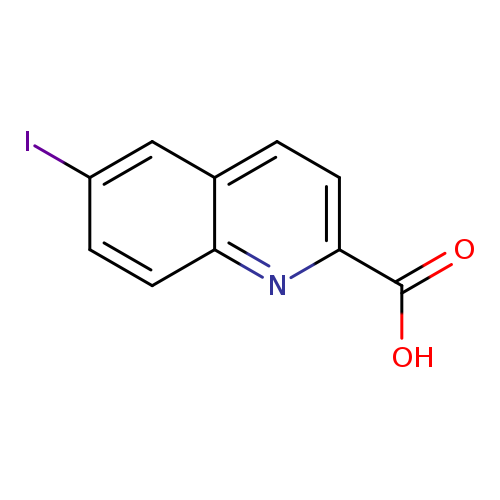
6-Iodoquinoline-2-carboxylic acidCatalog No.:AA01BG3J CAS No.:1017464-01-5 MDL No.:MFCD09881346 MF:C10H6INO2 MW:299.0646 |
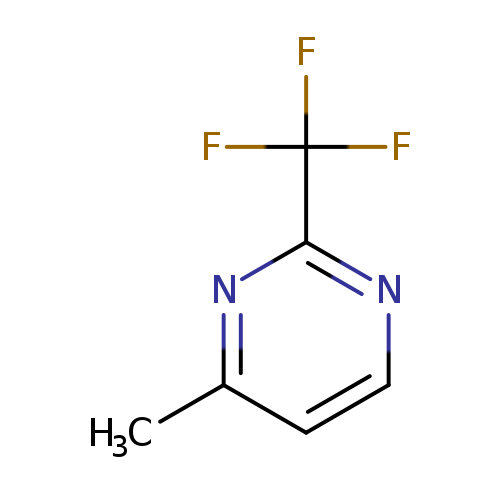
4-Methyl-2-(trifluoromethyl)pyrimidineCatalog No.:AA0005AY CAS No.:1017464-05-9 MDL No.:MFCD09881360 MF:C6H5F3N2 MW:162.1125 |
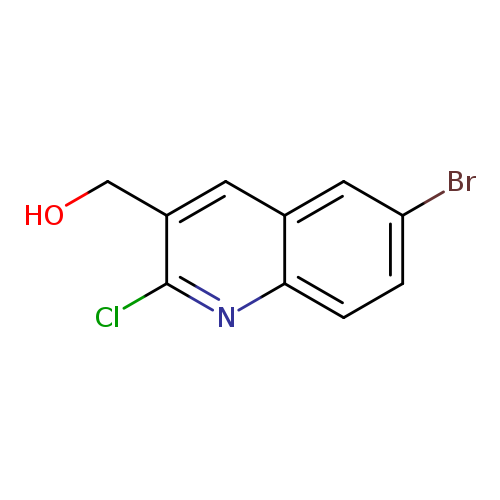
6-Bromo-2-chloroquinoline-3-methanolCatalog No.:AA0005AV CAS No.:1017464-16-2 MDL No.:MFCD09997953 MF:C10H7BrClNO MW:272.5257 |
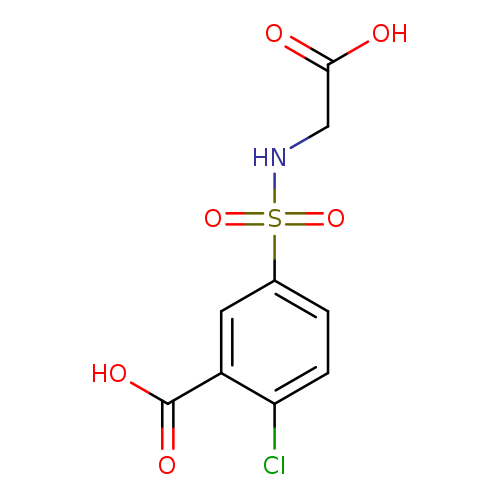
5-[(carboxymethyl)sulfamoyl]-2-chlorobenzoic acidCatalog No.:AA01BUUA CAS No.:1017464-69-5 MDL No.:MFCD09901850 MF:C9H8ClNO6S MW:293.6809 |
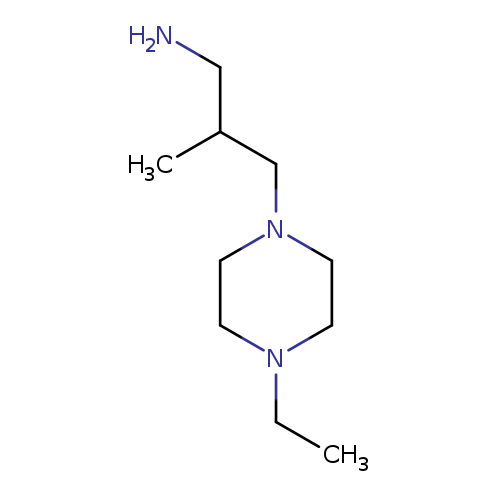
3-(4-ethylpiperazin-1-yl)-2-methylpropan-1-amineCatalog No.:AA019VA1 CAS No.:1017465-49-4 MDL No.:MFCD09901921 MF:C10H23N3 MW:185.3097 |
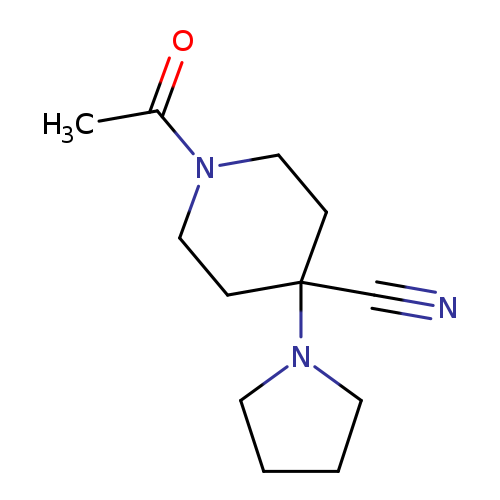
1-Acetyl-4-pyrrolidin-1-ylpiperidine-4-carbonitrileCatalog No.:AA008VKJ CAS No.:1017468-05-1 MDL No.:MFCD10005398 MF:C12H19N3O MW:221.2988 |

2-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)acetic acidCatalog No.:AA019WCN CAS No.:1017470-94-8 MDL No.:MFCD10003291 MF:C11H11N3O2 MW:217.2239 |
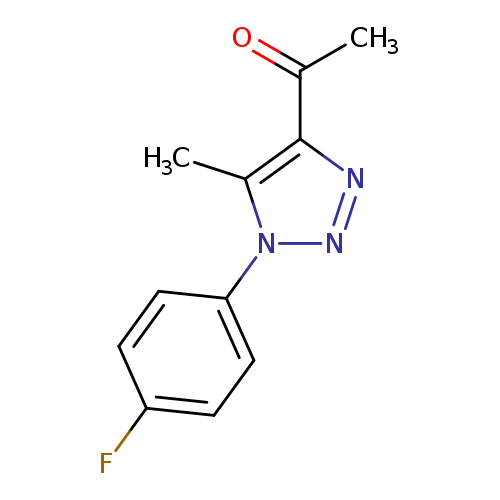
1-[1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl]ethan-1-oneCatalog No.:AA019WCP CAS No.:1017471-25-8 MDL No.:MFCD10003315 MF:C11H10FN3O MW:219.2150 |
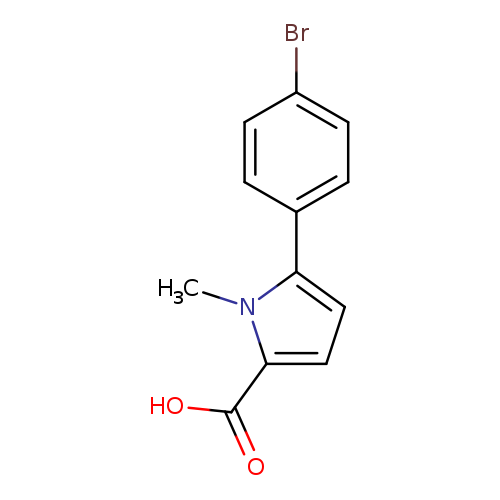
5-(4-bromophenyl)-1-methyl-1H-pyrrole-2-carboxylic acidCatalog No.:AA01A913 CAS No.:1017473-75-4 MDL No.:MFCD10006413 MF:C12H10BrNO2 MW:280.1173 |
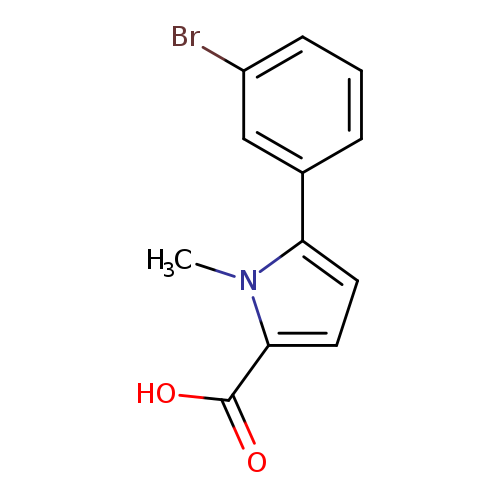
5-(3-bromophenyl)-1-methyl-1H-pyrrole-2-carboxylic acidCatalog No.:AA01A919 CAS No.:1017473-79-8 MDL No.:MFCD10006415 MF:C12H10BrNO2 MW:280.1173 |
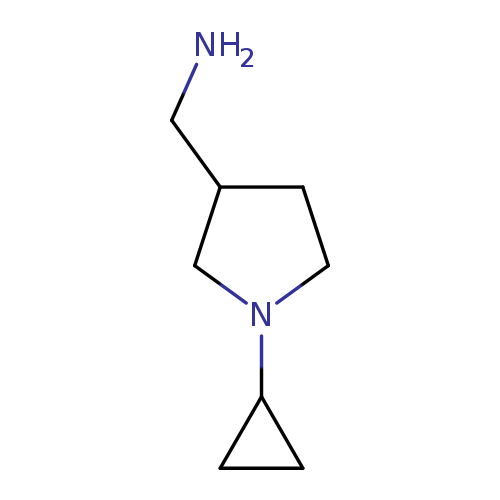
[(1-Cyclopropylpyrrolidin-3-yl)methyl]amine hydrochlorideCatalog No.:AA008VFN CAS No.:1017474-07-5 MDL No.:MFCD09932873 MF:C8H16N2 MW:140.2260 |
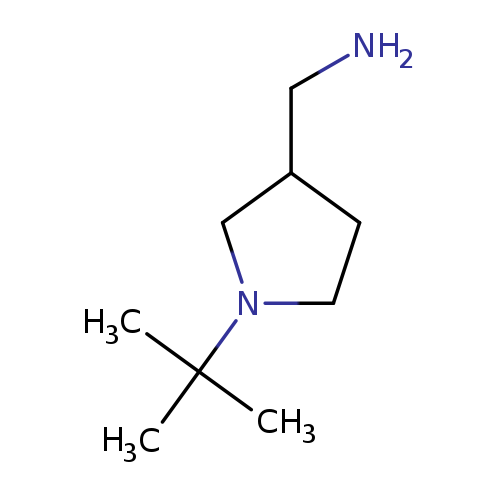
1-(1-tert-butylpyrrolidin-3-yl)methanamineCatalog No.:AA008V8T CAS No.:1017474-41-7 MDL No.:MFCD09937656 MF:C9H20N2 MW:156.2685 |
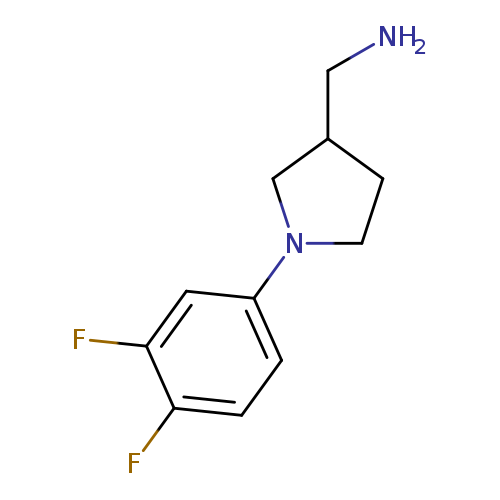
[1-(3,4-difluorophenyl)pyrrolidin-3-yl]methanamineCatalog No.:AA019XVP CAS No.:1017475-12-5 MDL No.:MFCD09927782 MF:C11H14F2N2 MW:212.2391 |
(1-Cyclopropylpyrrolidin-3-yl)methanolCatalog No.:AA0098XO CAS No.:1017476-51-5 MDL No.:MFCD10003882 MF:C8H15NO MW:141.2108 |
1-(4-chlorophenyl)-2-methylcyclopropan-1-amineCatalog No.:AA01BHNN CAS No.:1017478-72-6 MDL No.:MFCD09904018 MF:C10H12ClN MW:181.6620 |
1-(4-chlorophenyl)cyclohexan-1-amineCatalog No.:AA01A8R3 CAS No.:1017478-76-0 MDL No.:MFCD09904026 MF:C12H16ClN MW:209.7151 |
{1-[(2-chlorophenyl)methyl]pyrrolidin-2-yl}methanolCatalog No.:AA01AHV3 CAS No.:1017479-63-8 MDL No.:MFCD10004522 MF:C12H16ClNO MW:225.7145 |
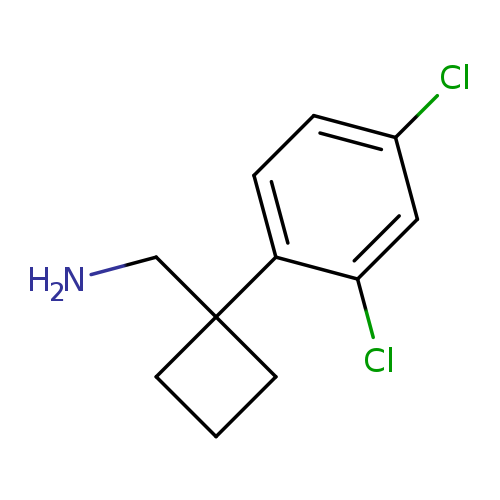
[1-(2,4-Dichlorophenyl)cyclobutyl]methanamineCatalog No.:AA01A410 CAS No.:1017479-81-0 MDL No.:MFCD09904135 MF:C11H13Cl2N MW:230.1336 |
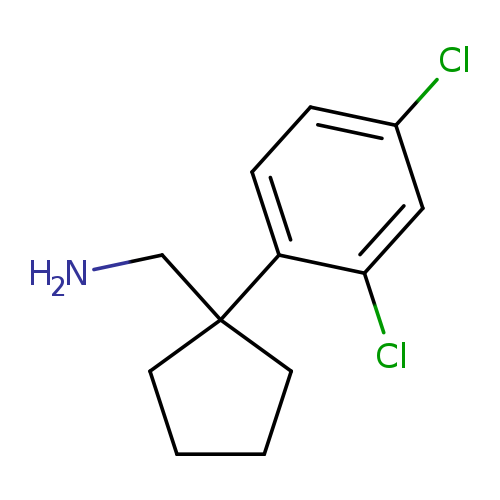
[1-(2,4-dichlorophenyl)cyclopentyl]methanamineCatalog No.:AA01A3QU CAS No.:1017479-85-4 MDL No.:MFCD09904136 MF:C12H15Cl2N MW:244.1602 |
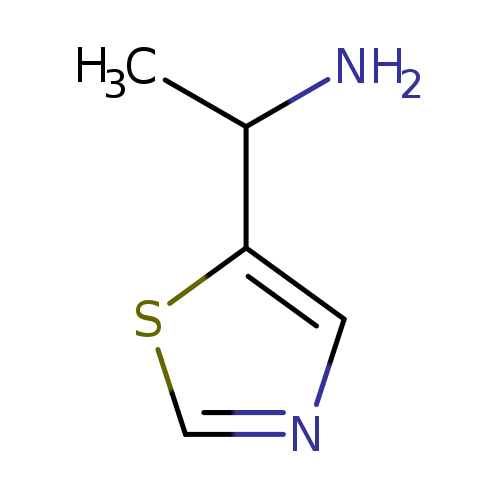
1-(Thiazol-5-yl)ethanamineCatalog No.:AA0098XP CAS No.:1017480-28-2 MDL No.:MFCD10007038 MF:C5H8N2S MW:128.1954 |
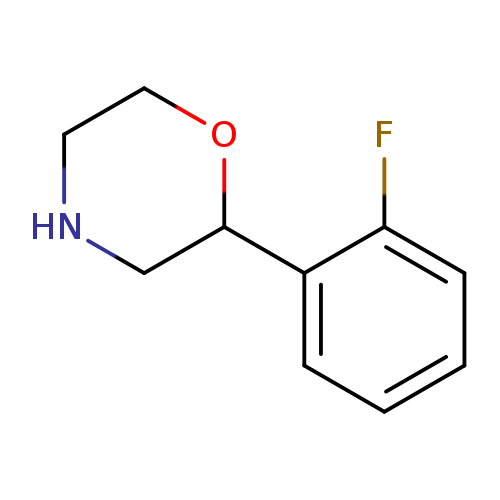
2-(2-Fluorophenyl)morpholineCatalog No.:AA00VSH7 CAS No.:1017480-65-7 MDL No.:MFCD09901449 MF:C10H12FNO MW:181.2068 |
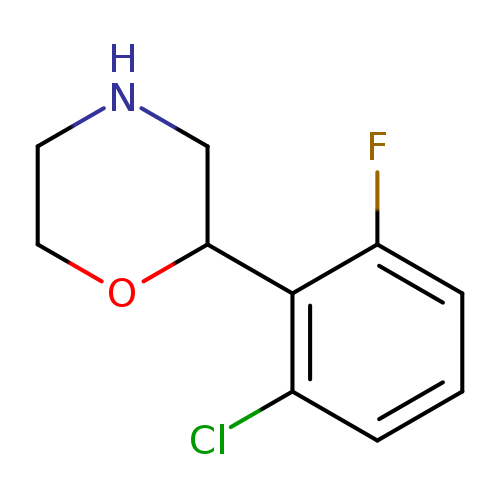
2-(2-chloro-6-fluorophenyl)morpholineCatalog No.:AA01C53E CAS No.:1017480-74-8 MDL No.:MFCD09901460 MF:C10H11ClFNO MW:215.6518 |
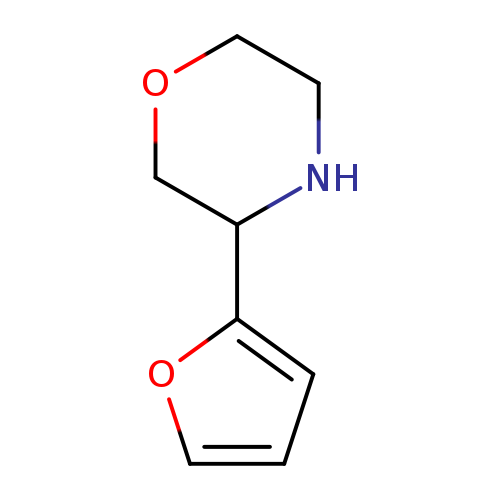
3-(furan-2-yl)morpholineCatalog No.:AA01AHAO CAS No.:1017481-25-2 MDL No.:MFCD09901522 MF:C8H11NO2 MW:153.1784 |
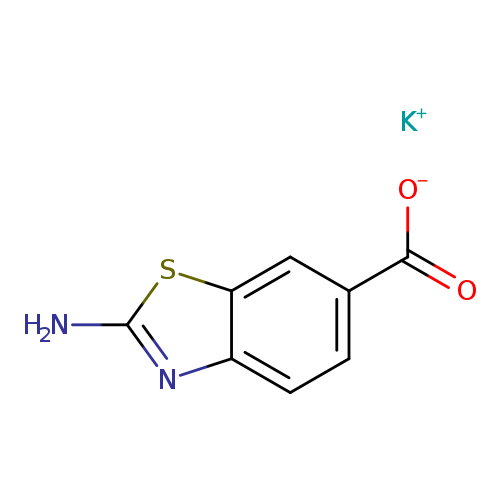
Potassium 2-amino-1,3-benzothiazole-6-carboxylateCatalog No.:AA01FMFT CAS No.:1017488-71-9 MDL No.:MFCD08137419 MF:C8H5KN2O2S MW:232.3008 |
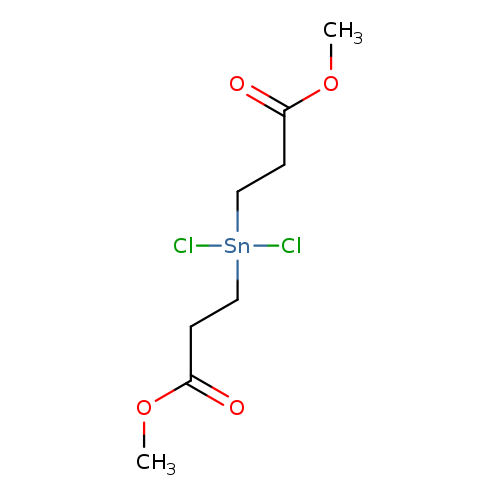
Propanoic acid, 3,3'-(dichlorostannylene)bis-, 1,1'-dimethyl esterCatalog No.:AA0005BE CAS No.:10175-01-6 MDL No.:MFCD00271043 MF:C8H14Cl2O4Sn MW:363.8014 |
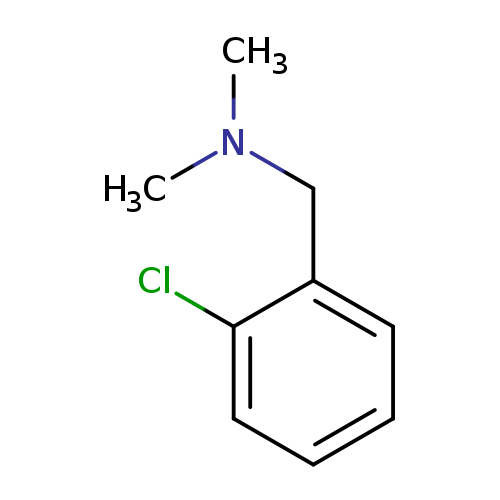
BenzeneMethanaMine, 2-chloro-N,N-diMethyl-Catalog No.:AA0098A3 CAS No.:10175-31-2 MDL No.:MFCD04037302 MF:C9H12ClN MW:169.6513 |
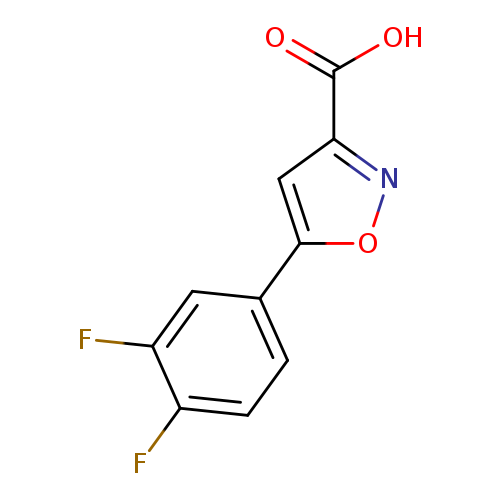
5-(3,4-Difluorophenyl)isoxazole-3-carboxylic acidCatalog No.:AA00JW16 CAS No.:1017513-51-7 MDL No.:MFCD07377121 MF:C10H5F2NO3 MW:225.1484 |
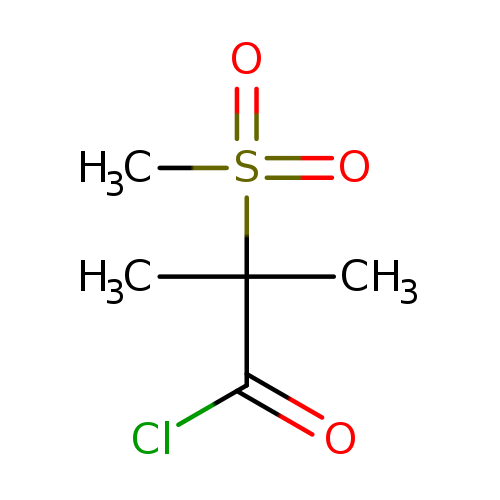
2-methanesulfonyl-2-methylpropanoyl chlorideCatalog No.:AA01BJAA CAS No.:1017540-45-2 MDL No.:MFCD19632278 MF:C5H9ClO3S MW:184.6412 |
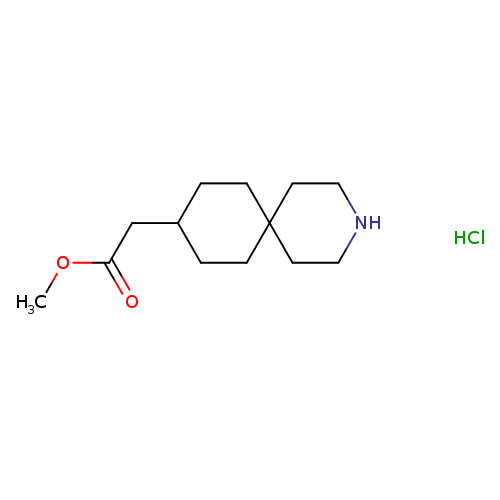
Methyl 2-(3-azaspiro[5.5]undecan-9-yl)acetate hydrochlorideCatalog No.:AA0005BK CAS No.:1017540-91-8 MDL No.:MFCD28501529 MF:C13H24ClNO2 MW:261.7882 |
4H,5H,6H,7H,8H-cyclohepta[b]thiophen-5-oneCatalog No.:AA01DUU2 CAS No.:1017589-87-5 MDL No.:MFCD24711886 MF:C9H10OS MW:166.2401 |
Boc-N-Me-Thr-OHCatalog No.:AA0005BM CAS No.:101759-72-2 MDL No.:MFCD02259477 MF:C10H19NO5 MW:233.2616 |
(S)-2-((tert-Butoxycarbonyl)(methyl)amino)butanoic acidCatalog No.:AA0005BL CAS No.:101759-74-4 MDL No.:MFCD05264091 MF:C10H19NO4 MW:217.2622 |
2,5-Dichloroisonicotinoyl chlorideCatalog No.:AA01DUU3 CAS No.:1017590-28-1 MDL No.:MFCD28679929 MF:C6H2Cl3NO MW:210.4452 |
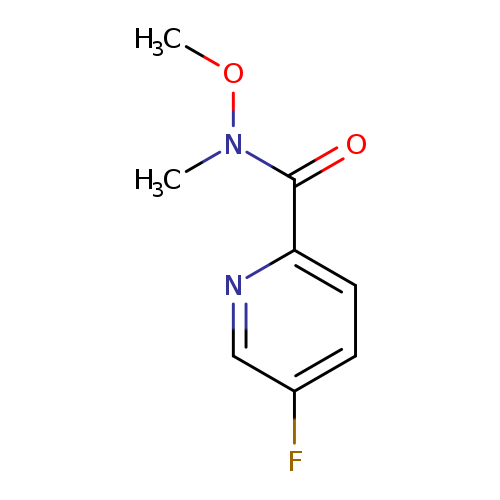
5-fluoro-N-methoxy-N-methylpyridine-2-carboxamideCatalog No.:AA01B9XQ CAS No.:1017598-58-1 MDL No.:MFCD18256777 MF:C8H9FN2O2 MW:184.1677 |
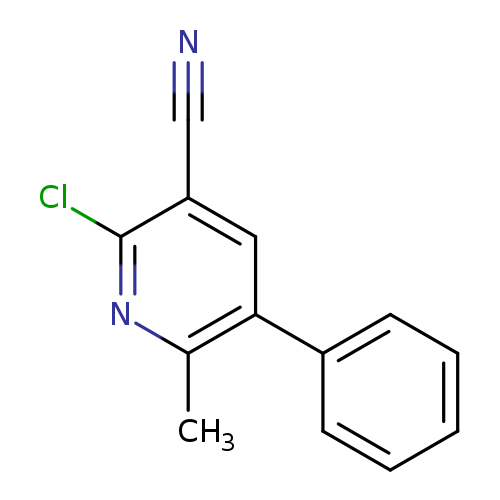
2-Chloro-6-methyl-5-phenylnicotinonitrileCatalog No.:AA0005CQ CAS No.:10176-63-3 MDL No.:MFCD00231559 MF:C13H9ClN2 MW:228.6770 |
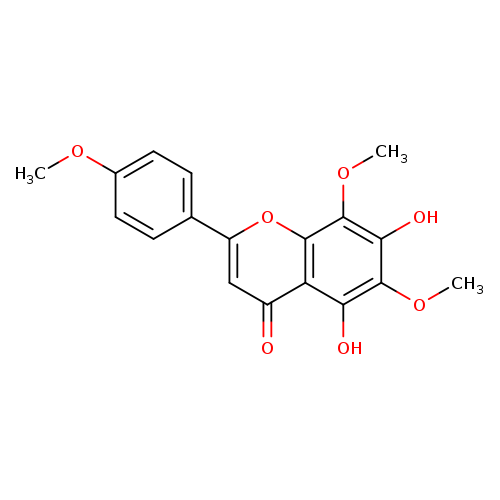
NevadensinCatalog No.:AA008XLE CAS No.:10176-66-6 MDL No.:MFCD08689947 MF:C18H16O7 MW:344.3154 |
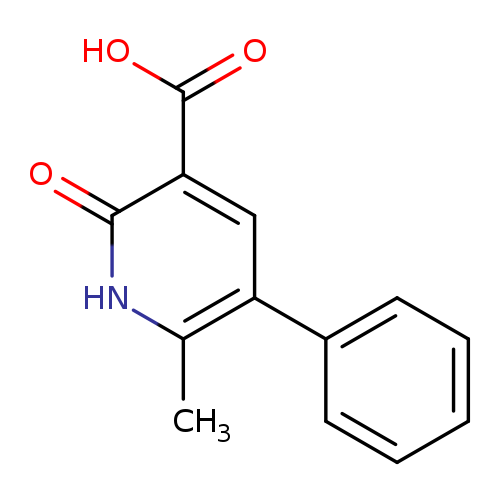
6-Methyl-2-oxo-5-phenyl-1,2-dihydropyridine-3-carboxylic acidCatalog No.:AA019YBS CAS No.:10176-79-1 MDL No.:MFCD22375125 MF:C13H11NO3 MW:229.2313 |
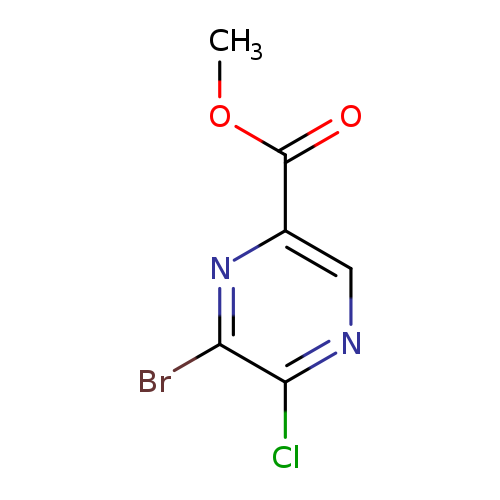
methyl 6-bromo-5-chloropyrazine-2-carboxylateCatalog No.:AA01DSVR CAS No.:1017603-87-0 MDL No.:MFCD31559718 MF:C6H4BrClN2O2 MW:251.4652 |
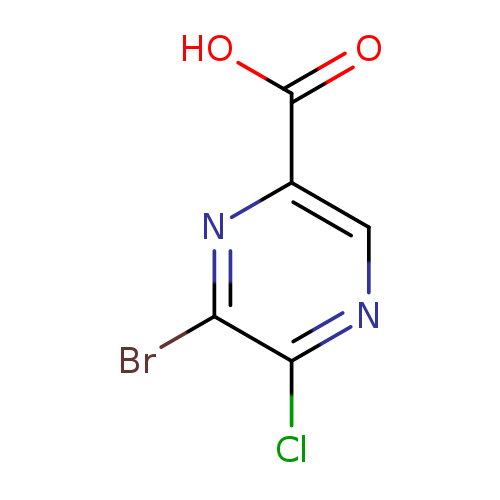
6-Bromo-5-chloropyrazine-2-carboxylic acidCatalog No.:AA01CA6S CAS No.:1017604-40-8 MDL No.:MFCD18073162 MF:C5H2BrClN2O2 MW:237.4386 |
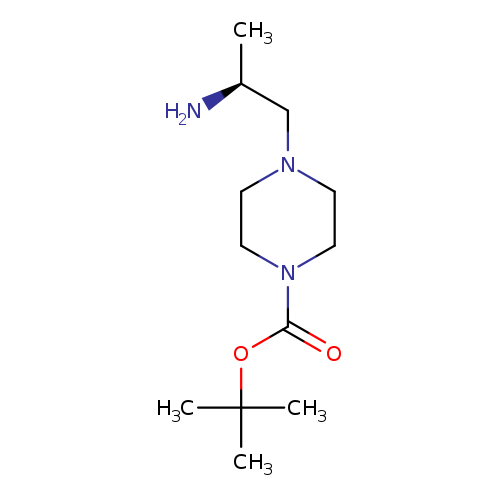
(S)-tert-Butyl 4-(2-aminopropyl)piperazine-1-carboxylateCatalog No.:AA0005C6 CAS No.:1017606-58-4 MDL No.:MFCD18249855 MF:C12H25N3O2 MW:243.3458 |
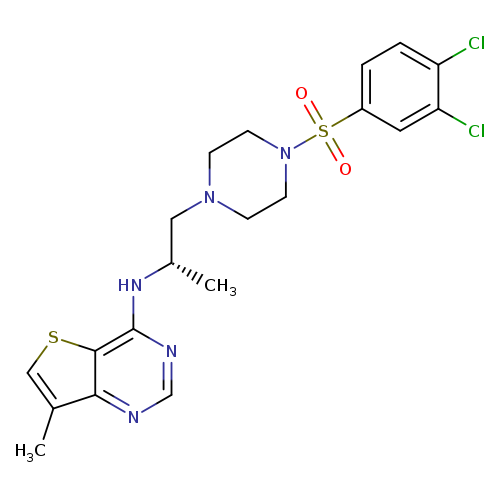
Lpa2 antagonist 1Catalog No.:AA008TEM CAS No.:1017606-66-4 MDL No.:MFCD28716107 MF:C20H23Cl2N5O2S2 MW:500.4649 |
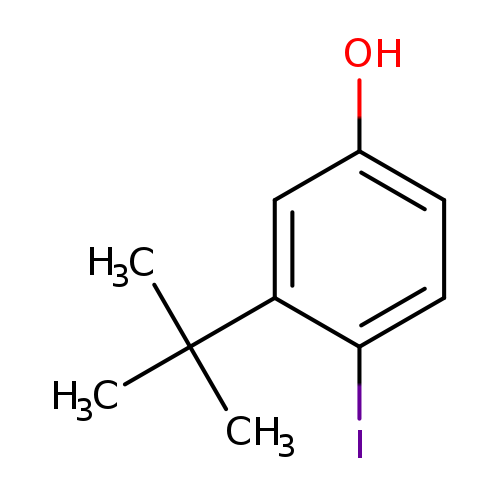
3-(tert-butyl)-4-iodophenolCatalog No.:AA01BS3Q CAS No.:1017608-22-8 MDL No.:MFCD15527203 MF:C10H13IO MW:276.1141 |
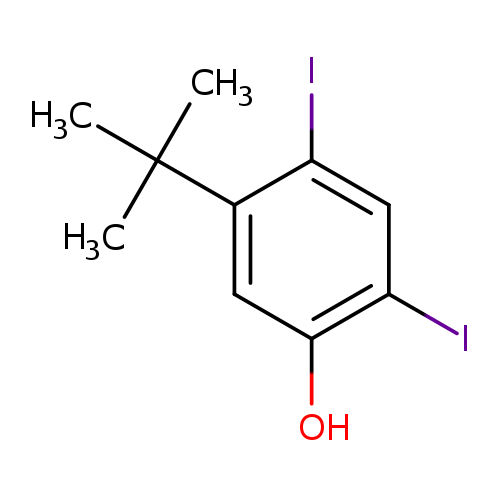
5-tert-butyl-2,4-diiodophenolCatalog No.:AA01E7C4 CAS No.:1017608-26-2 MDL No.:MFCD30537749 MF:C10H12I2O MW:402.0106 |
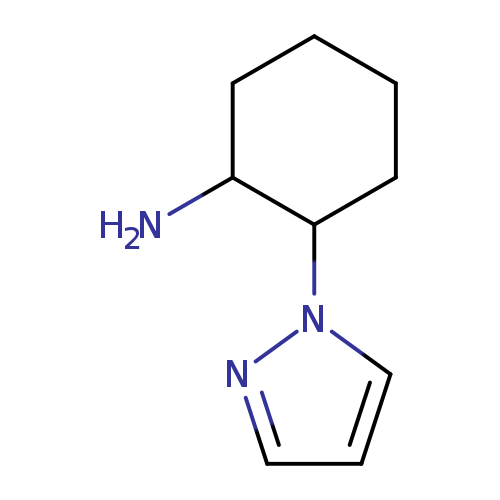
2-(1H-pyrazol-1-yl)cyclohexan-1-amineCatalog No.:AA019T8C CAS No.:1017665-07-4 MDL No.:MFCD09261658 MF:C9H15N3 MW:165.2355 |
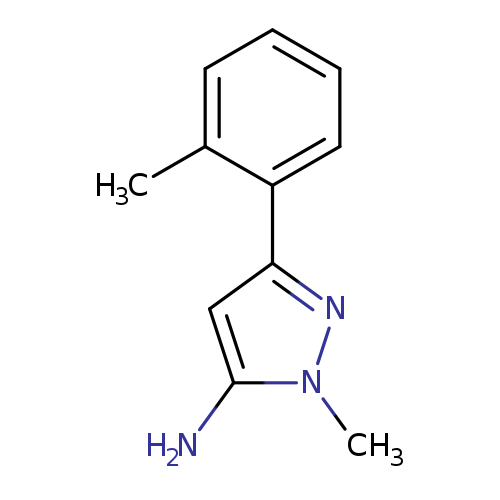
2-Methyl-5-(2-methylphenyl)pyrazol-3-amineCatalog No.:AA00949S CAS No.:1017665-59-6 MDL No.:MFCD06637387 MF:C11H13N3 MW:187.2410 |
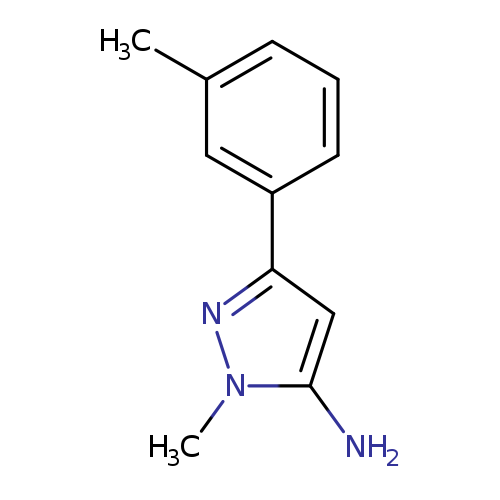
1-Methyl-3-(3-methylphenyl)-1h-pyrazol-5-amineCatalog No.:AA009N22 CAS No.:1017665-60-9 MDL No.:MFCD06637389 MF:C11H13N3 MW:187.2410 |
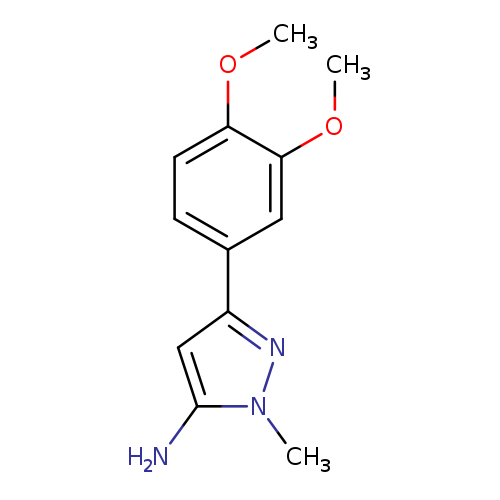
3-Amino-5-(3,4-dimethoxyphenyl)-2-methylpyrazoleCatalog No.:AA00946M CAS No.:1017665-64-3 MDL No.:MFCD09703235 MF:C12H15N3O2 MW:233.2664 |
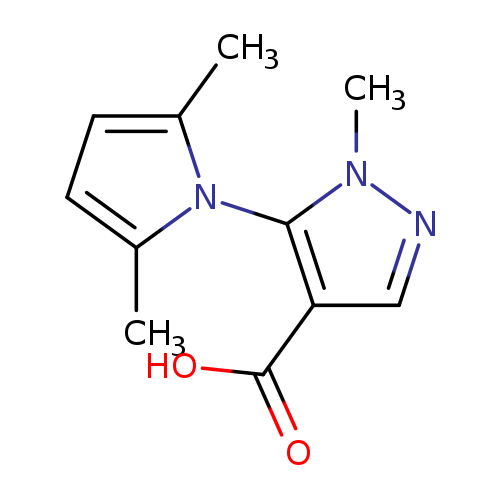
5-(2,5-Dimethyl-1h-pyrrol-1-yl)-1-methyl-1h-pyrazole-4-carboxylic acidCatalog No.:AA019WAM CAS No.:1017666-40-8 MDL No.:MFCD09703011 MF:C11H13N3O2 MW:219.2398 |
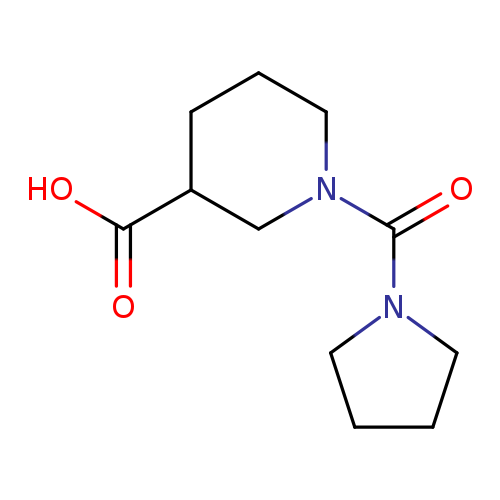
1-(pyrrolidine-1-carbonyl)piperidine-3-carboxylic acidCatalog No.:AA019MZK CAS No.:1017668-81-3 MDL No.:MFCD10012141 MF:C11H18N2O3 MW:226.2722 |
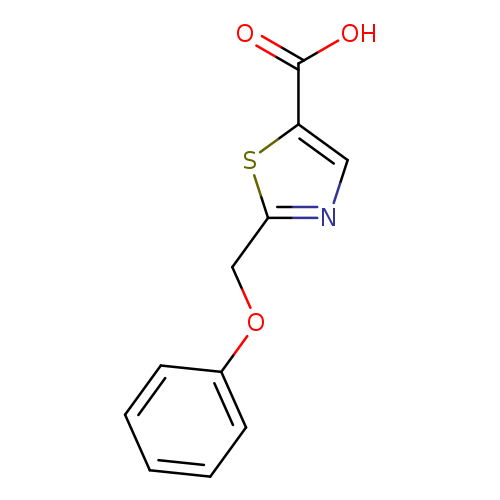
2-(Phenoxymethyl)thiazole-5-carboxylic acidCatalog No.:AA019XNJ CAS No.:1017669-06-5 MDL No.:MFCD10008839 MF:C11H9NO3S MW:235.2591 |
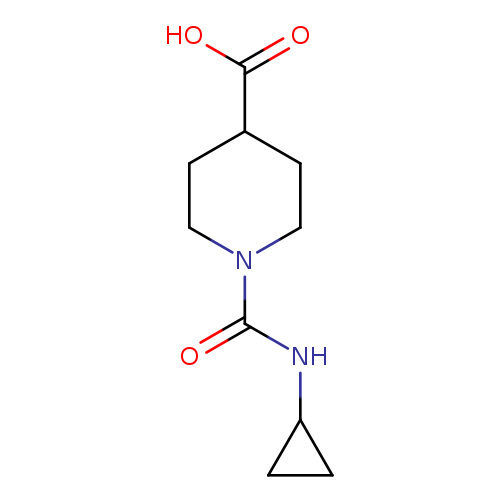
1-(cyclopropylcarbamoyl)piperidine-4-carboxylic acidCatalog No.:AA019MQC CAS No.:1017669-48-5 MDL No.:MFCD10012221 MF:C10H16N2O3 MW:212.2456 |
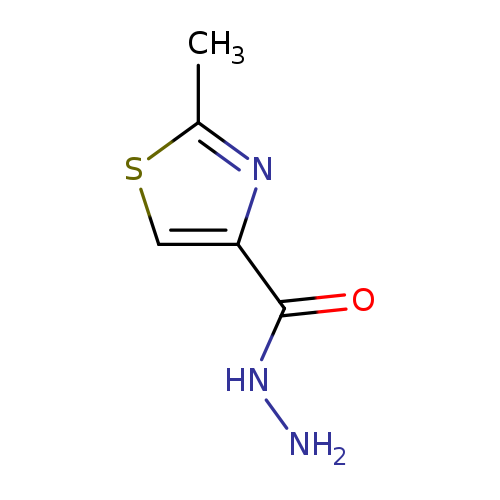
2-Methyl-thiazole-4-carboxylic acid hydrazideCatalog No.:AA0005CI CAS No.:101767-28-6 MDL No.:MFCD00662677 MF:C5H7N3OS MW:157.1936 |
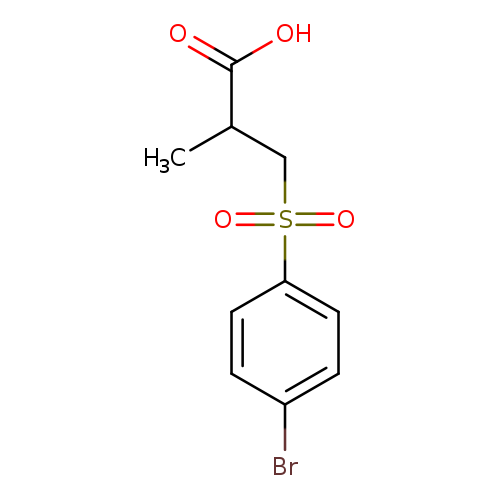
3-((4-Bromophenyl)sulfonyl)-2-methylpropanoic acidCatalog No.:AA019Z4Y CAS No.:1017674-08-6 MDL No.:MFCD10024398 MF:C10H11BrO4S MW:307.1609 |
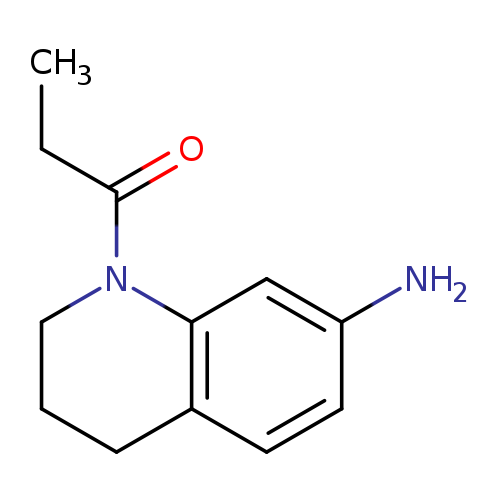
1-Propionyl-1,2,3,4-tetrahydroquinolin-7-amineCatalog No.:AA0192UZ CAS No.:1017674-20-2 MDL No.:MFCD10016585 MF:C12H16N2O MW:204.2682 |
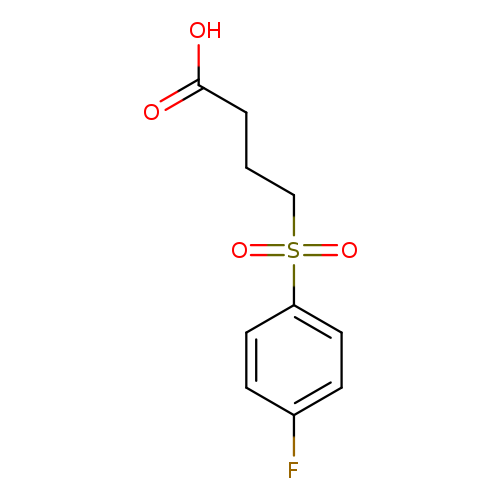
4-(4-fluorobenzenesulfonyl)butanoic acidCatalog No.:AA01FP7M CAS No.:1017674-75-7 MDL No.:MFCD10024474 MF:C10H11FO4S MW:246.2553 |
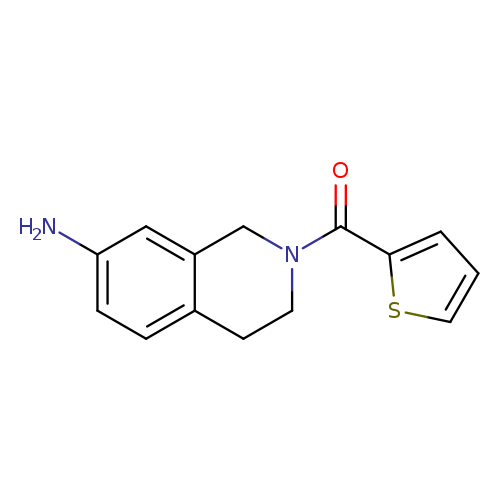
2-(2-Thienylcarbonyl)-1,2,3,4-tetrahydroisoquinolin-7-amineCatalog No.:AA01ARDC CAS No.:1017675-00-1 MDL No.:MFCD10016679 MF:C14H14N2OS MW:258.3388 |
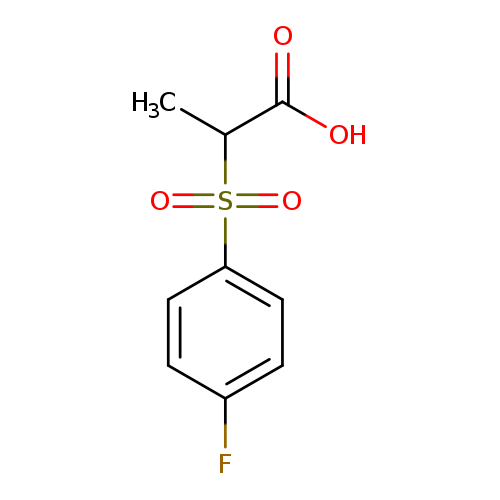
2-(4-fluorobenzenesulfonyl)propanoic acidCatalog No.:AA019VEV CAS No.:1017675-07-8 MDL No.:MFCD10024513 MF:C9H9FO4S MW:232.2288 |
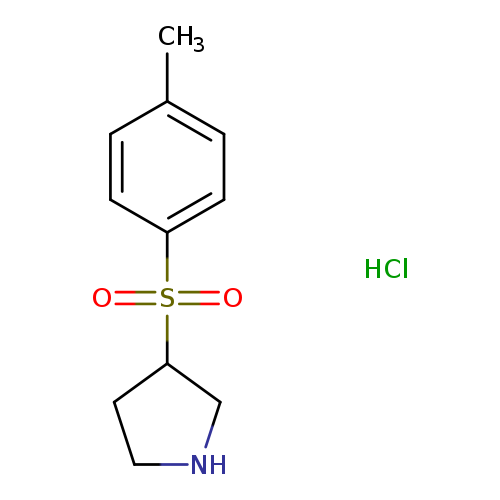
3-[(4-methylbenzene)sulfonyl]pyrrolidine, HClCatalog No.:AA008RVF CAS No.:101768-40-5 MDL No.:MFCD04117767 MF:C11H16ClNO2S MW:261.7682 |
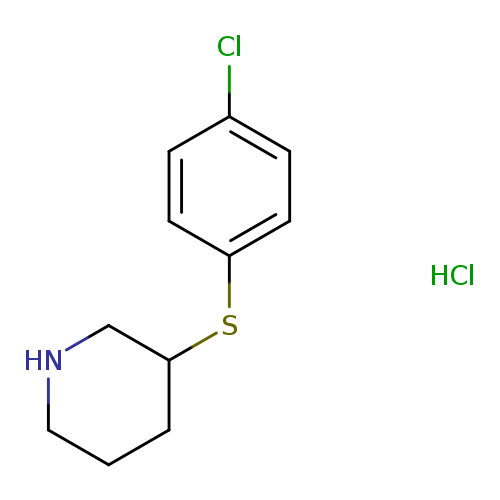
3-(4-Chlorophenylsulfanyl)piperidine, HClCatalog No.:AA00H9IG CAS No.:101768-48-3 MDL No.:MFCD05863874 MF:C11H15Cl2NS MW:264.2145 |

4-[(4-Chlorophenyl)sulfanyl]piperidineCatalog No.:AA0005CE CAS No.:101768-63-2 MDL No.:MFCD04115009 MF:C11H14ClNS MW:227.7536 |
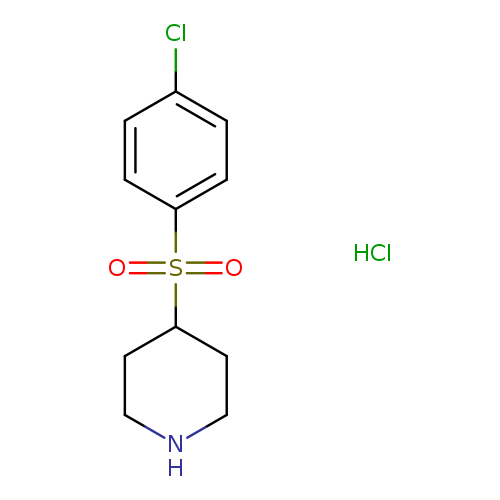
Piperidine, 4-[(4-chlorophenyl)sulfonyl]-, hydrochloride (1:1)Catalog No.:AA0005CD CAS No.:101768-64-3 MDL No.:MFCD04115824 MF:C11H15Cl2NO2S MW:296.2133 |
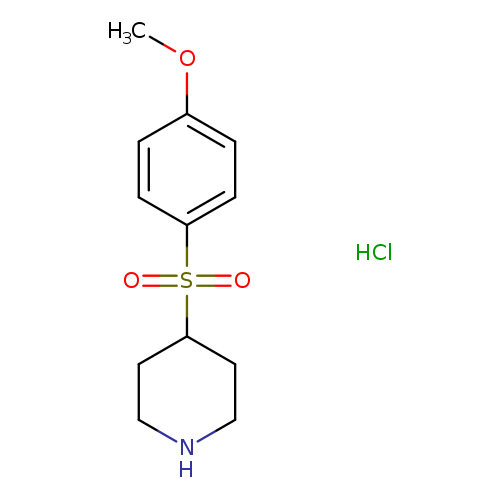
4-[(4-Methoxyphenyl)sulfonyl]piperidine hydrochlorideCatalog No.:AA01ARVC CAS No.:101768-77-8 MDL No.:MFCD24842733 MF:C12H18ClNO3S MW:291.7942 |
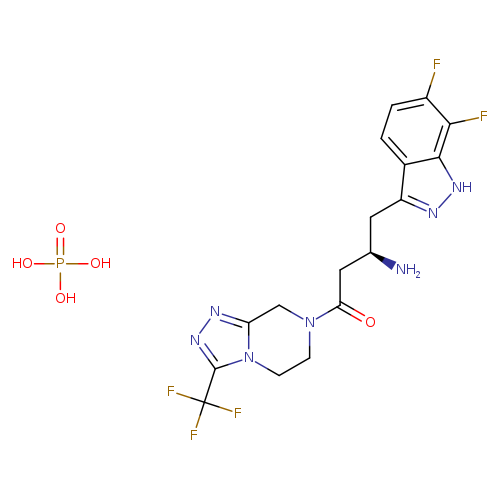
(3R)-3-Amino-4-(6,7-difluoro-1H-indazol-3-yl)-1-[5,6-dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazin-7(8H)-yl]-1-butanonephosphateCatalog No.:AA01ENLY CAS No.:1017682-66-4 MDL No.: MF:C17H19F5N7O5P MW:527.3424 |
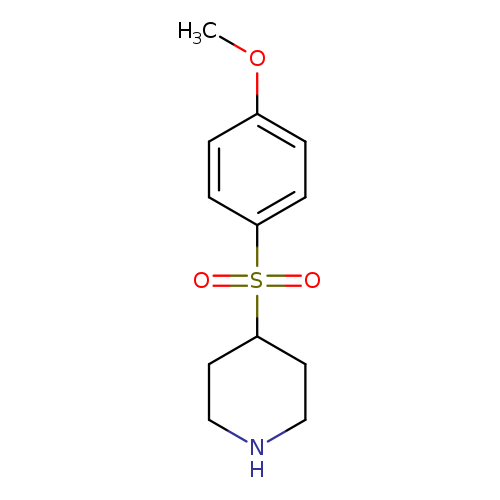
4-(4-methoxybenzenesulfonyl)piperidineCatalog No.:AA01ATK9 CAS No.:101769-01-1 MDL No.:MFCD15526094 MF:C12H17NO3S MW:255.3333 |
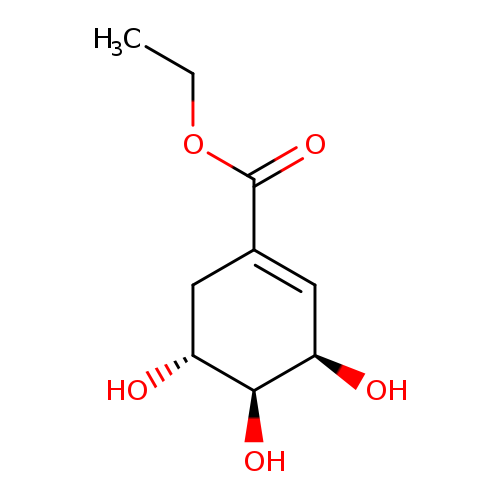
ShikiMic Acid Ethyl EsterCatalog No.:AA008X2V CAS No.:101769-63-5 MDL No.:MFCD30738184 MF:C9H14O5 MW:202.2045 |
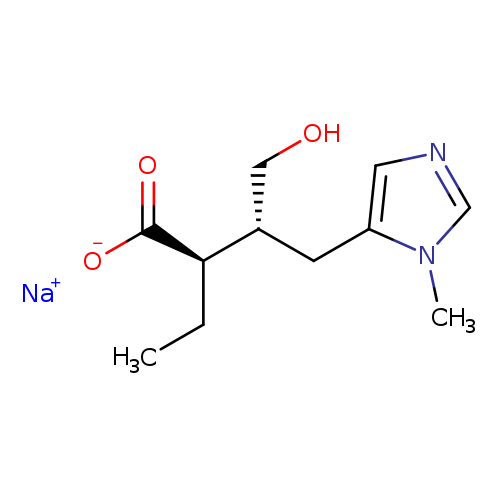
Isopilocarpic Acid SodiuM SaltCatalog No.:AA008VVQ CAS No.:101769-87-3 MDL No.:MFCD27967089 MF:C11H17N2NaO3 MW:248.2540 |
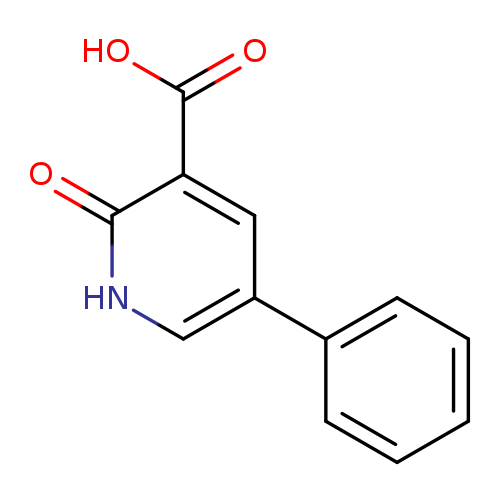
2-Hydroxy-5-phenylnicotinic acidCatalog No.:AA0005DG CAS No.:10177-08-9 MDL No.:MFCD22421597 MF:C12H9NO3 MW:215.2048 |
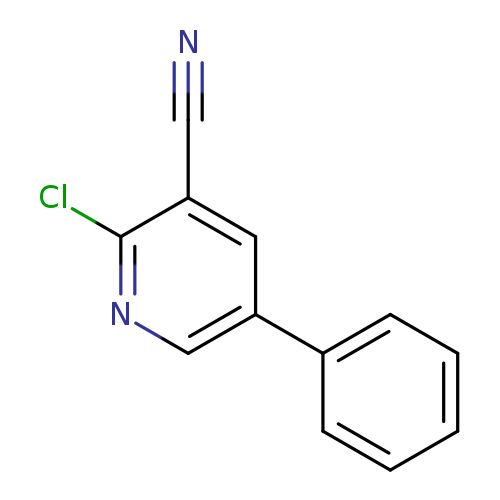
2-Chloro-5-phenylnicotinonitrileCatalog No.:AA0005DF CAS No.:10177-10-3 MDL No.:MFCD00231610 MF:C12H7ClN2 MW:214.6504 |
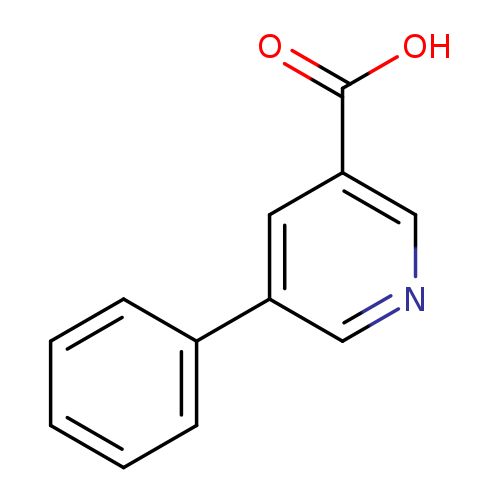
5-Phenylnicotinic acidCatalog No.:AA0005DD CAS No.:10177-12-5 MDL No.:MFCD03086176 MF:C12H9NO2 MW:199.2054 |
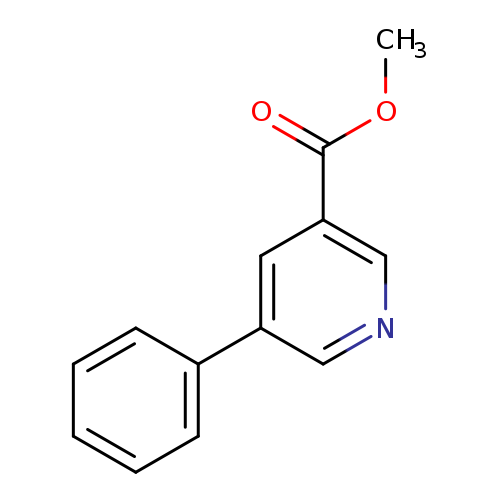
Methyl 5-phenylnicotinateCatalog No.:AA0005DC CAS No.:10177-13-6 MDL No.:MFCD06802742 MF:C13H11NO2 MW:213.2319 |
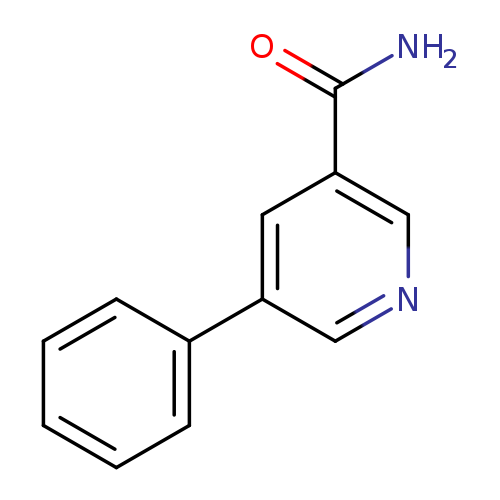
5-PhenylnicotinamideCatalog No.:AA0095MW CAS No.:10177-15-8 MDL No.:MFCD19688859 MF:C12H10N2O MW:198.2206 |
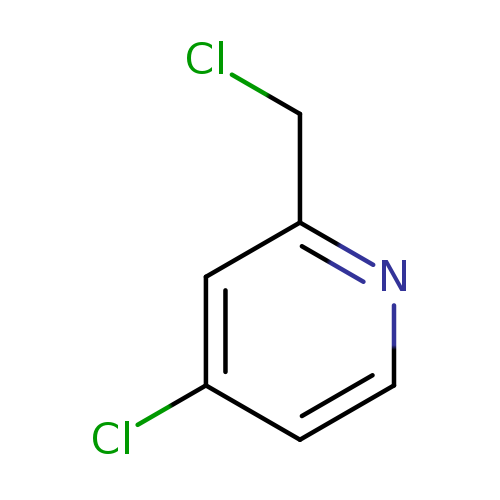
4-Chloro-2-(chloromethyl)pyridineCatalog No.:AA0005DB CAS No.:10177-21-6 MDL No.:MFCD07774098 MF:C6H5Cl2N MW:162.0166 |
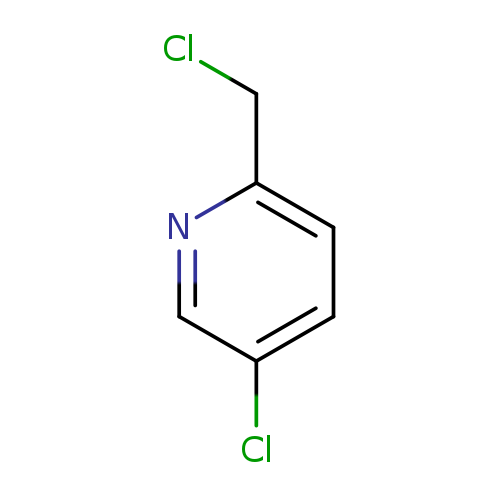
5-Chloro-2-(chloromethyl)pyridineCatalog No.:AA0005D9 CAS No.:10177-24-9 MDL No.:MFCD10697588 MF:C6H5Cl2N MW:162.0166 |
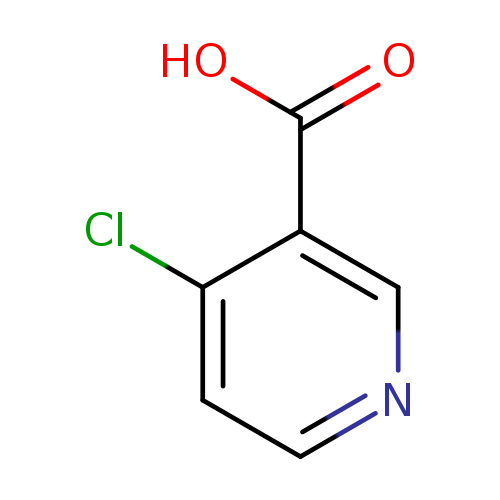
4-Chloronicotinic acidCatalog No.:AA0005D8 CAS No.:10177-29-4 MDL No.:MFCD00128860 MF:C6H4ClNO2 MW:157.5545 |
(Z)-3-Phenylacrylic acidCatalog No.:AA00066F CAS No.:102-94-3 MDL No.:MFCD00466869 MF:C9H8O2 MW:148.1586 |
(2-Nitrovinyl)benzeneCatalog No.:AA00066E CAS No.:102-96-5 MDL No.:MFCD00007402 MF:C8H7NO2 MW:149.1467 |
Benzenemethanamine, N-(1-methylethyl)-Catalog No.:AA00066D CAS No.:102-97-6 MDL No.:MFCD00008863 MF:C10H15N MW:149.2328 |

1-Propanone, 3-(4-morpholinyl)-1-phenyl-, hydrochloride (1:1)Catalog No.:AA00066B CAS No.:1020-16-2 MDL No.:MFCD00035332 MF:C13H18ClNO2 MW:255.7405 |
3,5-Di-tert-butylcatecholCatalog No.:AA00066A CAS No.:1020-31-1 MDL No.:MFCD00008819 MF:C14H22O2 MW:222.3233 |
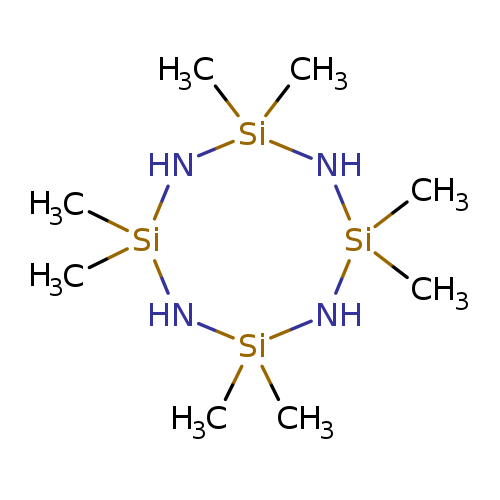
Cyclotetrasilazane, 2,2,4,4,6,6,8,8-octamethyl-Catalog No.:AA00067E CAS No.:1020-84-4 MDL No.:MFCD00046123 MF:C8H28N4Si4 MW:292.6767 |
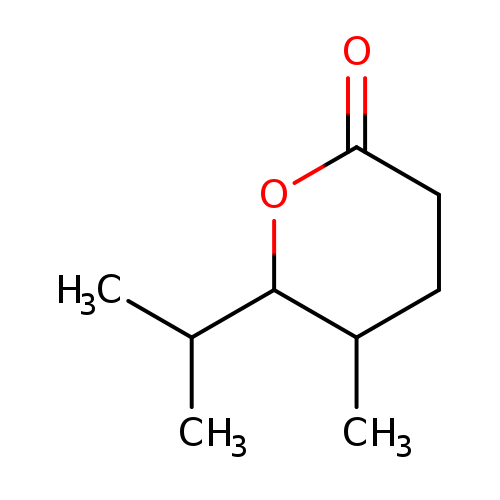
5-methyl-6-(propan-2-yl)oxan-2-one, Mixture of diastereomersCatalog No.:AA01EIMB CAS No.:10200-23-4 MDL No.:MFCD31665786 MF:C9H16O2 MW:156.2221 |
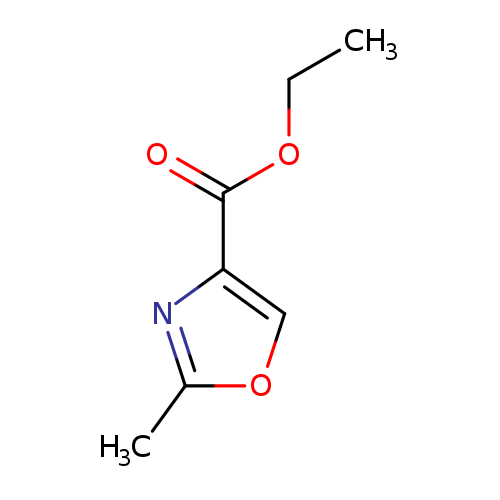
Ethyl-4-methyl-3,5-oxazolecarboxylateCatalog No.:AA000677 CAS No.:10200-43-8 MDL No.:MFCD07367959 MF:C7H9NO3 MW:155.1513 |
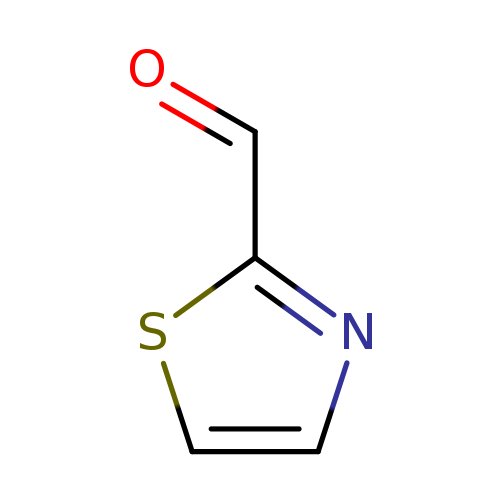
2-ThiazolecarboxaldehydeCatalog No.:AA000675 CAS No.:10200-59-6 MDL No.:MFCD00142924 MF:C4H3NOS MW:113.1377 |
1-(morpholin-4-yl)-2-(2-phenyl-1H-indol-3-yl)ethane-1,2-dioneCatalog No.:AA00IXK8 CAS No.:102003-06-5 MDL No.:MFCD02102454 MF:C20H18N2O3 MW:334.3685 |
(6-Fluoroimidazo[1,2-a]pyridin-3-yl)methanamineCatalog No.:AA00KKDA CAS No.:1020033-25-3 MDL No.:MFCD09994569 MF:C8H8FN3 MW:165.1676 |
C-(7-Methyl-[1,2,4]triazolo[4,3-a]pyridin-3-yl)-methylamineCatalog No.:AA00H9MW CAS No.:1020033-70-8 MDL No.:MFCD09994896 MF:C8H10N4 MW:162.1918 |
imidazo[1,2-a]pyrimidine-6-carbonitrileCatalog No.:AA01B6NG CAS No.:1020033-79-7 MDL No.:MFCD09994611 MF:C7H4N4 MW:144.1335 |
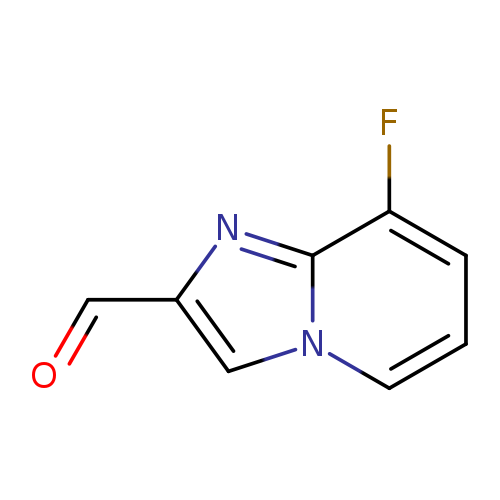
8-Fluoroimidazo[1,2-a]pyridine-2-carbaldehydeCatalog No.:AA01A2LC CAS No.:1020033-80-0 MDL No.:MFCD09994902 MF:C8H5FN2O MW:164.1365 |
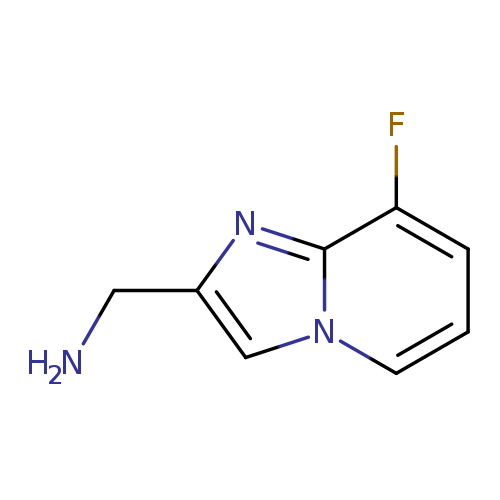
(8-fluoroimidazo[1,2-a]pyridin-2-yl)methanamineCatalog No.:AA01DTAQ CAS No.:1020033-88-8 MDL No.:MFCD09994906 MF:C8H8FN3 MW:165.1676 |
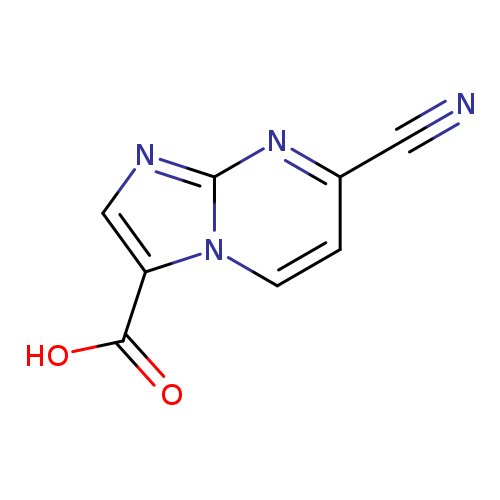
7-cyanoimidazo[1,2-a]pyrimidine-3-carboxylic acidCatalog No.:AA01C5C0 CAS No.:1020034-43-8 MDL No.:MFCD09994651 MF:C8H4N4O2 MW:188.1430 |
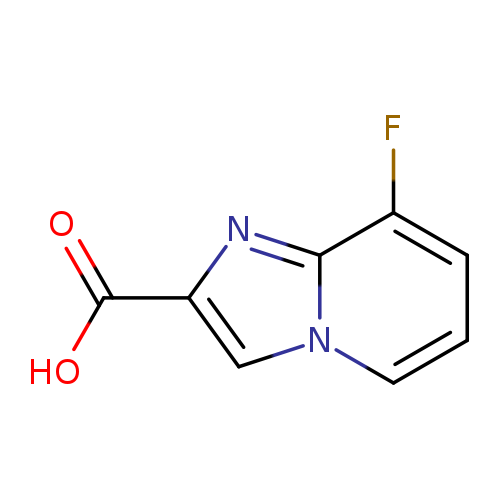
8-Fluoroimidazo[1,2-a]pyridine-2-carboxylic acidCatalog No.:AA000685 CAS No.:1020034-56-3 MDL No.:MFCD09994934 MF:C8H5FN2O2 MW:180.1359 |
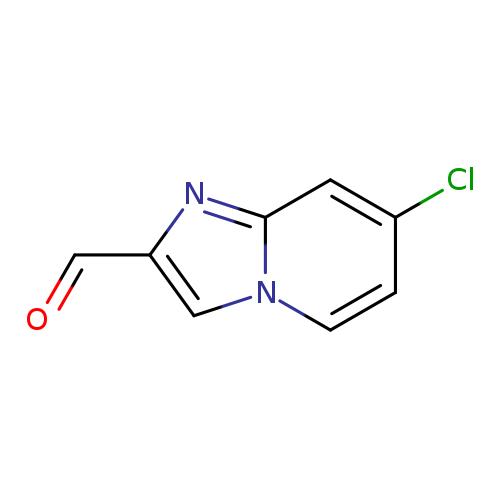
7-Chloroimidazo[1,2-a]pyridine-2-carbaldehydeCatalog No.:AA0093IR CAS No.:1020034-59-6 MDL No.:MFCD09994935 MF:C8H5ClN2O MW:180.5911 |
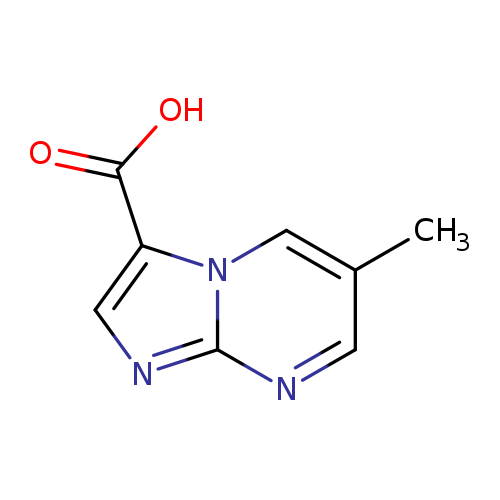
6-Methylimidazo[1,2-a]pyrimidine-3-carboxylic acidCatalog No.:AA000684 CAS No.:1020035-04-4 MDL No.:MFCD09994671 MF:C8H7N3O2 MW:177.1601 |
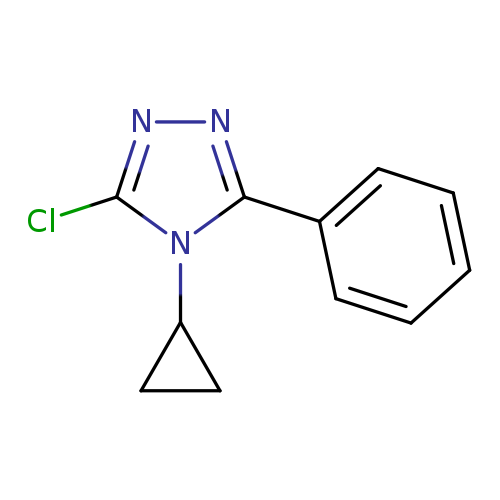
3-chloro-4-cyclopropyl-5-phenyl-4H-1,2,4-triazoleCatalog No.:AA019MWK CAS No.:1020035-36-2 MDL No.:MFCD09995819 MF:C11H10ClN3 MW:219.6702 |
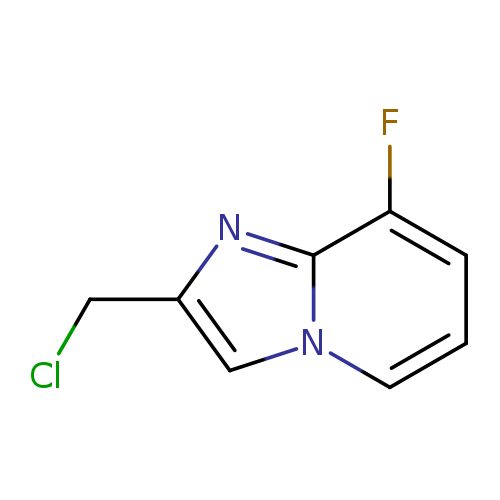
2-(Chloromethyl)-8-fluoroimidazo[1,2-a]pyridineCatalog No.:AA01CA9E CAS No.:1020035-40-8 MDL No.:MFCD09994963 MF:C8H6ClFN2 MW:184.5980 |
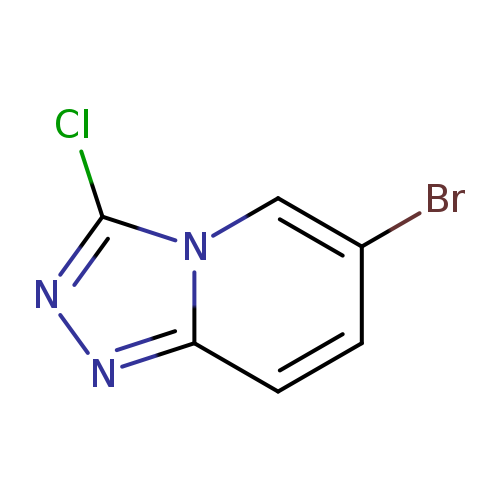
6-BroMo-3-chloro-[1,2,4]triazolo[4,3-a]pyridineCatalog No.:AA009LV9 CAS No.:1020036-34-3 MDL No.:MFCD09994026 MF:C6H3BrClN3 MW:232.4651 |
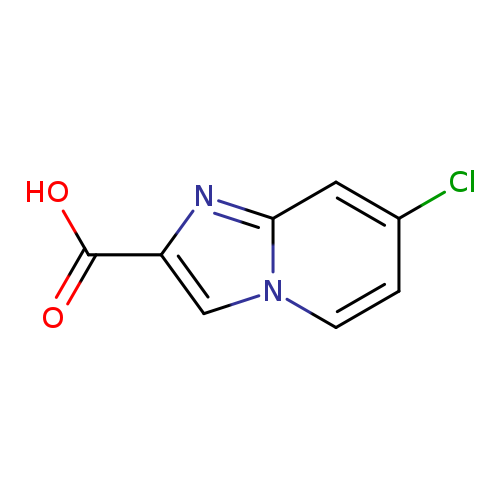
7-Chloroimidazo[1,2-a]pyridine-2-carboxylic acidCatalog No.:AA000683 CAS No.:1020038-42-9 MDL No.:MFCD09991784 MF:C8H5ClN2O2 MW:196.5905 |
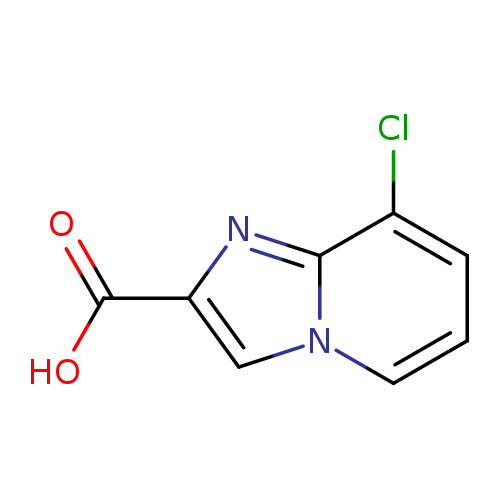
8-Chloroimidazo[1,2-a]pyridine-2-carboxylic acidCatalog No.:AA000682 CAS No.:1020038-45-2 MDL No.:MFCD09839010 MF:C8H5ClN2O2 MW:196.5905 |
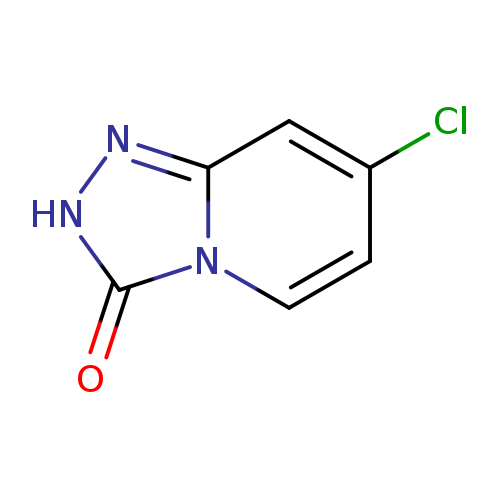
7-Chloro-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-oneCatalog No.:AA000680 CAS No.:1020039-12-6 MDL No.:MFCD23701114 MF:C6H4ClN3O MW:169.5685 |
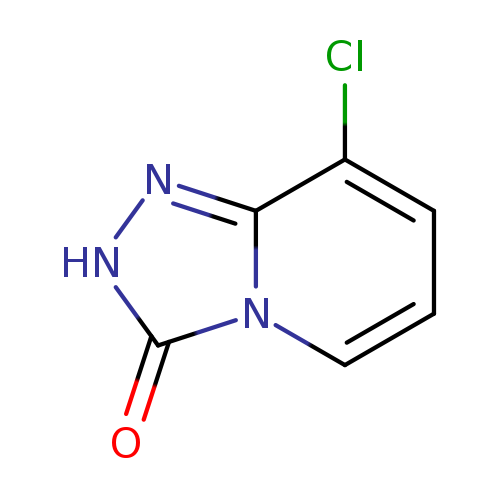
8-Chloro-[1,2,4]triazolo[4,3-a]pyridin-3(2h)-oneCatalog No.:AA00067Y CAS No.:1020042-77-6 MDL No.:MFCD21640309 MF:C6H4ClN3O MW:169.5685 |
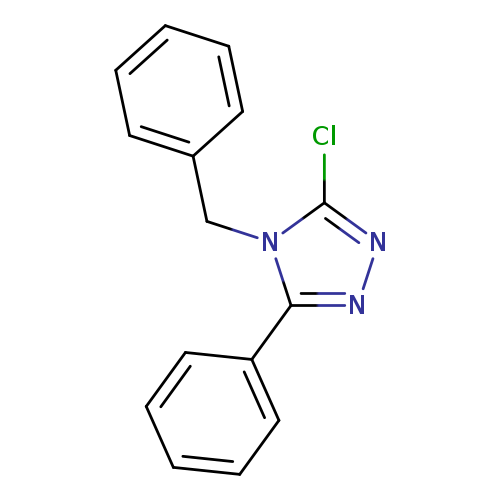
4-benzyl-3-chloro-5-phenyl-4H-1,2,4-triazoleCatalog No.:AA019MO4 CAS No.:1020043-28-0 MDL No.:MFCD09995976 MF:C15H12ClN3 MW:269.7289 |
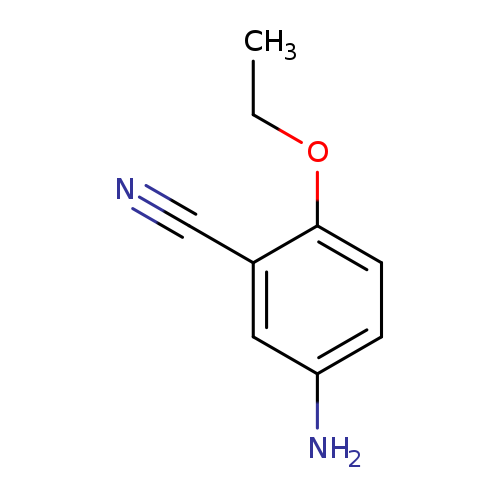
5-Amino-2-ethoxybenzonitrileCatalog No.:AA00067X CAS No.:1020046-39-2 MDL No.:MFCD10686562 MF:C9H10N2O MW:162.1885 |
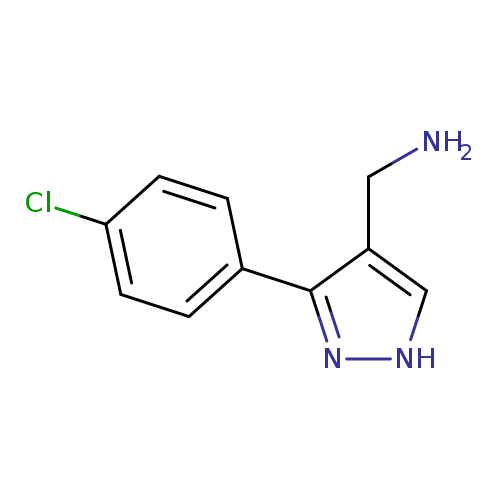
([3-(4-Chlorophenyl)-1h-pyrazol-4-yl]methyl)amine hydrochlorideCatalog No.:AA00067W CAS No.:1020052-19-0 MDL No.:MFCD08146630 MF:C10H10ClN3 MW:207.6595 |
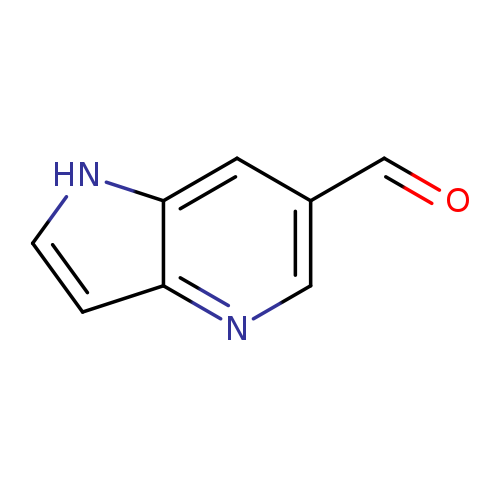
1H-Pyrrolo[3,2-b]pyridine-6-carbaldehydeCatalog No.:AA00067V CAS No.:1020056-33-0 MDL No.:MFCD09859114 MF:C8H6N2O MW:146.1460 |
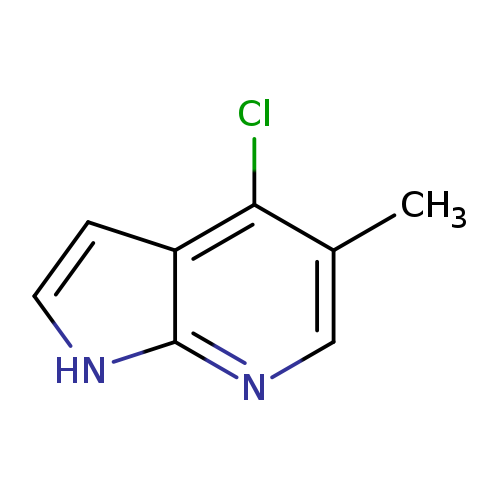
4-Chloro-5-methyl-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA00067U CAS No.:1020056-56-7 MDL No.:MFCD09965882 MF:C8H7ClN2 MW:166.6076 |
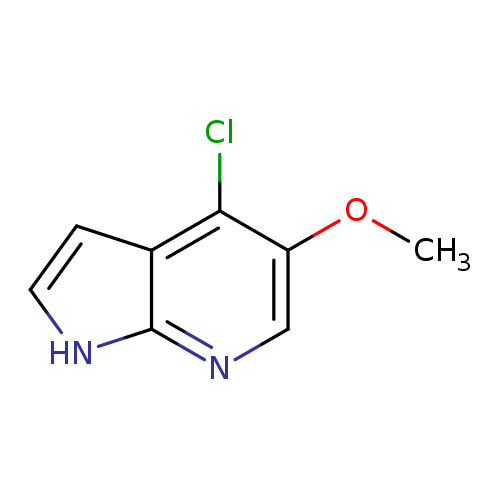
4-Chloro-5-methoxy-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA00067T CAS No.:1020056-72-7 MDL No.:MFCD09965889 MF:C8H7ClN2O MW:182.6070 |
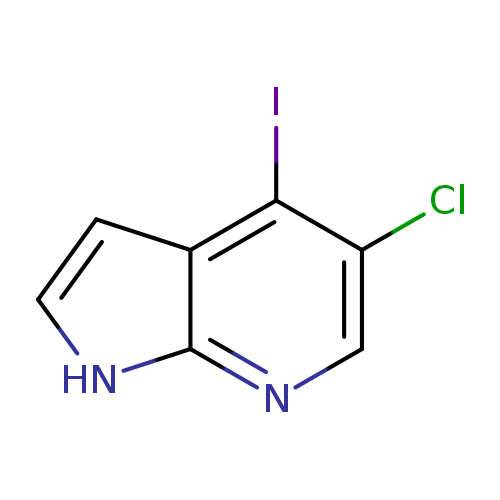
5-Chloro-4-iodo-1h-pyrrolo[2,3-b]pyridineCatalog No.:AA00067S CAS No.:1020056-77-2 MDL No.:MFCD09965892 MF:C7H4ClIN2 MW:278.4775 |
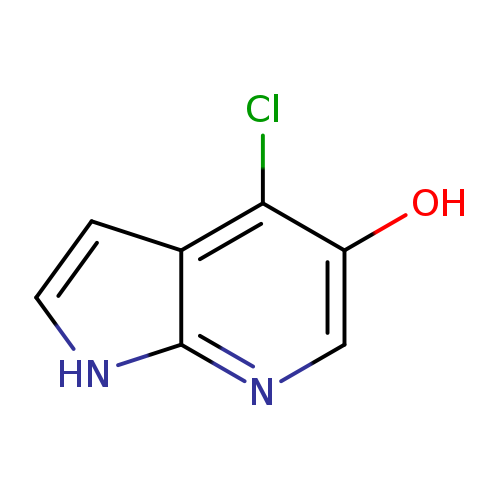
4-Chloro-1h-pyrrolo[2,3-b]pyridin-5-olCatalog No.:AA00067R CAS No.:1020056-82-9 MDL No.:MFCD09965894 MF:C7H5ClN2O MW:168.5804 |
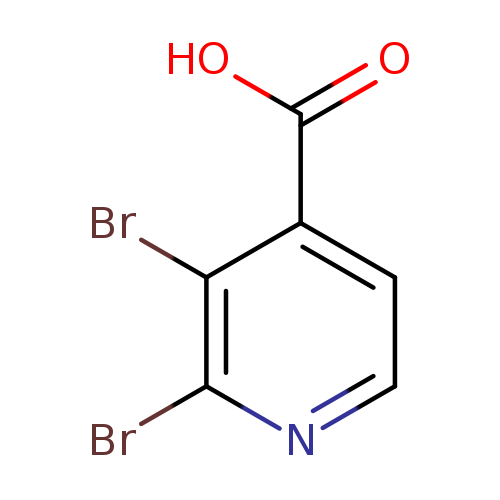
2,3-Dibromopyridine-4-carboxylic acidCatalog No.:AA00067P CAS No.:1020056-98-7 MDL No.:MFCD09997708 MF:C6H3Br2NO2 MW:280.9015 |
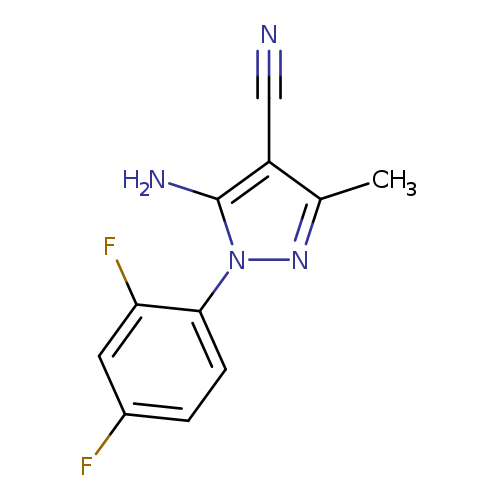
5-Amino-4-cyano-1-(2,4-difluorophenyl)-3-methyl-1H-pyrazoleCatalog No.:AA00067M CAS No.:1020057-92-4 MDL No.:MFCD08459254 MF:C11H8F2N4 MW:234.2048 |
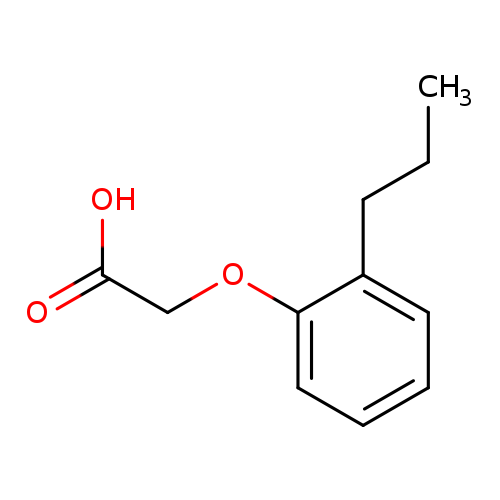
2-(2-propylphenoxy)acetic acidCatalog No.:AA00ITKF CAS No.:102008-91-3 MDL No.:MFCD05670711 MF:C11H14O3 MW:194.2271 |
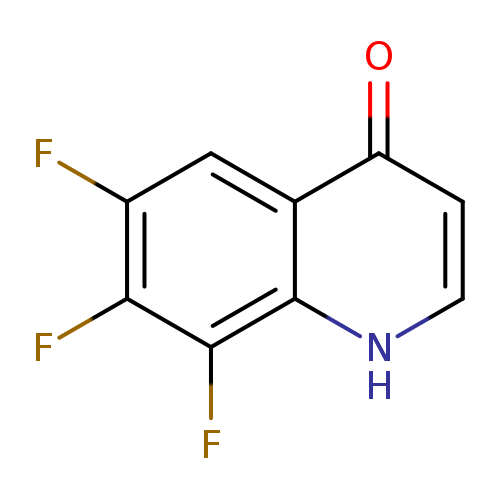
6,7,8-trifluoro-1,4-dihydroquinolin-4-oneCatalog No.:AA01BTLJ CAS No.:1020087-32-4 MDL No.:MFCD12192813 MF:C9H4F3NO MW:199.1294 |
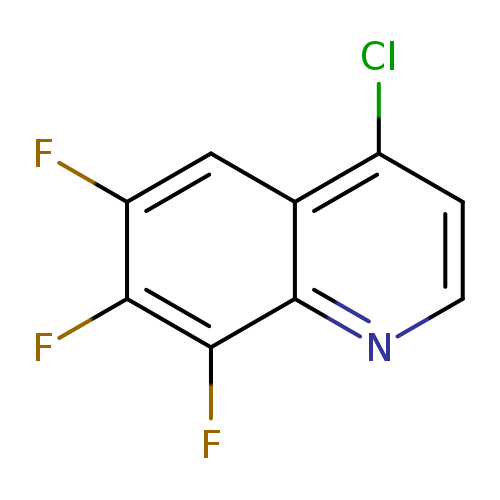
4-chloro-6,7,8-trifluoroquinolineCatalog No.:AA01AA8I CAS No.:1020087-33-5 MDL No.:MFCD12192768 MF:C9H3ClF3N MW:217.5750 |
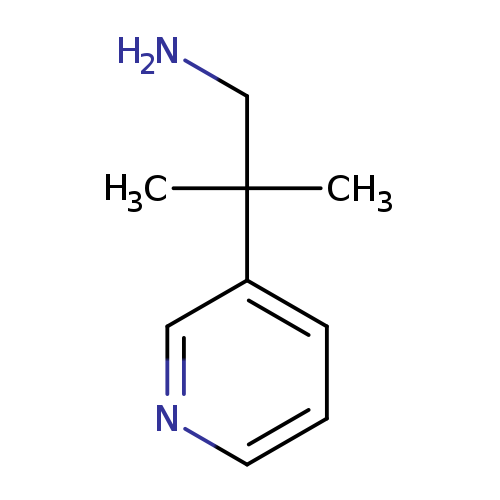
2-Methyl-2-(pyridin-3-yl)propan-1-amineCatalog No.:AA01C37L CAS No.:1020087-85-7 MDL No.:MFCD11898939 MF:C9H14N2 MW:150.2209 |
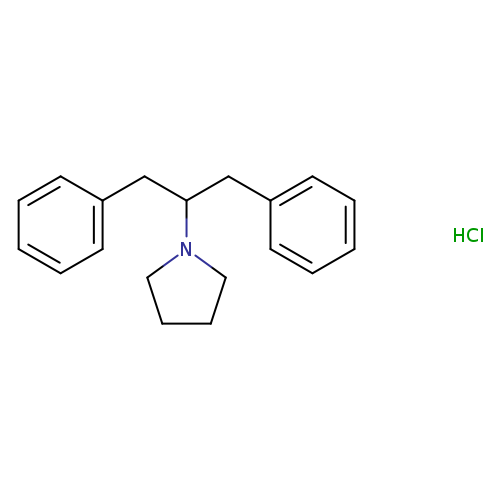
1-[2-phenyl-1-(phenylmethyl)ethyl]-pyrrolidine,monohydrochlorideCatalog No.:AA01EQBO CAS No.:102009-66-5 MDL No.: MF:C19H24ClN MW:301.8536 |

rac-methyl 2-[(1R,2S)-2-{[(tert-butoxy)carbonyl]amino}cyclopentyl]acetate, transCatalog No.:AA01EIGG CAS No.:1020097-79-3 MDL No.:MFCD29033855 MF: MW: |
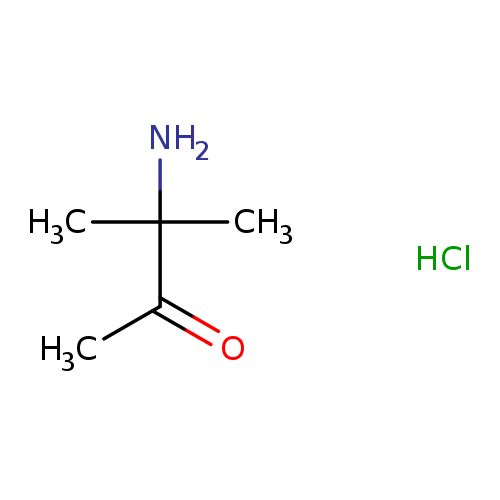
3-Amino-3-methyl-2-butanone hydrochlorideCatalog No.:AA00068W CAS No.:10201-15-7 MDL No.:MFCD09864171 MF:C5H12ClNO MW:137.6079 |
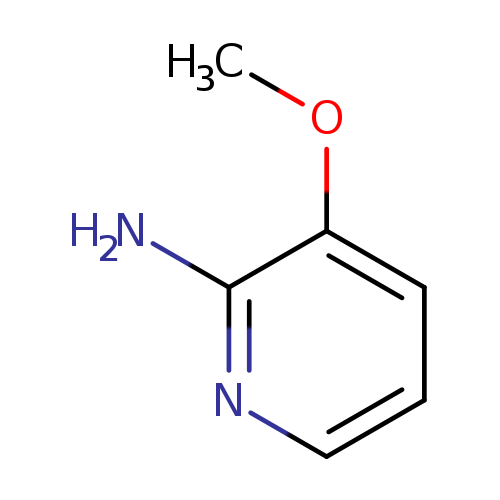
2-Amino-3-methoxypyridineCatalog No.:AA00068U CAS No.:10201-71-5 MDL No.:MFCD07374874 MF:C6H8N2O MW:124.1405 |
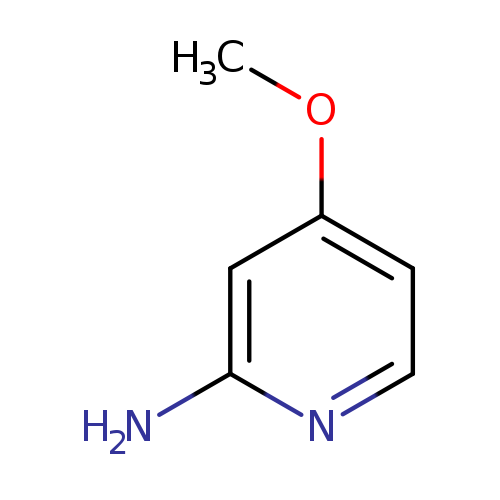
2-Amino-4-methoxypyridineCatalog No.:AA00068T CAS No.:10201-73-7 MDL No.:MFCD07437849 MF:C6H8N2O MW:124.1405 |
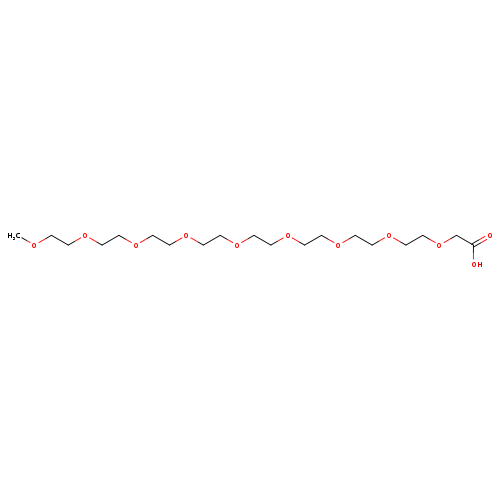
3,6,9,12,15,18,21,24,27-Nonaoxaoctacosanoic acidCatalog No.:AA00068I CAS No.:102013-72-9 MDL No.:MFCD16619222 MF:C19H38O11 MW:442.4984 |
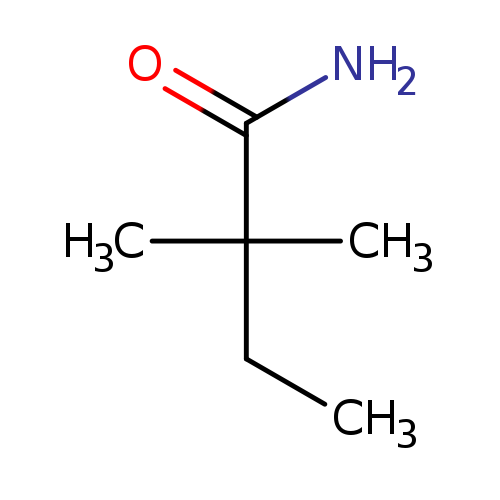
2,2-DimethylbutanamideCatalog No.:AA00068F CAS No.:102014-33-5 MDL No.:MFCD02860386 MF:C6H13NO MW:115.1735 |
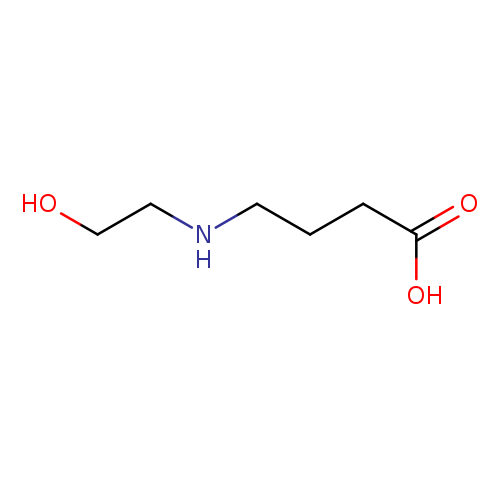
4-[(2-HYDROXYETHYL)AMINO]BUTANOIC ACIDCatalog No.:AA01DX49 CAS No.:102014-42-6 MDL No.:MFCD12177476 MF:C6H13NO3 MW:147.1723 |
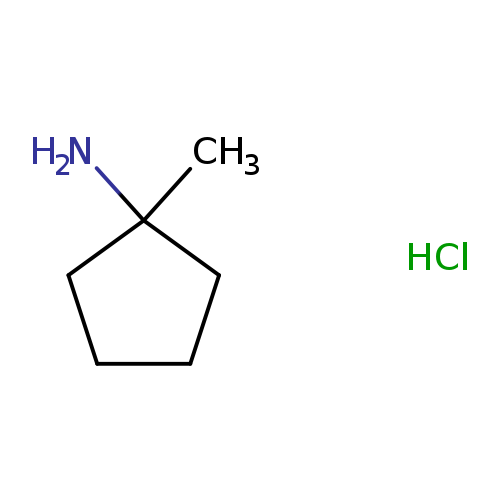
1-Methylcyclopentan-1-amine hydrochlorideCatalog No.:AA00069G CAS No.:102014-58-4 MDL No.:MFCD11858044 MF:C6H14ClN MW:135.6351 |
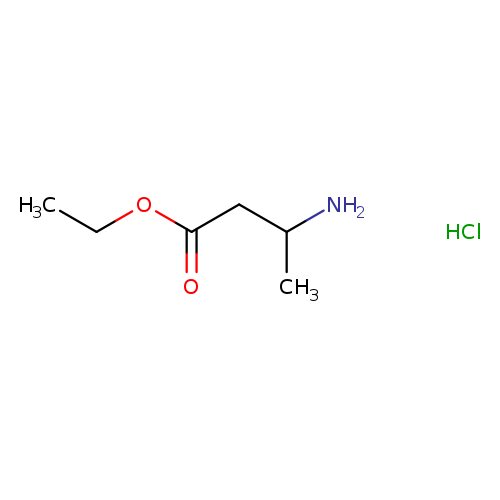
Ethyl 3-aminobutanoate hydrochlorideCatalog No.:AA00069F CAS No.:102014-64-2 MDL No.:MFCD22056243 MF:C6H14ClNO2 MW:167.6339 |
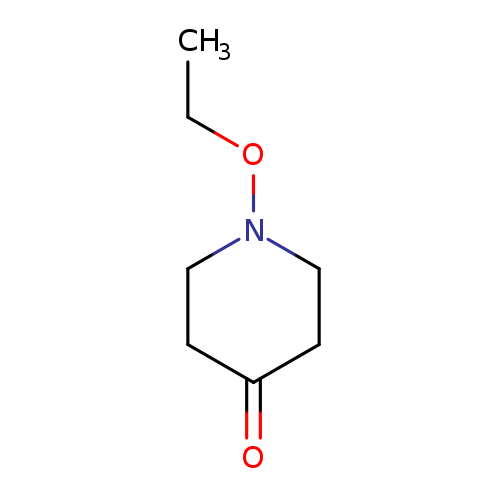
1-Ethoxy-piperidin-4-oneCatalog No.:AA009S0V CAS No.:102014-74-4 MDL No.:MFCD24396395 MF:C7H13NO2 MW:143.1836 |
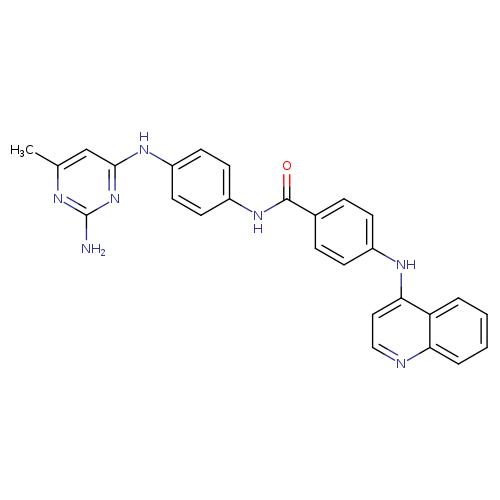
SGI-1027Catalog No.:AA000694 CAS No.:1020149-73-8 MDL No.:MFCD27937047 MF:C27H23N7O MW:461.5178 |
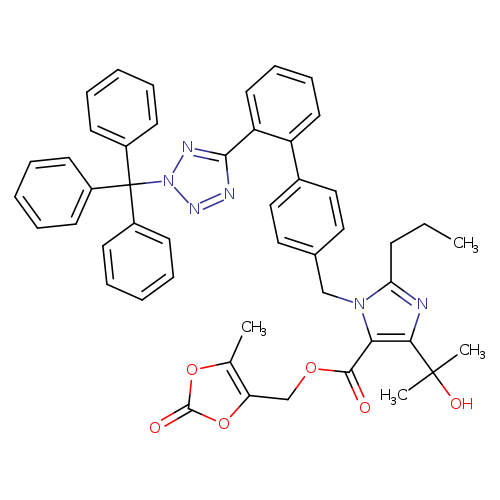
Trityl Olmesartan MedoxomilCatalog No.:AA0091TK CAS No.:1020157-01-0 MDL No.:MFCD20272387 MF:C48H44N6O6 MW:800.8996 |
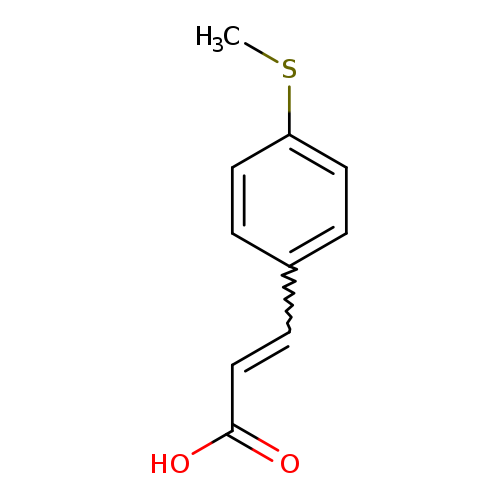
3-[4-(Methylsulfanyl)phenyl]acrylic acidCatalog No.:AA000699 CAS No.:102016-58-0 MDL No.:MFCD00266583 MF:C10H10O2S MW:194.2502 |

Dcc-2036Catalog No.:AA000692 CAS No.:1020172-07-9 MDL No.:MFCD19443646 MF:C30H28FN7O3 MW:553.5868 |
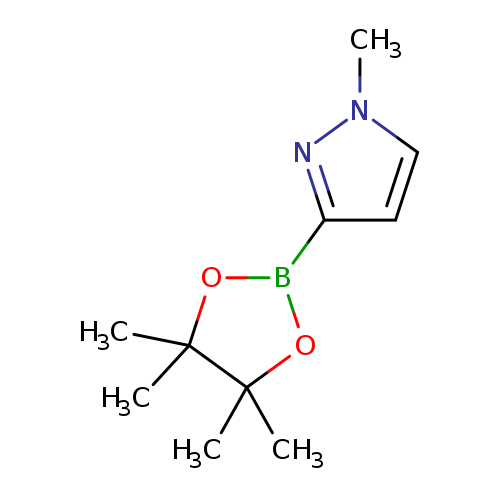
1-Methyl-1H-pyrazole-3-boronic acid pinacol esterCatalog No.:AA000690 CAS No.:1020174-04-2 MDL No.:MFCD04114000 MF:C10H17BN2O2 MW:208.0652 |
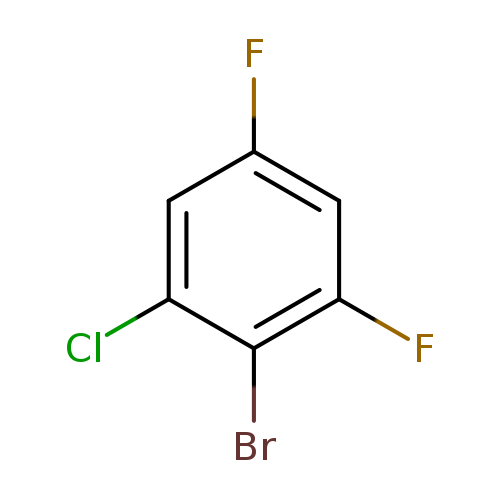
2-Bromo-1-chloro-3,5-difluorobenzeneCatalog No.:AA00068Z CAS No.:1020198-58-6 MDL No.:MFCD11849939 MF:C6H2BrClF2 MW:227.4339 |
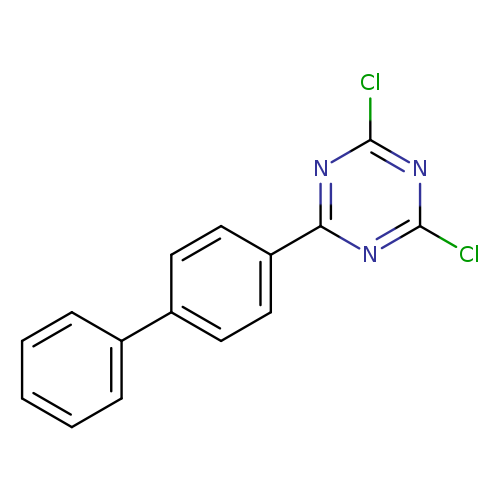
2-(4-Biphenylyl)-4,6-dichloro-1,3,5-triazineCatalog No.:AA008ZQ4 CAS No.:10202-45-6 MDL No.:MFCD28556865 MF:C15H9Cl2N3 MW:302.1581 |
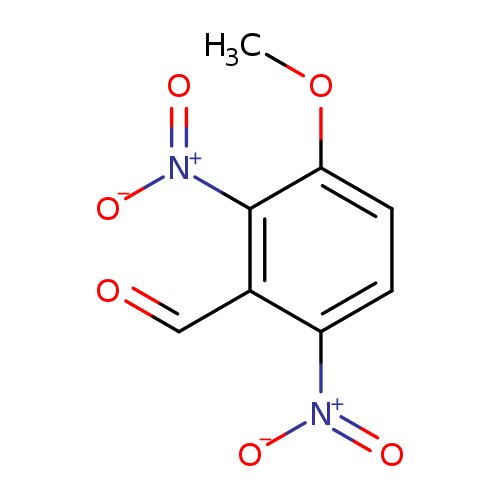
2,6-bisnitro-3-methoxybenzaldehydeCatalog No.:AA018TA3 CAS No.:10202-94-5 MDL No.:MFCD00666947 MF:C8H6N2O6 MW:226.1430 |
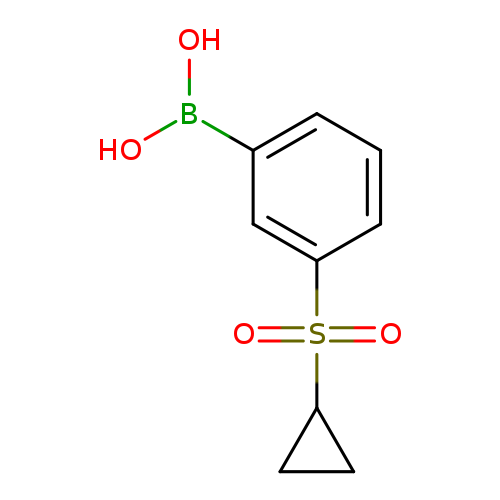
3-(Cyclopropylsulfonyl)phenylboronic acidCatalog No.:AA00068Y CAS No.:1020204-12-9 MDL No.:MFCD11617318 MF:C9H11BO4S MW:226.0572 |
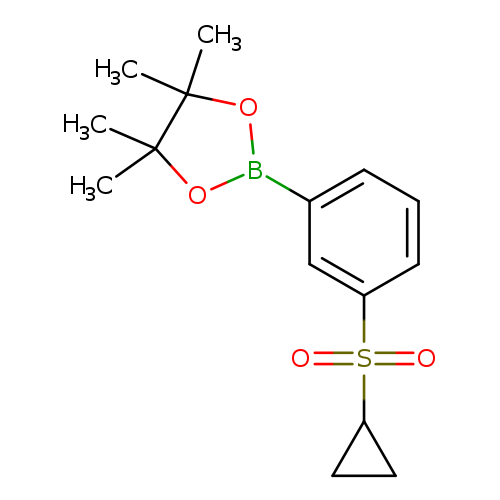
3-(Cyclopropylsulfonyl)phenylboronic acid, pinacol esterCatalog No.:AA00944W CAS No.:1020206-37-4 MDL No.:MFCD19237190 MF:C15H21BO4S MW:308.2008 |
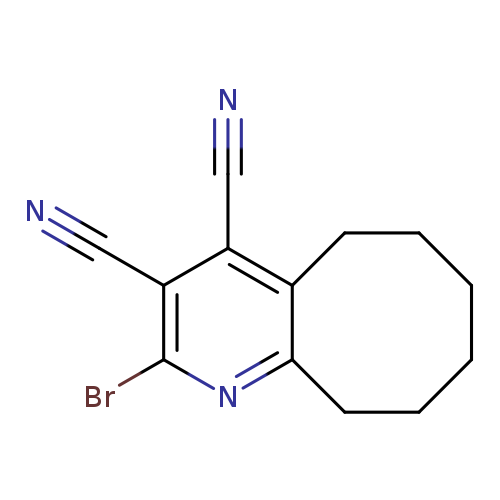
2-bromo-5H,6H,7H,8H,9H,10H-cycloocta[b]pyridine-3,4-dicarbonitrileCatalog No.:AA01BH3D CAS No.:1020244-24-9 MDL No.:MFCD12033780 MF:C13H12BrN3 MW:290.1585 |
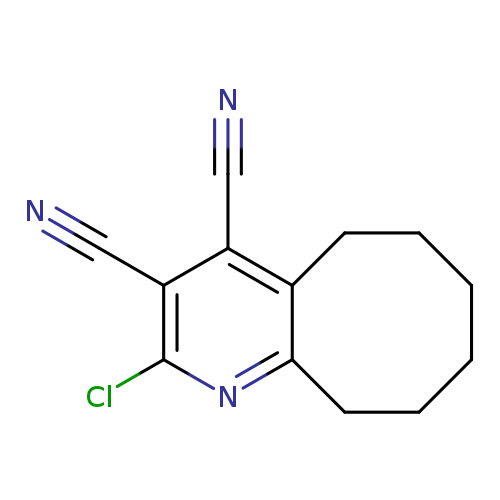
2-chloro-5H,6H,7H,8H,9H,10H-cycloocta[b]pyridine-3,4-dicarbonitrileCatalog No.:AA01BH4J CAS No.:1020244-32-9 MDL No.:MFCD12033785 MF:C13H12ClN3 MW:245.7075 |
2H-Tetrazole, 5-(4-bromo-3,5-dimethyl-1H-pyrazol-1-yl)-Catalog No.:AA0006A5 CAS No.:1020248-97-8 MDL No.:MFCD20502617 MF:C6H7BrN6 MW:243.0640 |
2-Propenoic acid, 2-(benzoylamino)-3-(phenylamino)-, methyl esterCatalog No.:AA0006A9 CAS No.:102025-82-1 MDL No.:MFCD00172672 MF:C17H16N2O3 MW:296.3205 |
Benzamide, N-[(3,4-dimethoxyphenyl)methyl]-2-[(2-hydroxy-1-methylethyl)amino]-5-nitro-Catalog No.:AA0006A4 CAS No.:1020251-53-9 MDL No.:MFCD09955575 MF:C19H23N3O6 MW:389.4024 |
3-phenyl-2-(thiophene-2-carbonyl)-2H,4H-indeno[1,2-c]pyrazol-4-oneCatalog No.:AA00IV1L CAS No.:1020251-78-8 MDL No.:MFCD00129340 MF:C21H12N2O2S MW:356.3972 |
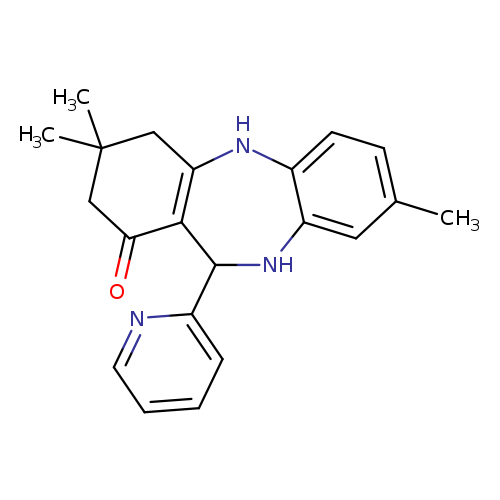
6,14,14-trimethyl-10-(pyridin-2-yl)-2,9-diazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6-tetraen-12-oneCatalog No.:AA00IX3F CAS No.:1020251-80-2 MDL No.:MFCD00170016 MF:C21H23N3O MW:333.4268 |
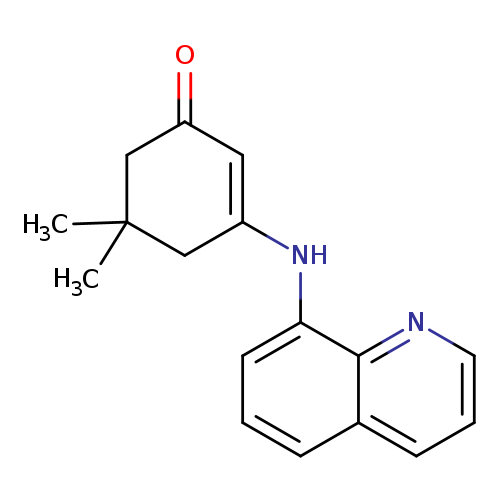
5,5-dimethyl-3-[(quinolin-8-yl)amino]cyclohex-2-en-1-oneCatalog No.:AA00IZVC CAS No.:1020251-81-3 MDL No.:MFCD00170042 MF:C17H18N2O MW:266.3376 |
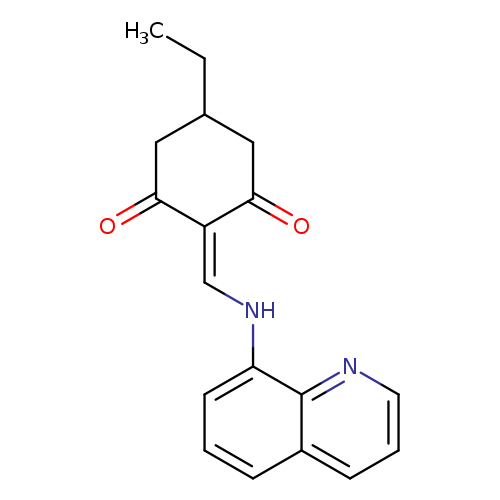
5-ethyl-2-{[(quinolin-8-yl)amino]methylidene}cyclohexane-1,3-dioneCatalog No.:AA00IV22 CAS No.:1020251-82-4 MDL No.:MFCD00170043 MF:C18H18N2O2 MW:294.3477 |
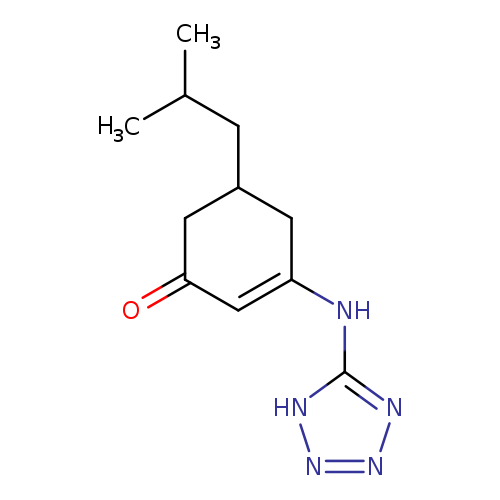
5-(2-methylpropyl)-3-[(1H-1,2,3,4-tetrazol-5-yl)amino]cyclohex-2-en-1-oneCatalog No.:AA00IV23 CAS No.:1020251-83-5 MDL No.:MFCD00129636 MF:C11H17N5O MW:235.2856 |
N-(4-bromonaphthalen-1-yl)-2-(1H-indol-3-yl)-2-oxoacetamideCatalog No.:AA00IT8J CAS No.:1020251-84-6 MDL No.:MFCD00170207 MF:C20H13BrN2O2 MW:393.2334 |
2-({[4-(naphthalen-2-yloxy)phenyl]amino}methylidene)propanedinitrileCatalog No.:AA00IT97 CAS No.:1020251-85-7 MDL No.:MFCD00170352 MF:C20H13N3O MW:311.3367 |
3-tert-butyl-1-(4-nitrophenyl)-1H,4H-indeno[1,2-c]pyrazol-4-oneCatalog No.:AA00IX4G CAS No.:1020251-86-8 MDL No.:MFCD00170363 MF:C20H17N3O3 MW:347.3673 |
8-[2-nitro-4-(trifluoromethyl)phenoxy]quinolineCatalog No.:AA00IV2M CAS No.:1020251-87-9 MDL No.:MFCD00170366 MF:C16H9F3N2O3 MW:334.2495 |
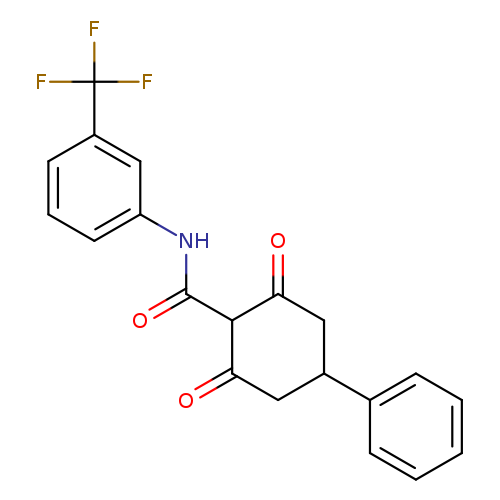
2,6-dioxo-4-phenyl-N-[3-(trifluoromethyl)phenyl]cyclohexane-1-carboxamideCatalog No.:AA00IX4H CAS No.:1020251-88-0 MDL No.:MFCD00170372 MF:C20H16F3NO3 MW:375.3411 |
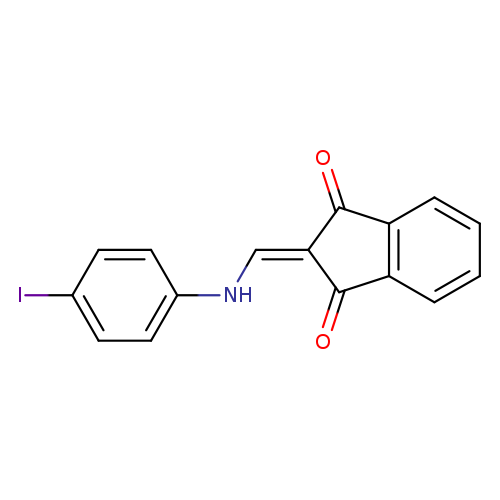
2-{[(4-iodophenyl)amino]methylidene}-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IX4I CAS No.:1020251-90-4 MDL No.:MFCD00129471 MF:C16H10INO2 MW:375.1606 |
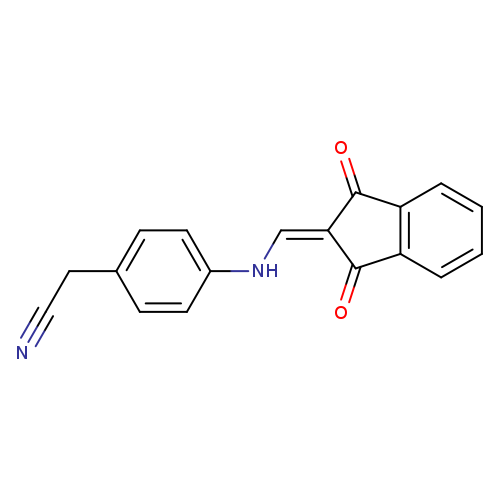
2-(4-{[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)methyl]amino}phenyl)acetonitrileCatalog No.:AA00IT98 CAS No.:1020251-91-5 MDL No.:MFCD00129459 MF:C18H12N2O2 MW:288.3001 |
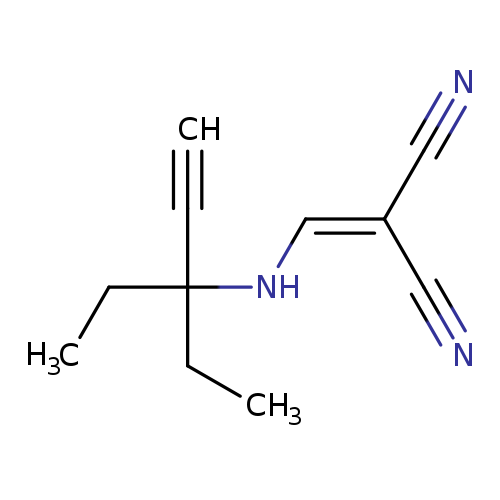
2-{[(3-ethylpent-1-yn-3-yl)amino]methylidene}propanedinitrileCatalog No.:AA00IT9A CAS No.:1020251-93-7 MDL No.:MFCD00170392 MF:C11H13N3 MW:187.2410 |
2-amino-3-{[(6-nitro-2H-1,3-benzodioxol-5-yl)methylidene]amino}but-2-enedinitrileCatalog No.:AA00IV2P CAS No.:1020251-94-8 MDL No.:MFCD00955491 MF:C12H7N5O4 MW:285.2151 |
N'-[1-(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)ethyl]benzohydrazideCatalog No.:AA00IZW9 CAS No.:1020251-95-9 MDL No.:MFCD00170413 MF:C18H14N2O3 MW:306.3154 |
2-(3,4-dimethoxyphenyl)-N'-[1-(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)ethyl]acetohydrazideCatalog No.:AA00IX4M CAS No.:1020251-96-0 MDL No.:MFCD00170414 MF:C21H20N2O5 MW:380.3939 |
5-{[(2,4-dichlorophenyl)methylidene]amino}-6-imino-2-oxo-1-phenyl-1,2,3,6-tetrahydropyrimidine-4-carbonitrileCatalog No.:AA00IX4N CAS No.:1020251-99-3 MDL No.:MFCD00170423 MF:C18H11Cl2N5O MW:384.2188 |
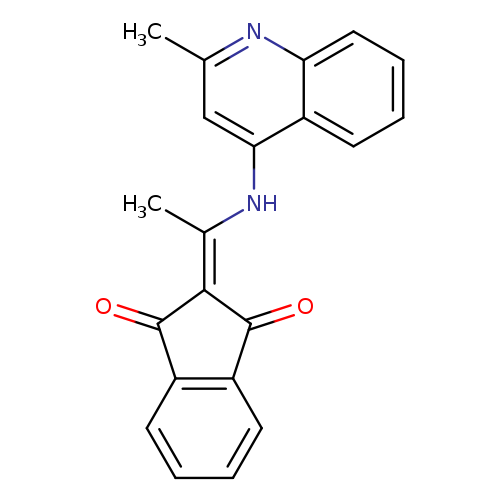
2-{1-[(2-methylquinolin-4-yl)amino]ethylidene}-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZWA CAS No.:1020252-01-0 MDL No.:MFCD00170425 MF:C21H16N2O2 MW:328.3639 |
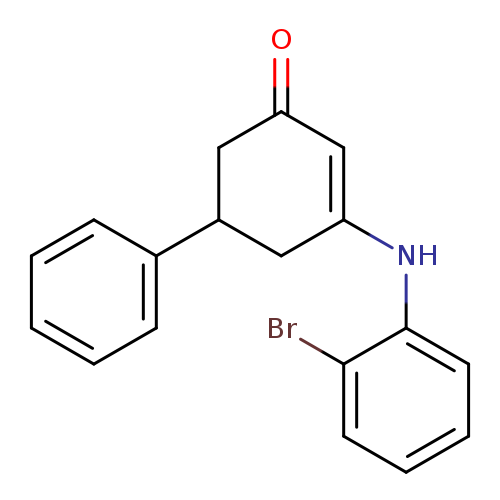
3-[(2-bromophenyl)amino]-5-phenylcyclohex-2-en-1-oneCatalog No.:AA00IT9D CAS No.:1020252-06-5 MDL No.:MFCD00170453 MF:C18H16BrNO MW:342.2297 |
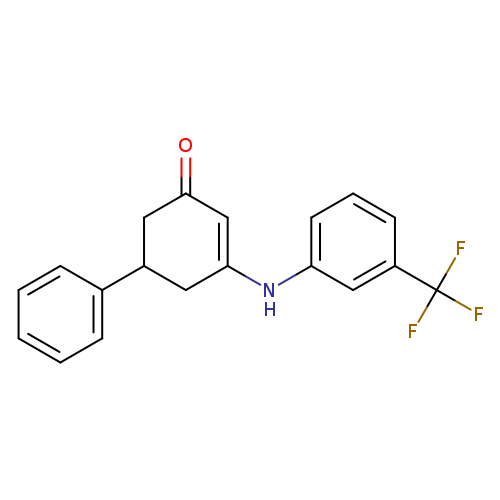
5-phenyl-3-{[3-(trifluoromethyl)phenyl]amino}cyclohex-2-en-1-oneCatalog No.:AA00IZWC CAS No.:1020252-08-7 MDL No.:MFCD00129502 MF:C19H16F3NO MW:331.3316 |
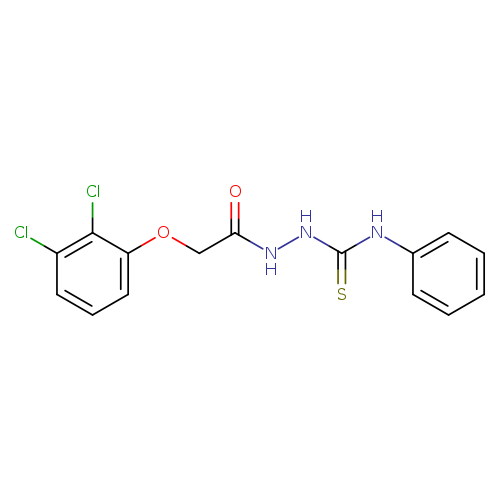
2-(2,3-dichlorophenoxy)-N-[(phenylcarbamothioyl)amino]acetamideCatalog No.:AA00IX4S CAS No.:1020252-10-1 MDL No.:MFCD00170470 MF:C15H13Cl2N3O2S MW:370.2536 |
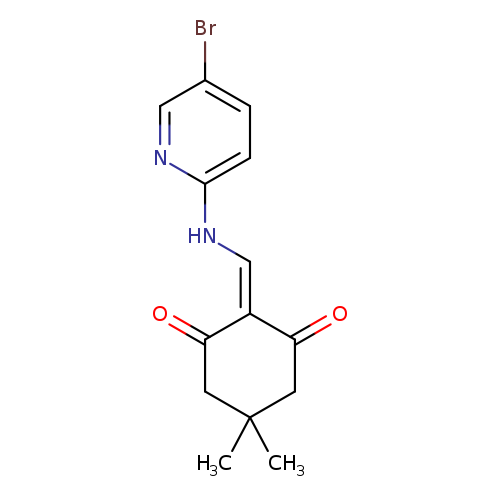
2-{[(5-bromopyridin-2-yl)amino]methylidene}-5,5-dimethylcyclohexane-1,3-dioneCatalog No.:AA00IX4T CAS No.:1020252-12-3 MDL No.:MFCD00170486 MF:C14H15BrN2O2 MW:323.1851 |
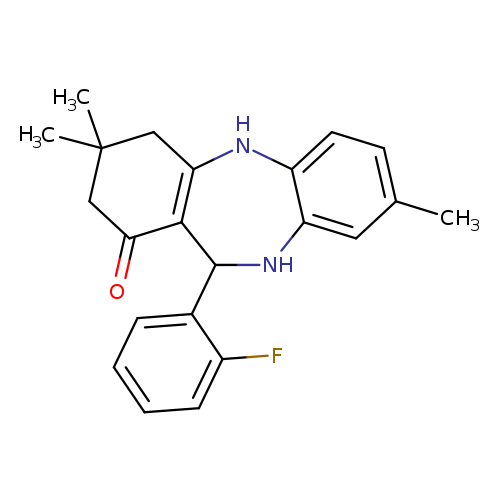
10-(2-fluorophenyl)-6,14,14-trimethyl-2,9-diazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6-tetraen-12-oneCatalog No.:AA00IT9I CAS No.:1020252-13-4 MDL No.:MFCD00170488 MF:C22H23FN2O MW:350.4292 |
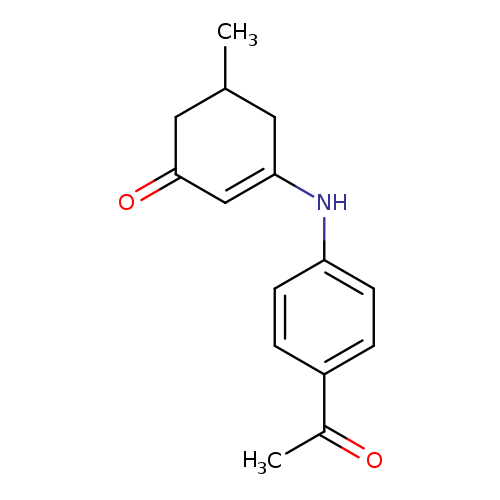
3-[(4-acetylphenyl)amino]-5-methylcyclohex-2-en-1-oneCatalog No.:AA00IV2W CAS No.:1020252-15-6 MDL No.:MFCD00129484 MF:C15H17NO2 MW:243.3010 |
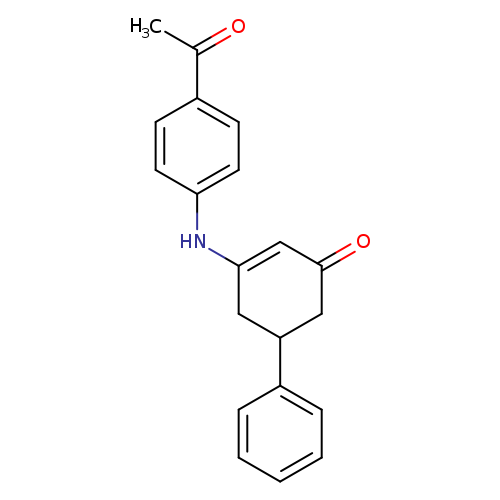
3-[(4-acetylphenyl)amino]-5-phenylcyclohex-2-en-1-oneCatalog No.:AA00IT9O CAS No.:1020252-16-7 MDL No.:MFCD00129499 MF:C20H19NO2 MW:305.3704 |
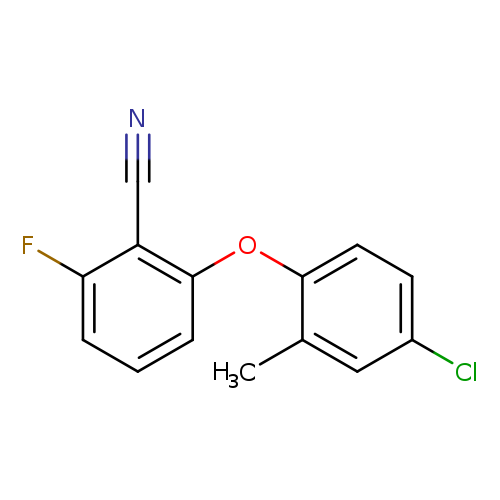
2-(4-Chloro-2-methylphenoxy)-6-fluorobenzonitrileCatalog No.:AA00IX4Y CAS No.:1020252-17-8 MDL No.:MFCD00170523 MF:C14H9ClFNO MW:261.6788 |
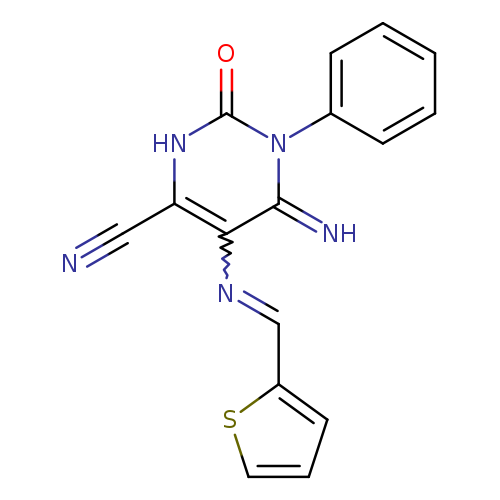
6-imino-2-oxo-1-phenyl-5-{[(thiophen-2-yl)methylidene]amino}-1,2,3,6-tetrahydropyrimidine-4-carbonitrileCatalog No.:AA00IX50 CAS No.:1020252-18-9 MDL No.:MFCD00170528 MF:C16H11N5OS MW:321.3564 |
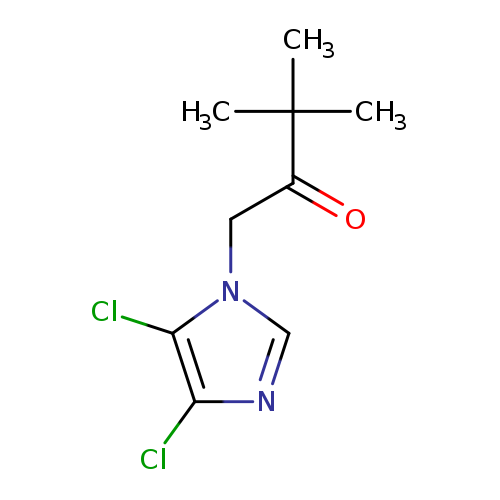
1-(4,5-dichloro-1H-imidazol-1-yl)-3,3-dimethylbutan-2-oneCatalog No.:AA00IV31 CAS No.:1020252-19-0 MDL No.:MFCD00170536 MF:C9H12Cl2N2O MW:235.1104 |