2019-11-21 08:35:33
Kamal Nayan Sharma, Munsaf Ali, Avinash Kumar Srivastava, Raj Kumar Joshi*
Department of Chemistry, Malaviya National Institute of Technology Jaipur, J.L.N. Marg, Jaipur, 302017, Rajasthan, India
1. Introduction
Amides are amongst the most important organic compounds which have been extensively explored as synthetic building blocks in various organic transformations [1e3]. The presence of amide functional group [4e9] is a key chemical connection in nitrogen containing biologically active compounds [10], various commercially available pharmaceutical drugs [11e13], and polymers [14,15], show as immense prevalence of amide bond formation in synthetic chemistry. Classically, amides are synthesized by a stoichiometric reaction of carboxylic acid or its derivatives (halides, esters or anhydrides) with amines [16]. The spontaneous formation of the amides is not possible through unifying these two functional groups at ambient temperature, because the essential water elimination step takes place at very high temperature (200 C) [17].
The formation of undesired products and low atom economy in such process restrict their employability in industrial applications. Hence, the development of advance atom-efficient catalytic methods for amide formation are highly desired in modern synthetic chemistry [18,19]. In this context, several metal-catalyzed approaches for amide synthesis have been developed [20]. Many of transition metals including scandium [21], nickel [22], copper [23e25], zinc [26], and palladium [27] based catalysts have been reported for the catalytic transformations of aldehydes [24,25,27e30] or oximes [22,23,28] into corresponding amides.
Alumina-supported rhodium [31,32], titanosilicates loaded with rhodium [33], and [Ir(Cp*)Cl2]2 [34] have been found to be potential candidates for amide synthesis. However, the prime requisite of an
inert atmosphere to handle the air-sensitive metal catalysts and harsh reaction conditions are some of the major disadvantages of these protocols. Moreover, some functional groups do not withstand under such severe ambience and the selectivity of the desire product decreases. Also, the high catalyst loading and stoichiometric amount of additional reagents produce the significant quantity of undesired products. Crabtree and co-workers has developed a ruthenium complex of terpyridine based NNN type pincer ligand which efficiently carry out an additives free one-pot conversion of amides from aldehydes [35]. The prime goal of present work is to develop an elegant and more efficient method for one-pot synthesis of amide from aldehyde which can effectively reduced the formation of hazardous wastage and use of detrimental additive reagents. The present ruthenium catalysts are economically better as compare to the reported Rh/Ir/Pd metals based catalysts for amide transformatios.
The half sandwich ruthenium(II) complex of tridentate N-heterocycles based organochalcogen ligands had shown promising catalytic activity in various catalytic reactions such as asymmetric catalysis and hydration of nitrile [36,37], transfer hydrogenation of ketones and oxidation of alcohols [38,39]. The promising catalytic potential of various metal complexes of pyrozole containing ligands has been already proven explicitly in various earlier reports [40e48]. The strong donor properties of chalcogen ligands make them suitable candidates for catalysis of organic transformations [49e56]. Apart from efficiency, organochalcogen based catalytic systems are quite attractive due to their insensitivity towards the air and moisture, good solubility in various organic solvents and high stability in organic solutions. The catalytic strength of metal complexes of organochalcogen ligands for this particular transformation has not been investigated so far. Also, to the best in our knowledge, we are the first to report the ruthenium complexes of pyrazole-based organochalcogen ligands.
Therefore, moving towards the ligand chemistry and application potential of N-heterocycles containing chalcogenated ligands, we have synthesized three new and novel mononuclear Ru(II) halfsandwich complexes of pyrazole based organochalcogen ligands and investigated their catalytic potential for aldehyde to amide transformations. The comparative study of the catalytic efficiency of these three complexes has been also investigated. Moreover, the present catalytic systems do not require any hazardous additives, and convert the aldehydes into corresponding amides in good to excellent amounts under aerobic reaction conditions.
2. Results and discussion
The systematic methodology adopted for the synthesis of Ru(II) complexes (1e3) is illustrated in Scheme 1. The previously reported methods [46,57] were used to prepare the pyrazole based thio/ seleno/telluro-ether functionalized bidentate ligands (L1-L3). Three new half-sandwich (h6-benzene)ruthenium(II) complexes (1e3) were synthesized by reacting [(h6-C6H6)RuCl(m-Cl)]2 with a , methanolic solution of L1/L2/L3 under ambient reaction conditions. All the complexes were characterized by using the NMR, MS, and FT-IR techniques. The NMR and mass spectra of complexes 1e3 have been provided in Supplementary data (Figs. S1eS9) which were found to be consistent with their molecular structures illustrated in Scheme 1.
Due to the low solubility of 1e3 in CDCl3, their NMR spectra were recorded in CD3CN. As compared to the corresponding free ligands [46,57], the deshielded NMR signals appeared in 1 H and 13C { 1
H} spectra of 1e3 at 0.9 ppm and 8.6 ppm, respectively, corroborating the coordination of ligand with Ru(II) in a bidentate chelate mode. The four methylene protons (each H5 and H6 group) of eNeCH2eCH2eE part of chelate ring were recorded as four multiplets in 1 H NMR spectra of complexe 1e3, which confirmed their diastereotopic nature which arised due to the rigid conformation of the coordinated ligand and inherent chirality associated with the asymmetric molecular structure of each of the three Ru complexes. Moreover, in 13C{1 H} NMR spectra, signals of C9, C6 and C5 were found more deshielded relative to those of other carbon atoms of the complex. The protons attached to these carbons also appeared somewhat more deshielded than other protons present in the complexes. The high magnitude of shift for these carbon atoms and proton is probably due to their closeness to the chalcogen donor atoms (N and S/Se/Te). Additionally, 1 H and 13C{1 H} NMR of each of the complexes 1e3 show a typical resonance (most intense signal) for six protons and carbons of h6-benzene in the range of 5.59e5.90 ppm and 86.2e87.3 ppm, respectively which is in close agreement with the earlier reported half-sandwich ruthenium complex of h6-benzene [58]. The intense mass peaks at (m/z) 496.9025, 544.8474 and 624.8472 appeared in mass spectra of 1, 2 and 3, respectively, are attributable to [MPF6] þ cations in each of the three complexes.
2.1. Crystal structures
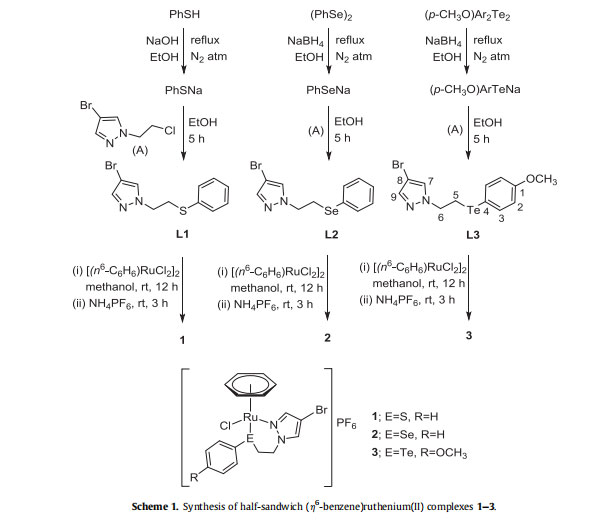
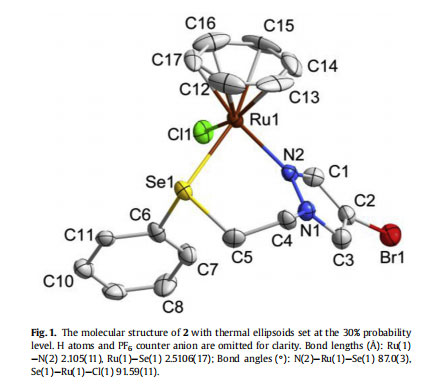
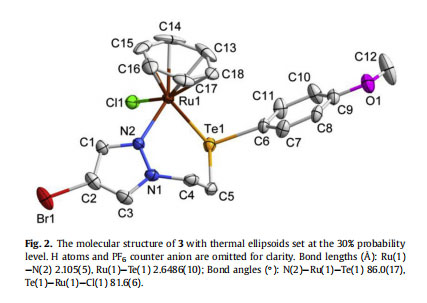
The solubility of complexes 1e3 was found to be good in acetonitrile, DMF and DMSO, while close to negligible in dichloromethane, chloroform and methanol, and completely insoluble in diethyl ether and n-hexane. The suitable quality single crystals of complexes 2 and 3 were grown by slow evaporation of their saturated solution in acetonitrile/methanol (1:1) and subjected to analysis through single crystal X-ray crystallography. The thermal ellipsoid diagrams of 2 and 3 are depicted in Figs. 1 and 2 with some selected bond lengths and bond angles. Additional parameters are provided in Table 1. The bidentate coordination of ligand through N of pyrazole ring and Se/Te with Ru results in the formation of a six membered chelate ring in each case. In the cation of each complex, Ru adopts a pseudo-octahedral half-sandwich “pianostool” geometry. The observed bond lengths for RueSe and RueTe bonds in 2 and 3, are 2.511 Å and 2.648 Å, respectively, which fall in the range of previously reported ruthenium complexes [38,59].
2.2. Evaluation of the catalytic potential of Ru(II) complexes (1e3) for amide synthesis
Previously, the half-sandwich Ru(II) complexes have been found to be promising candidates for the catalysis of various organic transformations including conversion of aldehyde to amides which were traditionally achieved by the reaction of carboxylic acid or its derivatives (anhydrides, esters or halides) with amines at high temperature [16,17]. In this context, metal-based homogeneous [20e25] as well as heterogeneous [31e33] catalysts have been developed. Most of the methods suffer from several disadvantages like requirement of inert atmosphere, high catalyst loading, a stoichiometric amount of additional reagents, harsh reaction conditions which are detrimental to the integrity of substrate. Therefore, for the employment of the strong s-donor properties of soft chalcogen donor sites of N-heterocycles based organochalcogen ligands, insensitivity of their metal complexes towards the air and moisture, the catalytic strength of present Ru(II) complexes (1e3) was explored for catalytic conversion of aryl aldehydes to corresponding amides (Scheme 2).
All three complexes were found to be highly efficient at the 0.1 mol% loading of catalyst at 100 C temperature under the aerobic conditions. Moreover, the present catalytic reaction does not demand any hazardous additives and produce the amides in good to excellent yields without generating any by-products. For the optimizations of reaction parameters, initially, the benzaldehyde was chosen as a model substrate and a series of the reactions using the ruthenium complexes 1, 2 and 3 as catalyst were performed (Table 2).
The maximum conversion of aldehyde to amides was obtained with the use of 0.1 mol% amount of the catalyst, while below to 0.1 mol%, the yield of the desired product was significantly reduced.
Moreover, the continuous increase in mol% of the catalyst (0.1e0.5) does not help in further improvement of yield of the desire product. Furthermore, during the solvent optimization, the highest yield of
product was obtained in toluene (Table 2, Entry 1). The desired product was also produced when solvents including the THF, acetonitrile and 1,4-dioxane were used (Table 2, Entry 2e4), but yield of the desire product was substantially reduced. Water and DMF were also checked as solvent but the reaction was not initiated and failed to produce the desire product (Table 2, Entry 4e5). The effect of various bases was also investigated, the highest yield (95%) of benzamide was obtained with NaOH (Table 2, Entry 1). Other bases including the NaHCO3, KOH, K2CO3 and Cs2CO3 afforded relatively low yield of desired product 80%, 40%, 50% and 10%, respectively (Table 2, Entries 7e10). It was also noticed that the reaction did not produce desired product in the absence of base (Table 2, Entry 11). Hence, careful selection of the base is a prime requisite for the present transformation reaction. Furthermore, the highest yield of the amide was obtained at 95e100 C temperature.
The drastic changes in the yield were observed when temperature was reduced to 80 C (Yield 17%, Table 2, entry 13), and further lowering the temperature of the reaction failed to bring the desired
transformations (Table 2, entry 14).
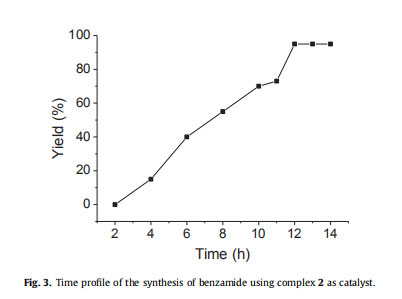
Fig. 3 shown at below, represents the time profile of catalytic reaction of model sustrate producing benzamide under optimized rection conditions. The initial formation of the desired compound
was detected after 4 h in the reaction. The continuous increase in the yield was observed with time and after 12 h the yield of the reaction was found to be constant (95%). In order to check the wide scope of the reaction, various functionally different aldehydes were used under the optimized reaction conditions and reaction was completely generalized (Table 3). One pot methodology for the synthesis of the primary amides from various aldehydes was developed by using the Ru(II) complexes (1e3). It seems that the substituent present on the aromatic ring of benzaldehyde do not affect the formation of product. However, some minor differences in the product's yields were observed.


The catalytic activity of 1, 2 and 3 was found to be in order of 2 (Se) > 1 (S) > 3 (Te). Such variations are arised due to the difference in the s-donor properties of S/Se/Te as the other donor sites (N of pyrazole moiety of L1-L3) are common in all the three complexes. A similar trend of catalytic activity for Pd complexes of S/Se/Te donor analogues ligands towards the CeC coupling reaction has also been
reported by Singh and co-workers [60]. An another Pd(II) complex with Te ligand [61] has also been reported which had shown much lower catalytic efficiency than the S/Se analogues [62,63]. The high
catalytic activity of the selenium based ligand over the sulphur analogue for transfer hydrogenation has also been established through the experimental as well as the theortical calculations by Singh and co-workers [64]. The catalytic studies on the Se based systems [63,65,66] have been indicated the excellent reactivity of selenium towards catalytic reactions [63] and sometimes, Se based systems had shown their outperformance to the phosphine based analogue [67]. The lower catalytic activity of present Te containg Ru complex is probably due to the steric effect of large size of Te which causes a lesser electronic charge density accumulation over central metal atom [60].

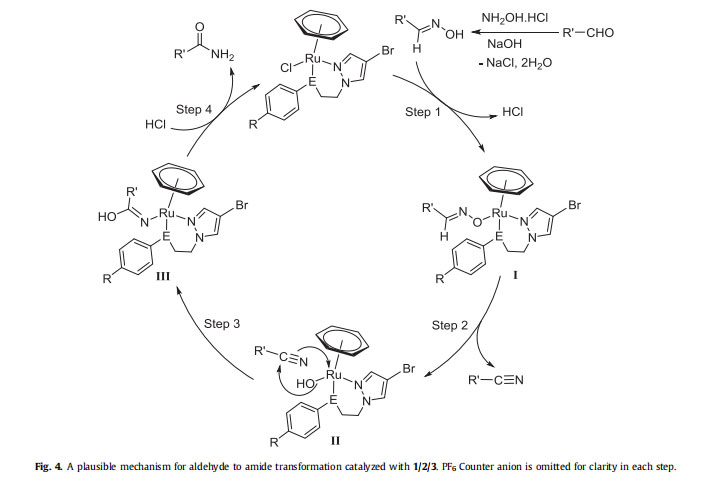
Based on the available literature evidences for such metal catalyzed reactions [31,68,69], the proposed plausible mechanistic pathway for the present ruthenium catalyzed transformation of aldehyde to amide is depicted in Fig. 4. Here, it is assumed that, the OH group of aldoxime which is generated in situ from the reaction of aldehyde and hydroxyl amine hydrochloride in the presence of base [35], gets coordinated to the ruthenium(II) catalyst (1/2/3) to form (h6-C6H6)Ru(L)(OeN¼CHR0) species I together with the elimination of HCl (step 1). In the next step, species I eliminates the
nitrile and resulted the intermediate (II) [(h6-C6H6)Ru(L)(OH)] [70].
The simultaneous nucleophilic attack of nitrile over the coordinated hydroxide resulted a ruthenium iminolate species (III) in step 3 [31,68]. Under the similar reaction conditions, the formation of benzamide was also noted when benzonitrile was directly used as a substrate with Ru catalyst (2). This experiment further strengthen and confirmed the reaction proceed through a nitrile intermediate. Finally, in the step 4, the hydrolysis of the ruthenium iminolate species (III) leads to regeneration of the catalyst with concomitant formation of the final product.
2.3. Comparison of catalytic efficiency of present Ru catalysts (1e3) with previously reported catalytic systems for aldehyde to amide transformations The catalytic transformation of aldehydes to amides with the present Ru(II) complexes 1/2/3 is quite efficient in terms of catalyst loading, reaction time, reaction temperature and use of additives in comparison to reported metal-based catalysts for amide synthesis
[22,34,70,71]. The first metal catalyzed transformation of aldoximes to amides was carried out in xylene at high temperature (138 C) with high catalyst loading of nickel acetate (5.6 mol%).
Moreover, the formation of by-products was also observed during the catalytic process [22]. The iridium catalyst [Ir(Cp*)Cl2]2 [34] has also been used for the amide synthesis with a higher catalyst loading (2.5 mol %). Williams and co-workers [70] have also reported a Ru catalyst that catalyzed the rearrangement of aldoximes to amides, but p-toluenesulfonic acid as an additive was used, and a considerable amount of nitrile was also obtained with amides. Crabtree and co-workers reported the ruthenium catalyst based on NNN pincer ligand [terpyRu(PPh3)Cl2], which was found to be effective in the catalysis of aldehyde to amide transformation in 17 h. The maximum yields were observed when an additive NaHCO3 was used with 1.0 mol% of catalyst loading. Moreover, a h6-areneruthenium(II) complex [RuCl2(h6
-C6Me6){P(NMe2)3}] has also been established [71] as an effective catalyst for aldoxime to primary amides in water at 100 C with high catalyst loading (5 mol%). The present ruthenium half-sandwich complexes (1e3) show the 95% amide formation in 12 h with 0.1 mol% of Ru catalyst under the aerobic reaction conditions, therefore, can be considered highly effective in the catalysis of aldehyde to amide transformation.
3. Conclusions
Three new Ru(II) half-sandwich complexes have been synthesized in high yield by reacting pyrazole-based chalcogenoethers with [(h6-C6H6)RuCl(m-Cl)]2 in methanol under ambient reaction conditions and authenticated with 1 H, 13C{1 H} NMR, mass and FT-IR analytical techniques. Single crystal X-ray diffraction analysis of 2 and 3 revealed pseudo-octahedral half sandwich piano-stool geometry at Ru metal centre in both the complexes. The complexes 1e3 have been found efficient, thermally robust and moisture/air insensitive catalysts for the transformation of aldehyde to primary amide in high yield (95%). Complex 2, consisting the selenium ligand has been found more efficient than their sulphur and tellurium analogues.
4. Experimental section
4.1. Physical measurement
The NMR spectra were recorded on a JEOL ECS-400 spectrometer operating at 400 MHz and 101 MHz for 1 H and 13C nuclei, respectively. FT-IR spectra were recorded on a Perkin-Elmer 10.4.00 FT-IR spectrometer within the range 4000e400 cm1 using KBr pellets of the sample. High-Resolution Electron Impact Mass Spectra (HR-EIMS) were obtained with Xevo G2-S Q-Tof (Waters, USA). The diffraction data on a single crystal of 2 and 3 were collected on a Bruker AXS SMART Apex CCD Diffractometer using Mo-Ka (0.71073 Å) radiation at 298(2) K. The software SADABS [72] was used for absorption correction (if needed) and SHELXTL for space group, structure determination, and refinements. All nonhydrogen atoms were refined anisotropically. Hydrogen atoms were included in idealized position with isotropic thermal parameters set at 1.2 times that of the carbon atom to which they are attached. The least-square refinement cycle on F2 was performed until the model converged. The melting point of solid compounds were determined in an open capillary and reported as such. The yields given are referred to isolated yields of compounds which have purity 95%.
4.2. Chemicals and reagents
4-Bromopyrazole, phenyl diselenide, thiophenol, sodium borohydride, ruthenium chloride, ammonium hexafluorophosphate were procured from Sigma-Aldrich (USA), and used as received. Bis(4-methoxyphenyl) ditelluride, L1, L2 and L3 were prepared by previously reported methods [46,47]. Prior to their use, all the solvents were dried and distilled by standard procedures [73]. The common chemicals and reagents which are available commercially within the country were used as received. AA Blocks offers a comprehensive range of building blocks and specially designed scaffolds to support your R&D. Located in San Diego, striving to provide efficient and cost effective solutions to scientists, AA blocks is a research chemical supplier the same as Sigma-Aldrich of speciality building blocks and intermediates to the pharmaceutical, biotechnology and agrochemical industry.
4.3. Synthesis of complexes [(h6-C6H6)Ru(L1/L2/L3)Cl].PF6 (1e3)
Brick red solid [Ru(h6-C6H6)Cl2]2 (0.050 g, 0.1 mmol) was added to a solution of L1 (0.057 g, 0.2 mmol)/L2 (0.066 g, 0.2 mmol)/L3 (0.082 g, 0.2 mmol) made in 25 mL of methanol, and the reaction mixture was stirred for 12 h at ambient temperature. The resulting reaction mixture was filtered, and the volume of the filtrate was reduced to 5 mL at rotary evaporator. It was mixed with solid NH4PF6 (0.032 g, 0.2 mmol) and further stirred at rt for 3h. The resulting precipitated solid was filtered, washed with 5 mL of icecold methanol, and dried in vacuo. Single crystals of complexes 2 and 3 were obtained by slow evaporation of solution made in methanol.
1. Yellow solid, Yield: 0.096 g, 75%. mp: 195 C. 1 H NMR (400 MHz, CD3CN) d (ppm): 8.08 (s, 1H, H9), 7.89 (s, 1H, H7), 7.63e7.46 (m, 5H, H1-3), 5.92 (s, 6H, h6-C6H6), 4.83e4.78 (m, 1H, H6), 4.46e4.35 (m, 1H, H6), 3.45e3.42 (m, 1H, H5), 2.98e2.92 (m, 1H, H5). 13C{1 H} NMR (101 MHz, CD3CN) d (ppm): 148.5 (C9), 136.7 (C7), 130.8 (C3), 130.6 (C2), 129.7 (C1), 125.9 (C4), 93.7 (C8), 86.2 (h6-C6H6), 49.9 (C6), 34.5 (C5). HR-MS (CH3CN) [MPF6] þ (m/z) Found: 496.9025; Calc. value for [C17H17BrClN2RuS]þ: 496.9028. FT-IR (KBr, nmax/cm1): 3093 (m, nCeH aromatic), 2926 (m, nCeH aliphatic), 1579 (m, nC]N aromatic), 1440 (s, nC]C aromatic), 1303 (m, nC‒N aliphatic), 823 (s, nCeH aromatic, bending).
2. Yellow solid, Yield: 0.099 g, 72%. mp: 190 C. 1 H NMR (400 MHz, CD3CN) d (ppm): 8.08 (s, 1H, H9), 7.90 (s, 1H, H7), 7.79e7.76 (m, 2H, H3), 7.62e7.60 (m, 3H, H1 and H2), 5.59 (s, 6H, h6-C6H6), 5.11e5.05 (m, 1H, H6), 4.57e4.51 (m, 1H, H6), 3.34e3.29 (m, 1H, H5), 3.02e2.95 (m, 1H, H5). 13C{1 H} NMR (101 MHz, CD3CN) d (ppm): 148.3 (C9), 136.8 (C7), 132.2 (C3), 130.8 (C2), 129.1 (C1), 124.8 (C4), 93.8 (C8), 87.3 (h6-C6H6), 52.0 (C6), 29.0 (C5). HR-MS (CH3CN) [MPF6] þ (m/z) Found: 544.8474; Calc. value for [C17H17BrClN2RuSe]þ: 544.8472. FT-IR (KBr, nmax/cm1): 3098 (m,
nCeH aromatic), 2958 (m, nCeH aliphatic), 1574 (m, nC]N aromatic), 1437 (s, nC]C aromatic), 1299 (m, nC‒N aliphatic), 834 (s, nCeH aromatic, bending).
3. Yellow solid, Yield: 0.114 g, 74%. mp: 193 C. 1 H NMR (400 MHz, CD3CN) d (ppm): 8.10 (s, 1H, H9), 7.89 (s, 1H, H7), 7.72 (d, 3J HH ¼ 6.6 Hz, 2H, H3), 7.12 (d, 3 JHH ¼ 6.7 Hz, 2H, H2), 5.60 (s, 6H h6
-C6H6), 5.33e5.29 (m, 1H, H6), 4.53e4.46 (m, 1H, H6), 3.85 (s, 3H, OCH3), 3.03e2.99 (m, 1H, H5), 2.92e2.85 (m, 1H, H5). 13C{1 H} NMR (101 MHz, CD3CN) d (ppm): 161.9 (C4), 148.2 (C9), 136.0 (C3), 128.4
(C7), 116.5 (C2), 103.1 (C1), 93.5 (C8), 87.3 (h6-C6H6), 55.4 (OCH3), 53.2 (C6), 13.0 (C5). HR-MS (CH3CN) [MPF6] þ (m/z) Found: 624.8472; Calc. value for [C18H19BrClN2ORuTe]þ: 624.8475. FT-IR (KBr, nmax/cm1): 3087 (m, nCeH aromatic), 2958 (m, nCeH aliphatic), 1580 (m, nC]N aromatic), 1491 (s, nC]C aromatic), 1297 (m, nC‒N aliphatic), 822 (s, nCeH aromatic (bending)).
4.4. Procedure for the catalytic reaction
In an oven-dried 100 mL two-neck round bottom flask, a mixture of aryl-aldehyde (1.0 mmol), NH2OH.HCl (1.0 mmol), NaOH (1.0 mmol), catalyst (0.1 mol%) and solvent (5 ml) were heated at 100 C with continuous stirring for 12 h in air. The progress of the reaction was continuously monitored by TLC until the maximum conversion of an aldehyde to the desired product observed. After completion, the reaction mixture was cooled to room temperature and extracted in ethyl acetate (2 25 mL). This extract was further washed with water and dried over anhydrous Na2SO4. The product was purified by column chromatography after removing the solvent on a rotary evaporator under reduced pressure. All the desired product obtained as white solid was authenticated by HR-MS, 1 H, and 13C{1
H} NMR spectroscopy. 1 H and 13C NMR of aldehyde to amides conversion products (Table 3, Entries 1e12).


Acknowledgements
K.N.S. thanks Science and Engineering Research Board, New Delhi for start-up research grant (YSS/2015/000698). M.A. and A.K.S. thank MANF-UGC, New Delhi and MNIT Jaipur, respectively for providing fellowships. R.K.J. thanks the DST New Delhi, for providing financial assistance under the INT/RUS/RFBR/P0222 scheme. Authors acknowledge MRC, MNIT Jaipur for providing characterization facilities. IIT Delhi is acknowledged for single crystal X-ray analysis.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jorganchem.2018.09.019.
N-(CyclopropylMethyl)-4-fluoro-benzylaMineCatalog No.:AA0098Y2 CAS No.:1019538-79-4 MDL No.:MFCD11140258 MF:C11H14FN MW:179.2340 |
(cyclopropylmethyl)[(2,4-dimethoxyphenyl)methyl]amineCatalog No.:AA01B8RA CAS No.:1019538-81-8 MDL No.:MFCD11140262 MF:C13H19NO2 MW:221.2955 |
(cyclopropylmethyl)({[2-(trifluoromethyl)phenyl]methyl})amineCatalog No.:AA01BA9J CAS No.:1019538-83-0 MDL No.:MFCD11140266 MF:C12H14F3N MW:229.2415 |
2-(ethylsulfanyl)-3-methylbutanoic acidCatalog No.:AA01A9XM CAS No.:1019538-92-1 MDL No.:MFCD11136006 MF:C7H14O2S MW:162.2499 |
2-(tert-butylsulfanyl)pyridine-4-carboxylic acidCatalog No.:AA01A50M CAS No.:1019539-10-6 MDL No.:MFCD11136038 MF:C10H13NO2S MW:211.2807 |
2-(tert-butylsulfanyl)-5-nitrobenzoic acidCatalog No.:AA01A4WA CAS No.:1019539-13-9 MDL No.:MFCD11136042 MF:C11H13NO4S MW:255.2902 |
4-(butan-2-ylsulfanyl)-2-(trifluoromethyl)anilineCatalog No.:AA01A8D0 CAS No.:1019539-28-6 MDL No.:MFCD11136074 MF:C11H14F3NS MW:249.2958 |
[2-(pyridin-2-yl)ethyl](pyridin-3-ylmethyl)amineCatalog No.:AA01BHKB CAS No.:1019542-07-4 MDL No.:MFCD11143801 MF:C13H15N3 MW:213.2783 |
N-(3,4-Dimethoxyphenyl)-4-fluorobenzylamineCatalog No.:AA00901N CAS No.:1019542-69-8 MDL No.:MFCD11924932 MF:C15H16FNO2 MW:261.2914 |
1-Ethyl-n-(2-methoxyphenyl)piperidin-4-amineCatalog No.:AA01AA9E CAS No.:1019543-38-4 MDL No.:MFCD11141277 MF:C14H22N2O MW:234.3373 |
3-[(propan-2-yl)amino]benzonitrileCatalog No.:AA01A8KP CAS No.:1019543-98-6 MDL No.:MFCD11144762 MF:C10H12N2 MW:160.2157 |

5-{[(4-bromophenyl)amino]methyl}-2-methoxyphenolCatalog No.:AA009M55 CAS No.:1019545-38-0 MDL No.:MFCD11144878 MF: MW: |
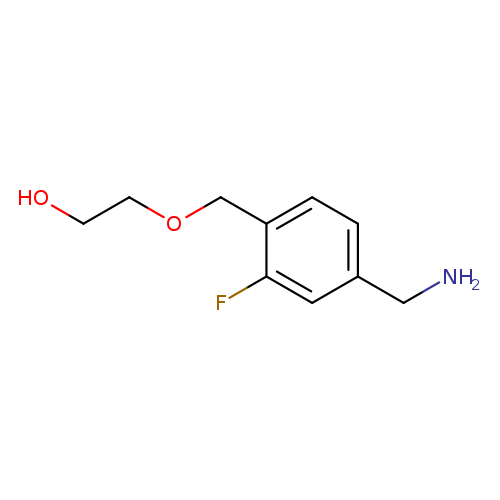
2-{[4-(aminomethyl)-2-fluorophenyl]methoxy}ethan-1-olCatalog No.:AA01AGX3 CAS No.:1019546-26-9 MDL No.:MFCD13808635 MF:C10H14FNO2 MW:199.2221 |
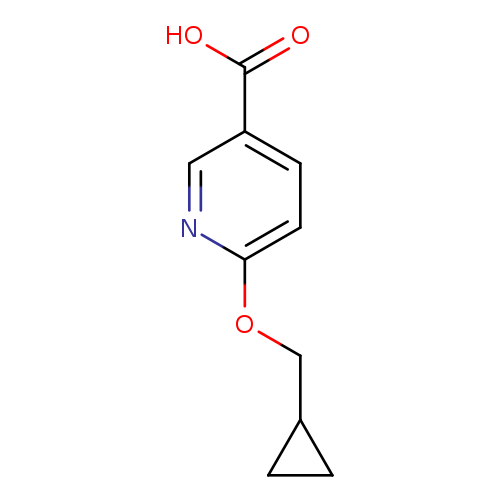
6-(Cyclopropylmethoxy)pyridine-3-carboxylic acidCatalog No.:AA0005ZM CAS No.:1019546-29-2 MDL No.:MFCD11136670 MF:C10H11NO3 MW:193.1992 |
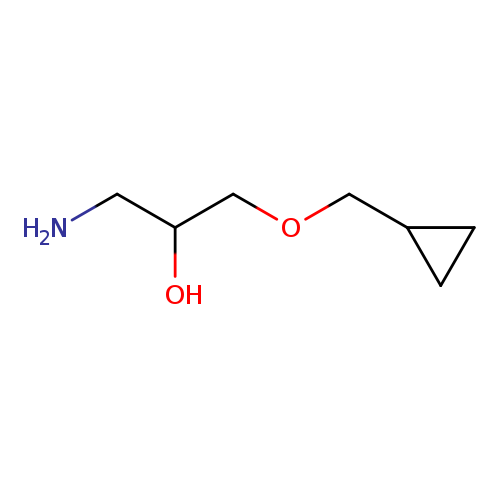
1-amino-3-(cyclopropylmethoxy)propan-2-olCatalog No.:AA01A8AP CAS No.:1019546-35-0 MDL No.:MFCD11136682 MF:C7H15NO2 MW:145.1995 |
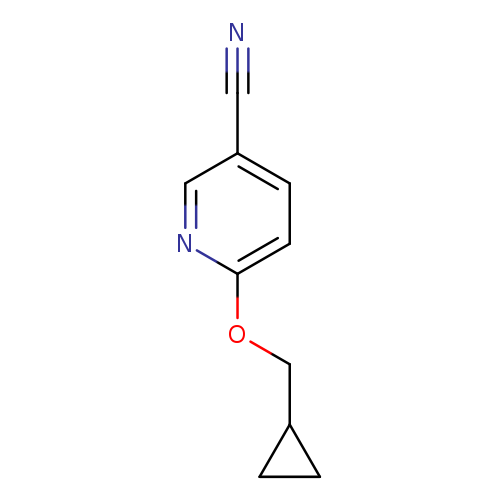
6-(cyclopropylmethoxy)pyridine-3-carbonitrileCatalog No.:AA01A7XI CAS No.:1019546-41-8 MDL No.:MFCD11136692 MF:C10H10N2O MW:174.1992 |
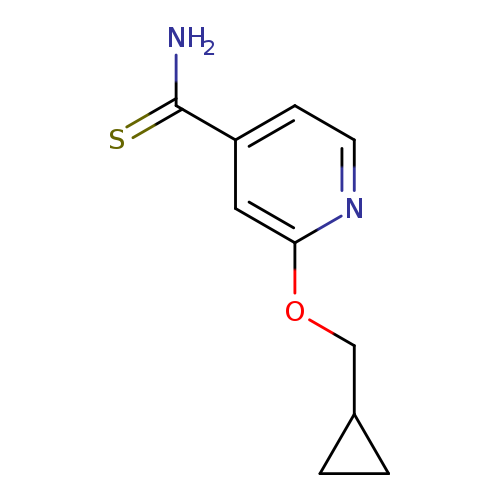
2-(cyclopropylmethoxy)pyridine-4-carbothioamideCatalog No.:AA00IMMH CAS No.:1019546-78-1 MDL No.:MFCD11136716 MF:C10H12N2OS MW:208.2801 |
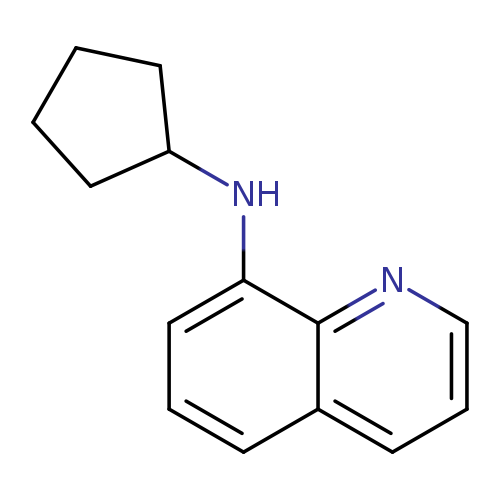
N-Cyclopentylquinolin-8-amineCatalog No.:AA01AAN9 CAS No.:1019549-62-2 MDL No.:MFCD11141625 MF:C14H16N2 MW:212.2902 |
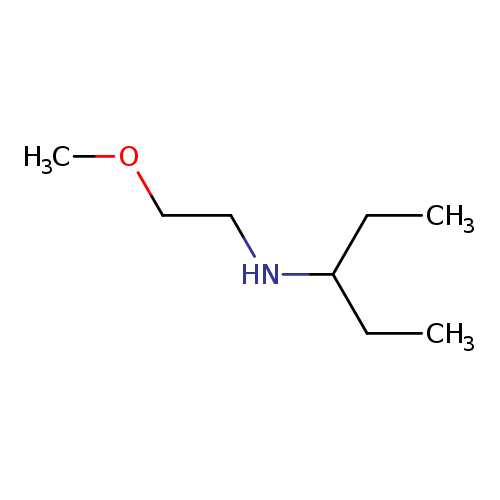
(2-Methoxyethyl)(pentan-3-yl)amineCatalog No.:AA019N1M CAS No.:1019551-00-8 MDL No.:MFCD11505424 MF:C8H19NO MW:145.2426 |
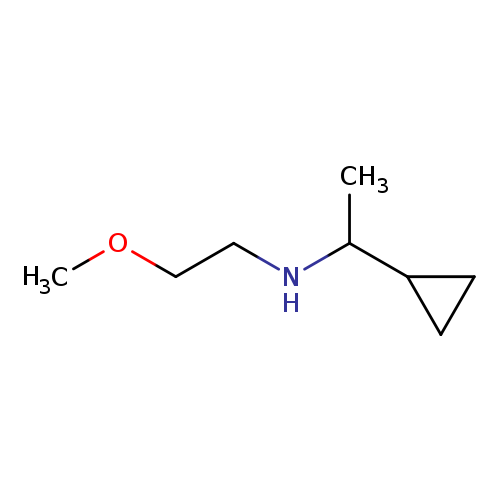
(1-cyclopropylethyl)(2-methoxyethyl)amineCatalog No.:AA019UQY CAS No.:1019551-02-0 MDL No.:MFCD11139373 MF:C8H17NO MW:143.2267 |
1-[3-Fluoro-4-(4-methylpiperazin-1-yl)phenyl]ethan-1-amineCatalog No.:AA019WKJ CAS No.:1019552-56-7 MDL No.:MFCD11137053 MF:C13H20FN3 MW:237.3164 |
1-[2-(4-Ethylpiperazin-1-yl)-5-fluorophenyl]ethan-1-amineCatalog No.:AA01A8AV CAS No.:1019553-69-5 MDL No.:MFCD11137149 MF:C14H22FN3 MW:251.3430 |
3-([(Cyclopropylmethyl)amino]methyl)phenolCatalog No.:AA01A865 CAS No.:1019553-83-3 MDL No.:MFCD11140433 MF:C11H15NO MW:177.2429 |
Methyl 2-[(1-phenylpropyl)amino]acetateCatalog No.:AA01A15W CAS No.:1019554-60-9 MDL No.:MFCD11140549 MF:C12H17NO2 MW:207.2689 |
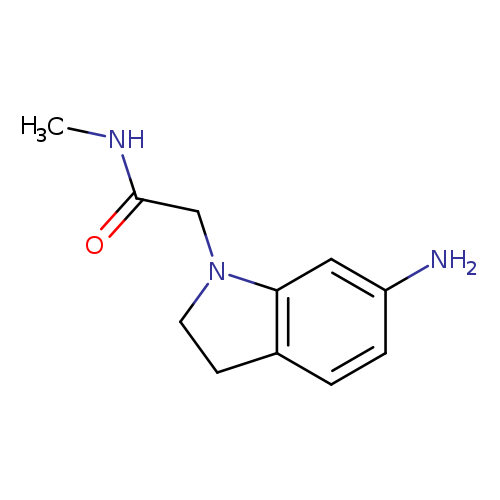
2-(6-amino-2,3-dihydro-1H-indol-1-yl)-N-methylacetamideCatalog No.:AA01AHBW CAS No.:1019555-45-3 MDL No.:MFCD11137672 MF:C11H15N3O MW:205.2563 |
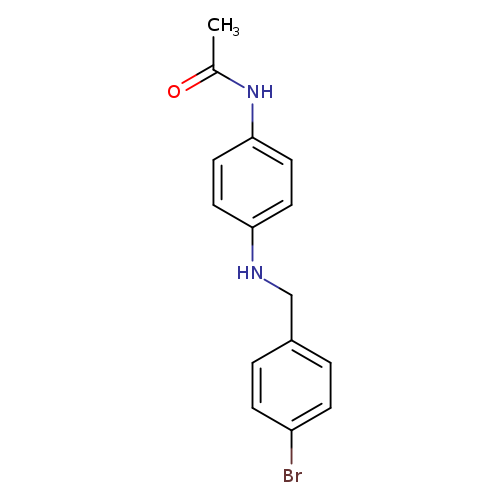
N-(4-{[(4-bromophenyl)methyl]amino}phenyl)acetamideCatalog No.:AA019UYQ CAS No.:1019555-56-6 MDL No.:MFCD11858083 MF:C15H15BrN2O MW:319.1964 |
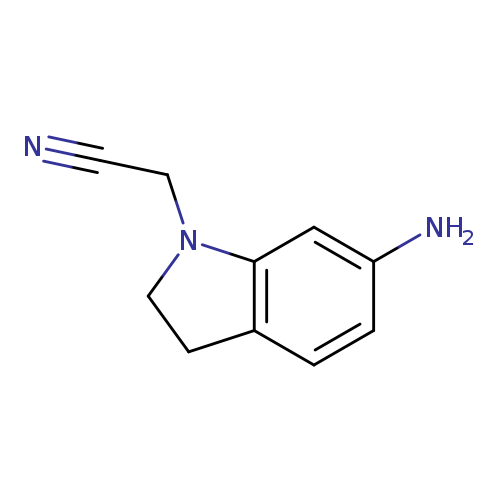
2-(6-amino-2,3-dihydro-1H-indol-1-yl)acetonitrileCatalog No.:AA01A87Z CAS No.:1019555-70-4 MDL No.:MFCD11137687 MF:C10H11N3 MW:173.2144 |
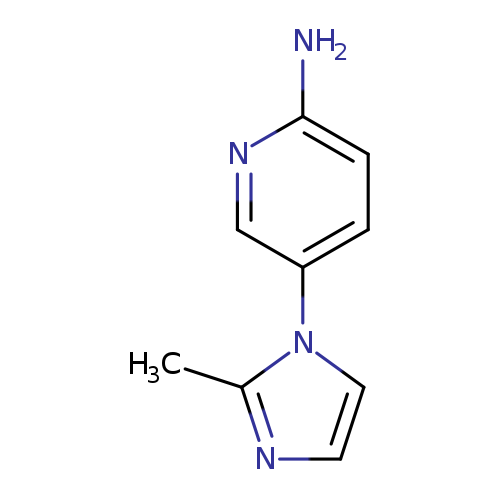
5-(2-Methyl-1h-imidazol-1-yl)pyridin-2-amineCatalog No.:AA0005ZK CAS No.:1019558-27-0 MDL No.:MFCD11135732 MF:C9H10N4 MW:174.2025 |
2-(Benzylaminomethyl)-4-bromo-1-fluorobenzeneCatalog No.:AA0005ZJ CAS No.:1019558-55-4 MDL No.:MFCD11140720 MF:C14H13BrFN MW:294.1621 |
N-(thiophen-3-ylmethyl)pyridin-3-amineCatalog No.:AA01A96S CAS No.:1019559-79-5 MDL No.:MFCD11140123 MF:C10H10N2S MW:190.2648 |
1,4-Bis(3-morpholinopropoxy)benzeneCatalog No.:AA01CC57 CAS No.:101956-13-2 MDL No.: MF:C20H32N2O4 MW:364.4791 |
3-[(Prop-2-en-1-ylamino)methyl]benzonitrileCatalog No.:AA019Y4S CAS No.:1019561-15-9 MDL No.:MFCD11138034 MF:C11H12N2 MW:172.2264 |
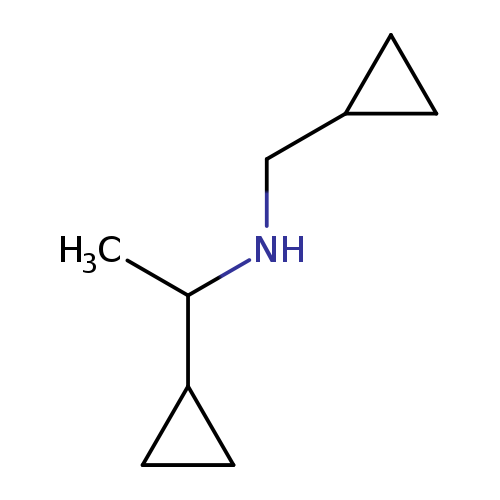
(1-cyclopropylethyl)(cyclopropylmethyl)amineCatalog No.:AA01B8QS CAS No.:1019561-65-9 MDL No.:MFCD11140362 MF:C9H17N MW:139.2380 |
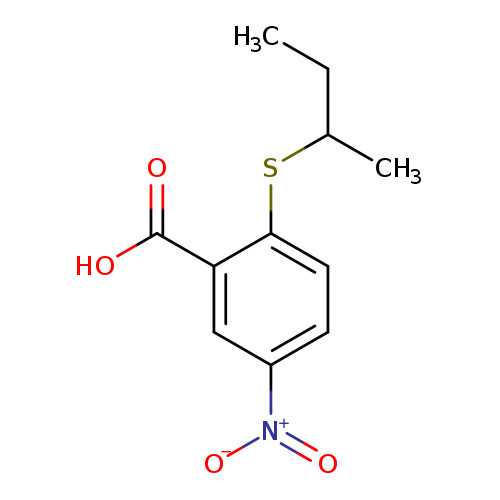
2-(butan-2-ylsulfanyl)-5-nitrobenzoic acidCatalog No.:AA01A4WB CAS No.:1019562-00-5 MDL No.:MFCD11136032 MF:C11H13NO4S MW:255.2902 |
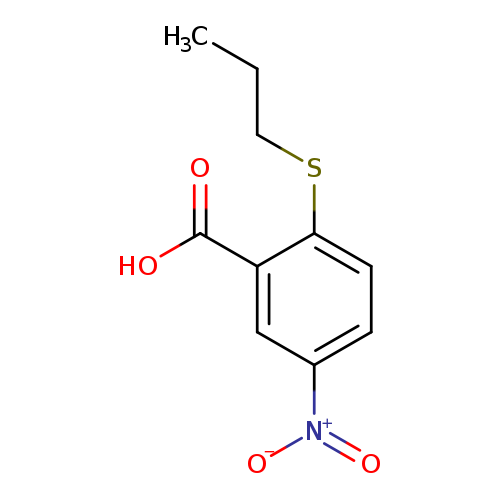
5-nitro-2-(propylsulfanyl)benzoic acidCatalog No.:AA01A5JY CAS No.:1019562-08-3 MDL No.:MFCD11136048 MF:C10H11NO4S MW:241.2636 |
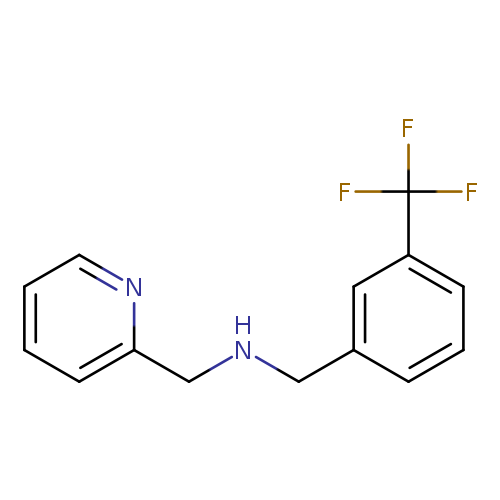
(pyridin-2-ylmethyl)({[3-(trifluoromethyl)phenyl]methyl})amineCatalog No.:AA019ZKL CAS No.:1019564-99-8 MDL No.:MFCD12447218 MF:C14H13F3N2 MW:266.2616 |
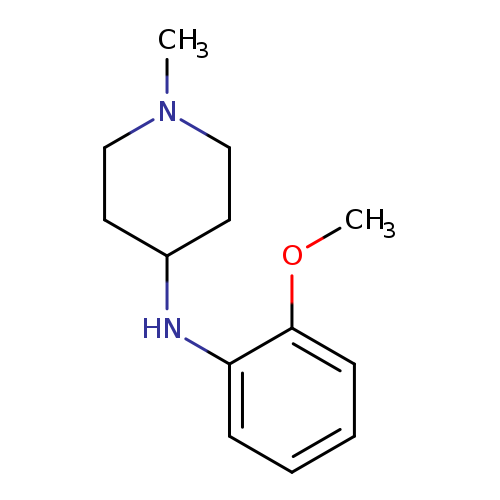
N-(2-methoxyphenyl)-1-methylpiperidin-4-amineCatalog No.:AA01AA9D CAS No.:1019565-48-0 MDL No.:MFCD11141275 MF:C13H20N2O MW:220.3107 |
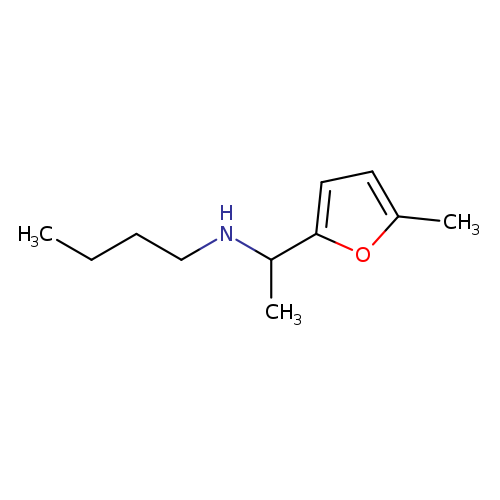
butyl[1-(5-methylfuran-2-yl)ethyl]amineCatalog No.:AA019LRR CAS No.:1019566-19-8 MDL No.:MFCD11142495 MF:C11H19NO MW:181.2747 |
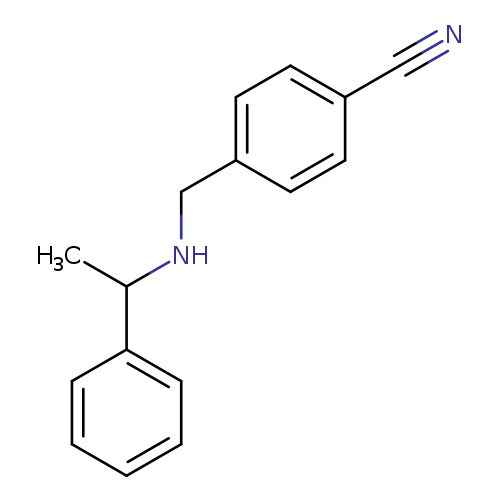
4-[(1-Phenyl-ethylamino)-methyl]-benzonitrile HClCatalog No.:AA0005ZI CAS No.:1019567-85-1 MDL No.:MFCD19442007 MF:C16H16N2 MW:236.3116 |
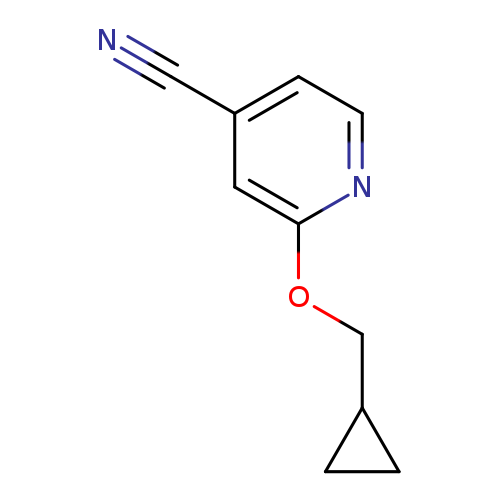
2-(Cyclopropylmethoxy)isonicotinonitrileCatalog No.:AA01AGX1 CAS No.:1019568-08-1 MDL No.:MFCD11136691 MF:C10H10N2O MW:174.1992 |
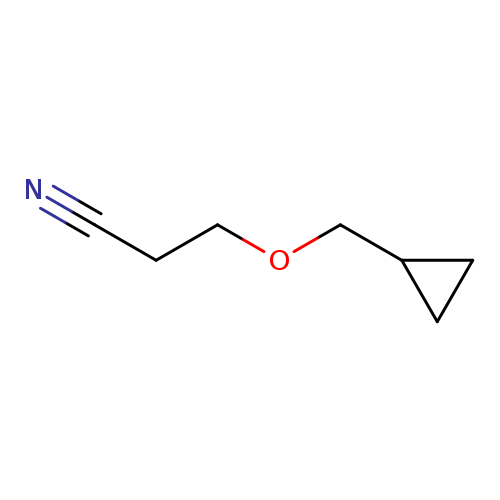
3-(Cyclopropylmethoxy)propanenitrileCatalog No.:AA00944A CAS No.:1019568-17-2 MDL No.:MFCD11136705 MF:C7H11NO MW:125.1683 |
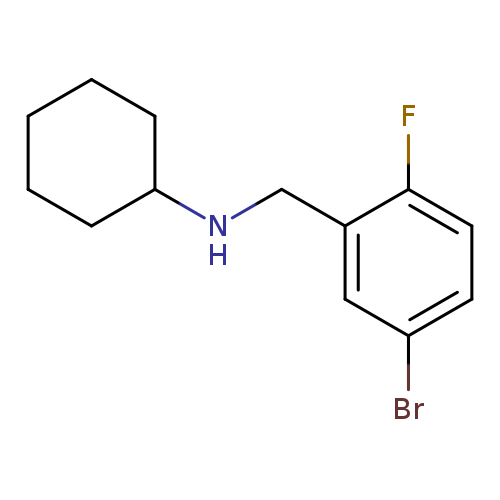
4-Bromo-2-(cyclohexylaminomethyl)-1-fluorobenzeneCatalog No.:AA0005ZH CAS No.:1019568-30-9 MDL No.:MFCD11138548 MF:C13H17BrFN MW:286.1832 |
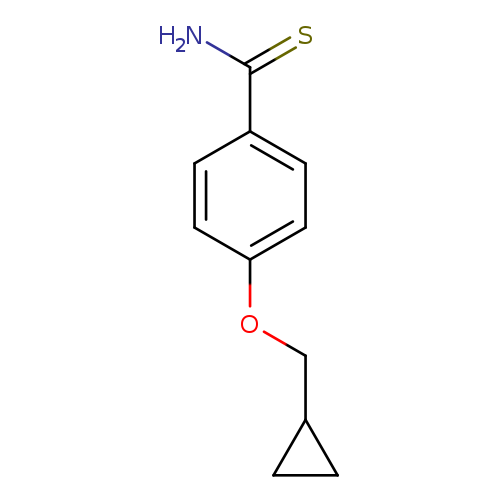
4-(cyclopropylmethoxy)benzene-1-carbothioamideCatalog No.:AA01BFFX CAS No.:1019568-41-2 MDL No.:MFCD11136718 MF:C11H13NOS MW:207.2920 |
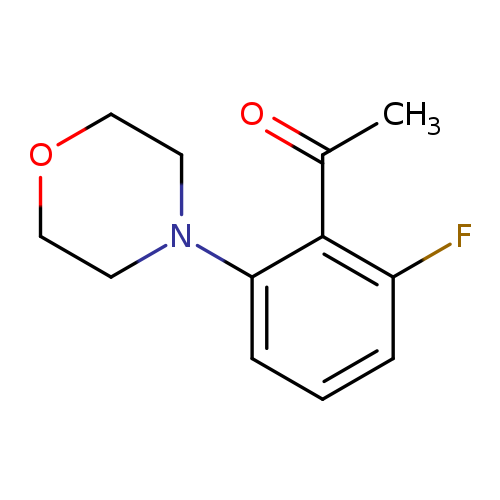
1-[2-fluoro-6-(morpholin-4-yl)phenyl]ethan-1-oneCatalog No.:AA01AKF5 CAS No.:1019569-01-7 MDL No.:MFCD11136757 MF:C12H14FNO2 MW:223.2435 |
4-ethyl-N-propylanilineCatalog No.:AA01C142 CAS No.:1019570-75-2 MDL No.:MFCD11146072 MF:C11H17N MW:163.2594 |
3-{[(quinolin-8-yl)amino]methyl}phenolCatalog No.:AA01BEI3 CAS No.:1019573-28-4 MDL No.:MFCD12623553 MF:C16H14N2O MW:250.2952 |
1-(2-phenoxyphenyl)ethan-1-amineCatalog No.:AA01A92Z CAS No.:1019573-79-5 MDL No.:MFCD11137326 MF:C14H15NO MW:213.2750 |
4-(Aminomethyl)-n-ethyl-n-phenylanilineCatalog No.:AA019RNK CAS No.:1019575-69-9 MDL No.:MFCD11135265 MF:C15H18N2 MW:226.3168 |
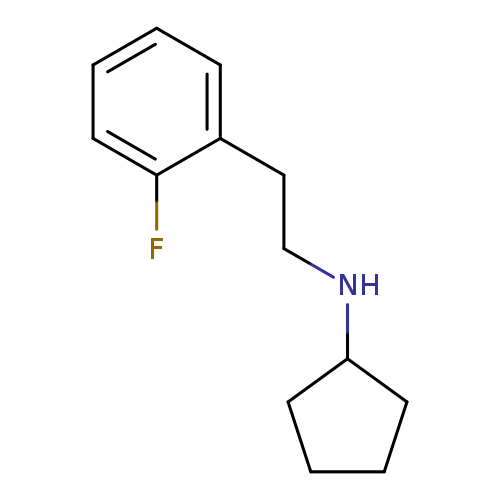
N-[2-(2-fluorophenyl)ethyl]cyclopentanamineCatalog No.:AA019LS7 CAS No.:1019577-12-8 MDL No.:MFCD11143328 MF:C13H18FN MW:207.2871 |
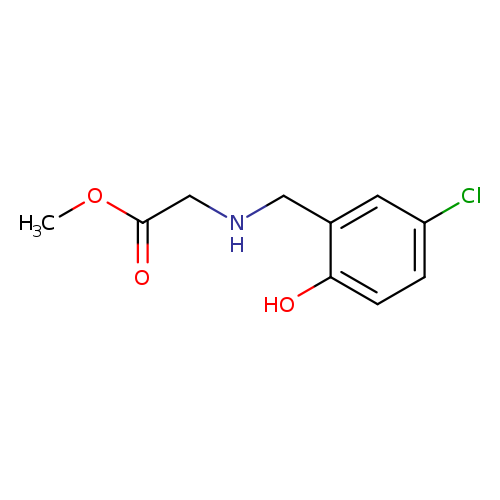
Methyl 2-([(5-chloro-2-hydroxyphenyl)methyl]amino)acetateCatalog No.:AA01AGSU CAS No.:1019577-98-0 MDL No.:MFCD11140602 MF:C10H12ClNO3 MW:229.6602 |
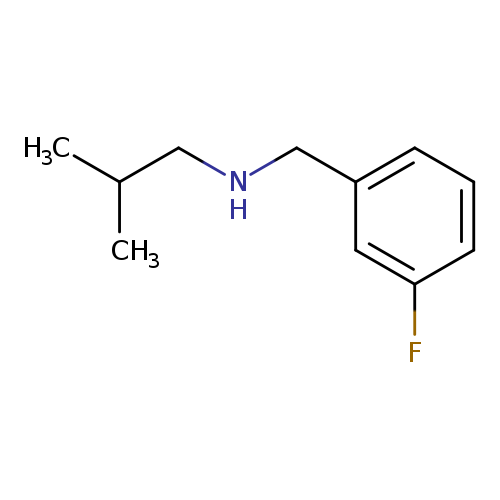
N-Isobutyl 3-fluorobenzylamineCatalog No.:AA000605 CAS No.:1019578-68-7 MDL No.:MFCD11139645 MF:C11H16FN MW:181.2498 |
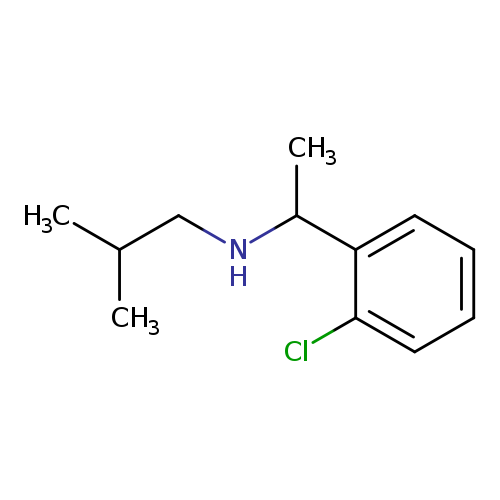
[1-(2-Chlorophenyl)ethyl](2-methylpropyl)amineCatalog No.:AA01B0SQ CAS No.:1019578-92-7 MDL No.:MFCD11139688 MF:C12H18ClN MW:211.7310 |
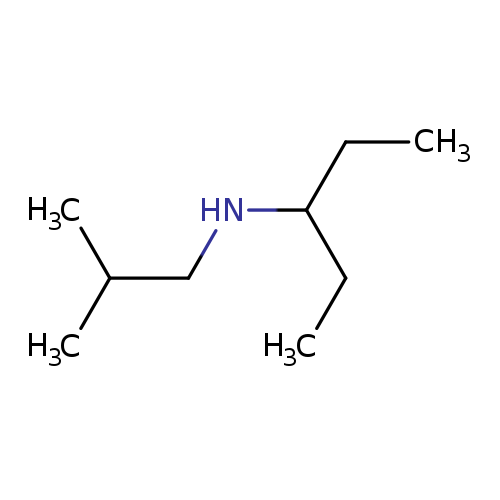
(2-methylpropyl)(pentan-3-yl)amineCatalog No.:AA019TS8 CAS No.:1019579-04-4 MDL No.:MFCD11139705 MF:C9H21N MW:143.2697 |
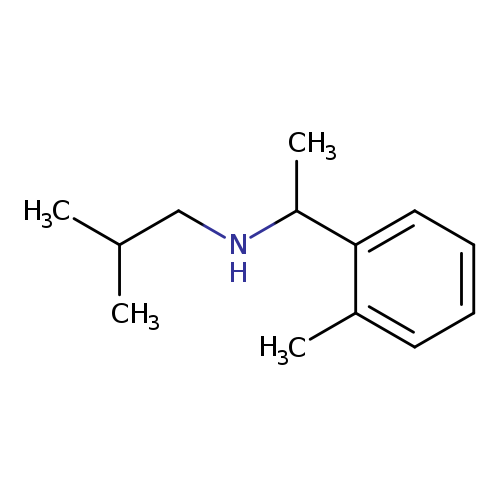
[1-(2-Methylphenyl)ethyl](2-methylpropyl)amineCatalog No.:AA01B0SA CAS No.:1019579-25-9 MDL No.:MFCD11139735 MF:C13H21N MW:191.3125 |
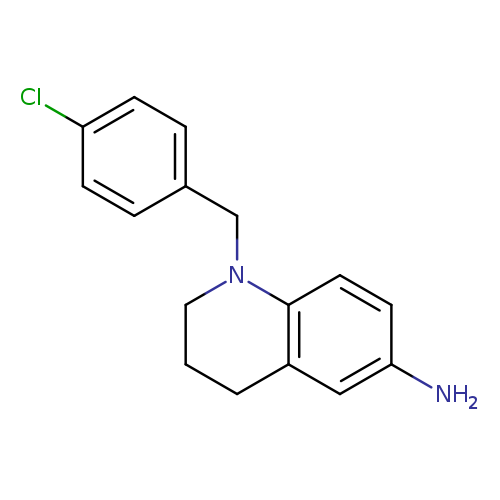
1-[(4-chlorophenyl)methyl]-1,2,3,4-tetrahydroquinolin-6-amineCatalog No.:AA01BDZM CAS No.:1019580-89-2 MDL No.:MFCD12551407 MF:C16H17ClN2 MW:272.7726 |
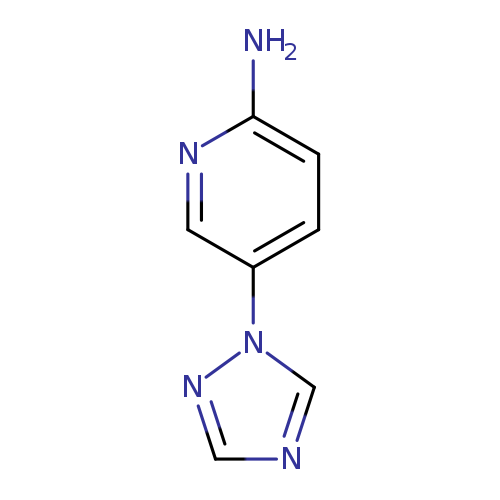
5-(1H-1,2,4-triazol-1-yl)pyridin-2-amineCatalog No.:AA01BDDU CAS No.:1019582-75-2 MDL No.:MFCD11135726 MF:C7H7N5 MW:161.1640 |
2-(2-Bromophenoxy)-5-fluoroanilineCatalog No.:AA00947N CAS No.:1019583-99-3 MDL No.:MFCD11135855 MF:C12H9BrFNO MW:282.1084 |
7-(phenylsulfanyl)-2,3-dihydro-1,4-benzodioxin-6-amineCatalog No.:AA01A947 CAS No.:1019584-14-5 MDL No.:MFCD11135869 MF:C14H13NO2S MW:259.3235 |
3-ethynyl-N-(thiophen-2-ylmethyl)anilineCatalog No.:AA01AABD CAS No.:1019586-19-6 MDL No.:MFCD11138203 MF:C13H11NS MW:213.2981 |
3-methyl-N-(pentan-3-yl)anilineCatalog No.:AA01AXIH CAS No.:1019587-47-3 MDL No.:MFCD11138318 MF:C12H19N MW:177.2860 |
(1-Phenylpropyl)(propan-2-yl)amineCatalog No.:AA01AXJT CAS No.:1019596-43-0 MDL No.:MFCD11139183 MF:C12H19N MW:177.2860 |
1-(furan-3-carbonyl)-2,3-dihydro-1H-indol-5-amineCatalog No.:AA01A8SJ CAS No.:1019597-66-0 MDL No.:MFCD11137558 MF:C13H12N2O2 MW:228.2466 |
[(2,4-dimethylphenyl)methyl](2-methoxyethyl)amineCatalog No.:AA019LOO CAS No.:1019598-18-5 MDL No.:MFCD11139308 MF:C12H19NO MW:193.2854 |
(2-methoxyethyl)({1-[3-(trifluoromethyl)phenyl]ethyl})amineCatalog No.:AA019LPB CAS No.:1019598-73-2 MDL No.:MFCD11139382 MF:C12H16F3NO MW:247.2567 |
methyl 5-amino-2,3-dihydro-1H-indole-1-carboxylateCatalog No.:AA019WEL CAS No.:1019599-43-9 MDL No.:MFCD11137592 MF:C10H12N2O2 MW:192.2145 |
4-fluoro-N-[(5-methylfuran-2-yl)methyl]anilineCatalog No.:AA01AGSZ CAS No.:1019599-93-9 MDL No.:MFCD11145039 MF:C12H12FNO MW:205.2282 |
Nitric acid, zinc salt, hydrate (2:1:6)Catalog No.:AA00060S CAS No.:10196-18-6 MDL No.:MFCD00011293 MF:H12N2O12Zn MW:297.4815 |
1-Butanol, 2-amino-2-methyl-Catalog No.:AA00060Q CAS No.:10196-30-2 MDL No.:MFCD09759242 MF:C5H13NO MW:103.1628 |
2,2,4-Trimethyl-1-oxa-4-aza-2-silacyclohexaneCatalog No.:AA00060M CAS No.:10196-49-3 MDL No.:MFCD09263699 MF:C6H15NOSi MW:145.2749 |
4-[(2-Methoxyethyl)amino]benzonitrileCatalog No.:AA01A7UO CAS No.:1019600-58-8 MDL No.:MFCD11135500 MF:C10H12N2O MW:176.2151 |
4-(3-Oxopiperazin-1-yl)benzonitrileCatalog No.:AA019MT8 CAS No.:1019601-14-9 MDL No.:MFCD11099554 MF:C11H11N3O MW:201.2245 |
4-[(4-methyl-1,3-thiazol-2-yl)amino]benzonitrileCatalog No.:AA01A7PE CAS No.:1019601-20-7 MDL No.:MFCD11135568 MF:C11H9N3S MW:215.2743 |
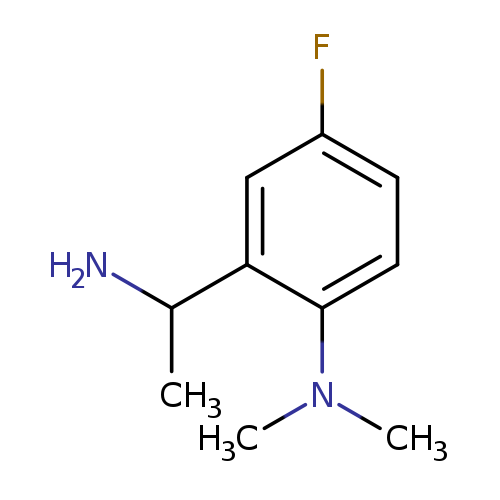
2-(1-aminoethyl)-4-fluoro-N,N-dimethylanilineCatalog No.:AA01AHAM CAS No.:1019602-10-8 MDL No.:MFCD11137153 MF:C10H15FN2 MW:182.2379 |
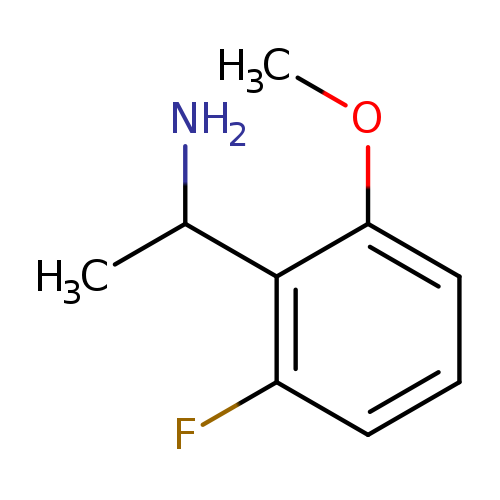
1-(2-fluoro-6-methoxyphenyl)ethan-1-amineCatalog No.:AA019RXA CAS No.:1019602-82-4 MDL No.:MFCD11137212 MF:C9H12FNO MW:169.1961 |
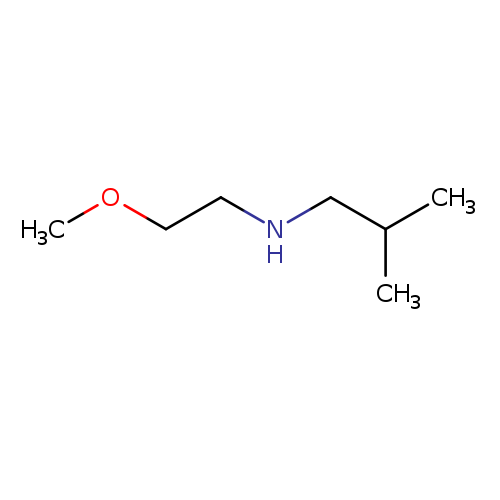
N-(2-Methoxyethyl)-2-methylpropan-1-amineCatalog No.:AA019LVH CAS No.:1019603-73-6 MDL No.:MFCD09971623 MF:C7H17NO MW:131.2160 |
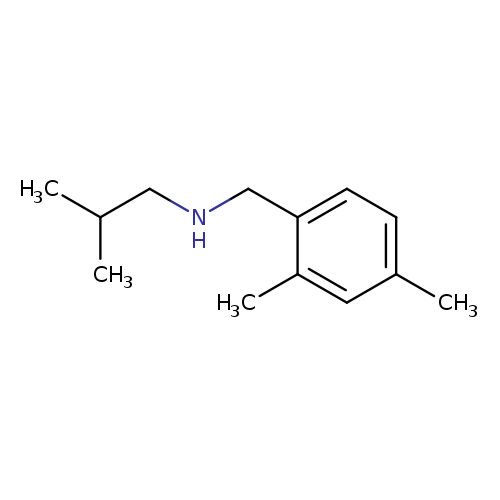
[(2,4-dimethylphenyl)methyl](2-methylpropyl)amineCatalog No.:AA01B08N CAS No.:1019606-02-0 MDL No.:MFCD11139636 MF:C13H21N MW:191.3125 |
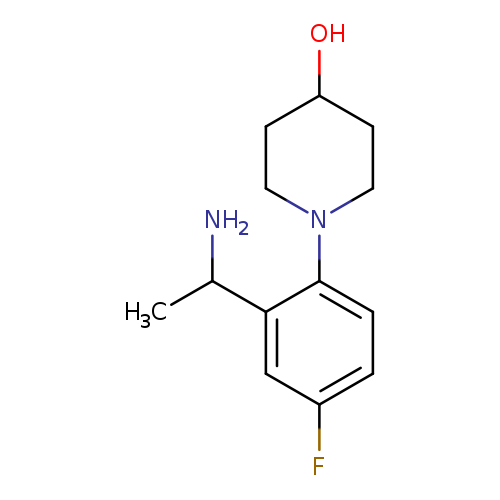
1-[2-(1-Aminoethyl)-4-fluorophenyl]piperidin-4-olCatalog No.:AA01AAU2 CAS No.:1019606-10-0 MDL No.:MFCD11137129 MF:C13H19FN2O MW:238.3012 |
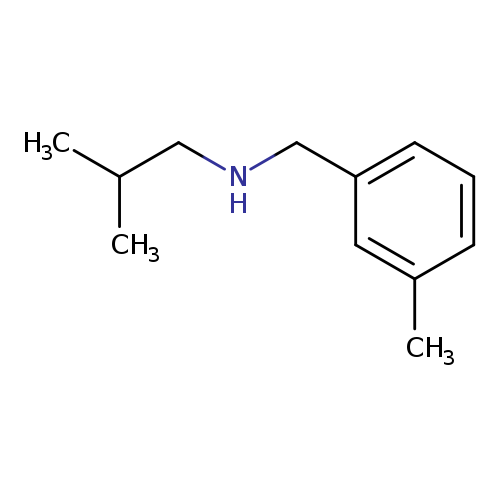
[(3-methylphenyl)methyl](2-methylpropyl)amineCatalog No.:AA00JTP2 CAS No.:1019606-15-5 MDL No.:MFCD11139648 MF:C12H19N MW:177.2860 |
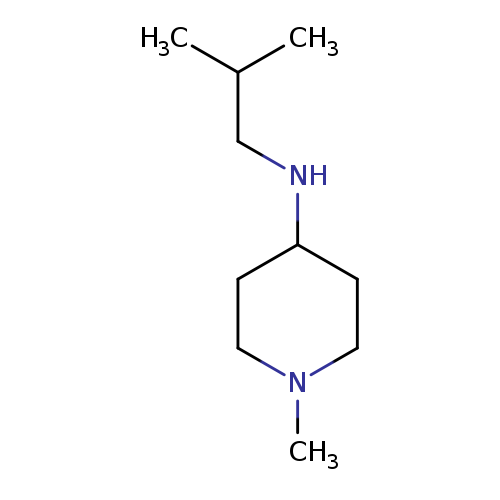
N-Isobutyl-1-methylpiperidin-4-amineCatalog No.:AA019WST CAS No.:1019606-19-9 MDL No.:MFCD11139654 MF:C10H22N2 MW:170.2951 |
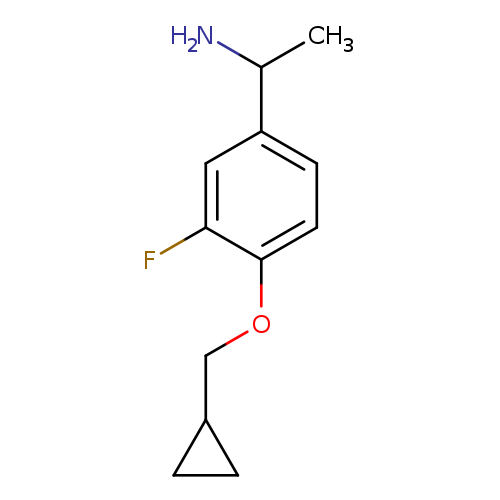
1-[4-(cyclopropylmethoxy)-3-fluorophenyl]ethan-1-amineCatalog No.:AA019ZOZ CAS No.:1019606-90-6 MDL No.:MFCD11137192 MF:C12H16FNO MW:209.2599 |
4-(Cyclopropylamino)benzonitrileCatalog No.:AA000611 CAS No.:1019607-55-6 MDL No.:MFCD11135501 MF:C10H10N2 MW:158.1998 |
4-[(cyclopropylmethyl)amino]benzonitrileCatalog No.:AA01AXHU CAS No.:1019607-58-9 MDL No.:MFCD11135508 MF:C11H12N2 MW:172.2264 |
4-{[(cyclopropylmethyl)amino]methyl}-N,N-dimethylanilineCatalog No.:AA01AAOS CAS No.:1019611-27-8 MDL No.:MFCD11140253 MF:C13H20N2 MW:204.3113 |
2-(ethylsulfanyl)-5-nitrobenzoic acidCatalog No.:AA01A3ET CAS No.:1019613-59-2 MDL No.:MFCD11136036 MF:C9H9NO4S MW:227.2371 |
2-[(pyrimidin-2-ylsulfanyl)methyl]anilineCatalog No.:AA019UMB CAS No.:1019613-83-2 MDL No.:MFCD11136110 MF:C11H11N3S MW:217.2901 |
2,4-difluoro-N-(pyridin-2-ylmethyl)anilineCatalog No.:AA019VWU CAS No.:1019614-22-2 MDL No.:MFCD11145862 MF:C12H10F2N2 MW:220.2180 |
2-bromo-4-methyl-N-(propan-2-yl)anilineCatalog No.:AA01AXP7 CAS No.:1019616-06-8 MDL No.:MFCD11141242 MF:C10H14BrN MW:228.1289 |
1-Bromo-4-fluoro-2-(butylaminomethyl)benzeneCatalog No.:AA00060Y CAS No.:1019618-98-4 MDL No.:MFCD11151689 MF:C11H15BrFN MW:260.1459 |
butyl[(2,4-difluorophenyl)methyl]amineCatalog No.:AA019LRK CAS No.:1019619-01-2 MDL No.:MFCD11142429 MF:C11H15F2N MW:199.2403 |
1-[5-fluoro-2-(4-hydroxypiperidin-1-yl)phenyl]ethan-1-oneCatalog No.:AA01E7B0 CAS No.:1019621-94-3 MDL No.:MFCD11136814 MF:C13H16FNO2 MW:237.2700 |
(2-Hydroxy-3-[2-(propan-2-yloxy)ethoxy]propyl)(methyl)amineCatalog No.:AA019X0F CAS No.:1019624-79-3 MDL No.:MFCD11135173 MF:C9H21NO3 MW:191.2679 |
1-[2-fluoro-6-(1-phenylethoxy)phenyl]ethan-1-oneCatalog No.:AA01ABC4 CAS No.:1019624-98-6 MDL No.:MFCD12446502 MF:C16H15FO2 MW:258.2875 |
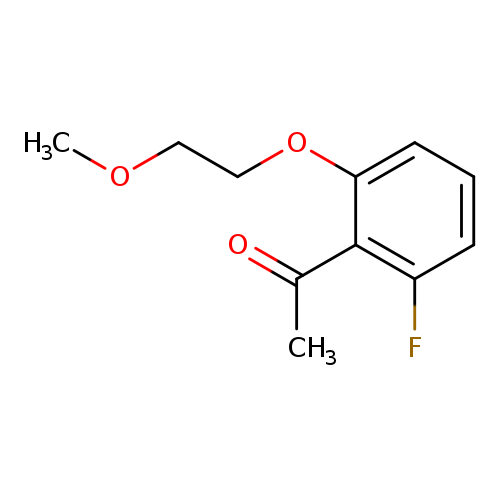
1-[2-fluoro-6-(2-methoxyethoxy)phenyl]ethan-1-oneCatalog No.:AA01AAIK CAS No.:1019625-48-9 MDL No.:MFCD11136922 MF:C11H13FO3 MW:212.2175 |
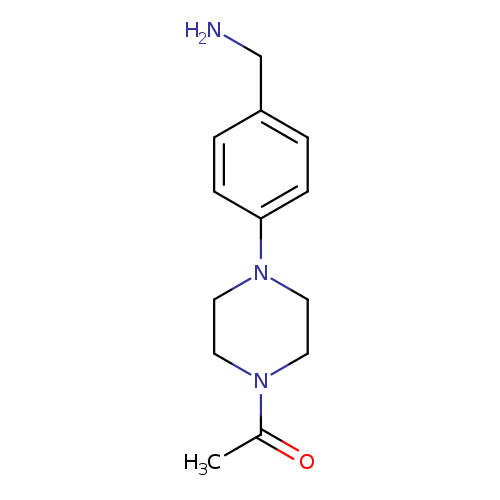
1-(4-[4-(Aminomethyl)phenyl]piperazin-1-yl)ethan-1-oneCatalog No.:AA019YHN CAS No.:1019625-52-5 MDL No.:MFCD11135267 MF:C13H19N3O MW:233.3095 |
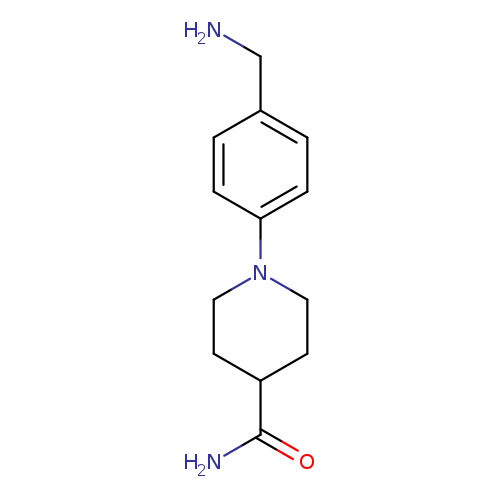
1-[4-(aminomethyl)phenyl]piperidine-4-carboxamideCatalog No.:AA01E81B CAS No.:1019625-55-8 MDL No.:MFCD11135270 MF:C13H19N3O MW:233.3095 |
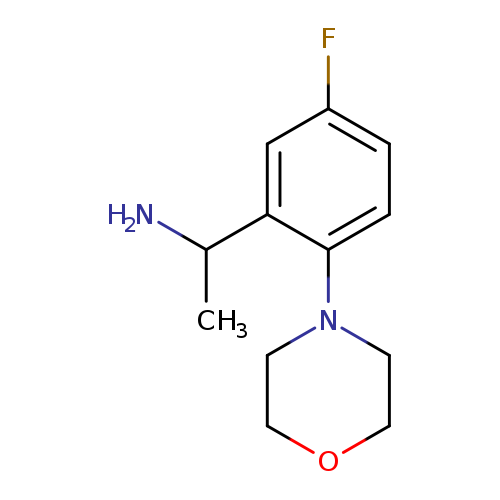
1-[5-Fluoro-2-(morpholin-4-yl)phenyl]ethan-1-amineCatalog No.:AA01AAU1 CAS No.:1019627-92-9 MDL No.:MFCD11137041 MF:C12H17FN2O MW:224.2746 |
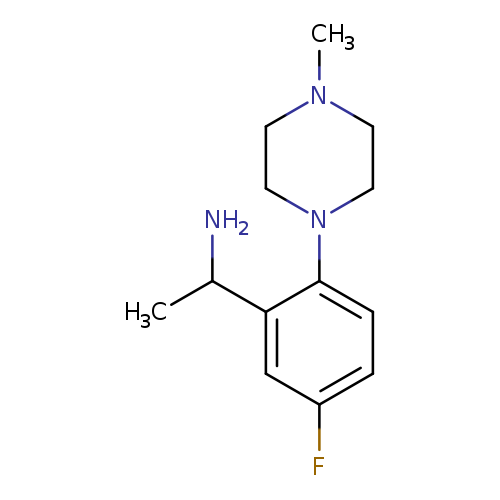
1-[5-Fluoro-2-(4-methylpiperazin-1-yl)phenyl]ethan-1-amineCatalog No.:AA01A8K0 CAS No.:1019628-04-6 MDL No.:MFCD11137054 MF:C13H20FN3 MW:237.3164 |
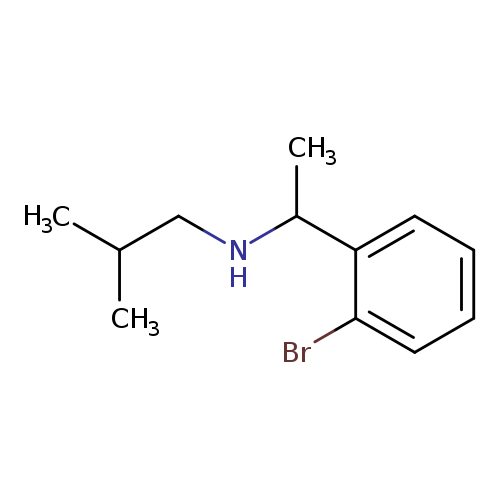
[1-(2-bromophenyl)ethyl](2-methylpropyl)amineCatalog No.:AA01B0SC CAS No.:1019628-97-7 MDL No.:MFCD11139707 MF:C12H18BrN MW:256.1820 |
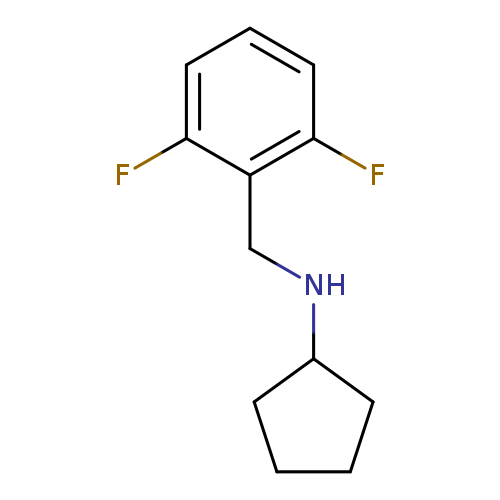
N-[(2,6-difluorophenyl)methyl]cyclopentanamineCatalog No.:AA01AAR9 CAS No.:1019629-72-1 MDL No.:MFCD11139802 MF:C12H15F2N MW:211.2510 |
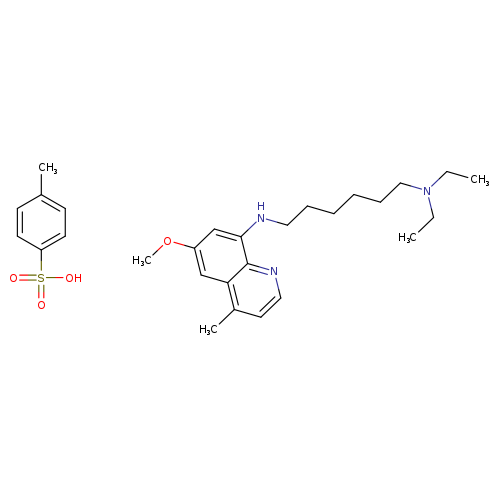
N1,N1-Diethyl-N6-(6-methoxy-4-methyl-8-quinolinyl)-1,6-hexanediamine 4-methylbenzenesulfonateCatalog No.:AA008ZDD CAS No.:1019640-33-5 MDL No.:MFCD17392572 MF:C28H41N3O4S MW:515.7078 |
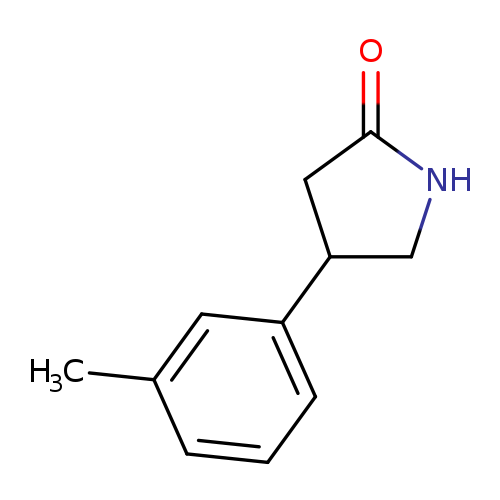
2-Pyrrolidinone, 4-(3-methylphenyl)Catalog No.:AA00H9MK CAS No.:1019650-80-6 MDL No.:MFCD19347433 MF:C11H13NO MW:175.2270 |
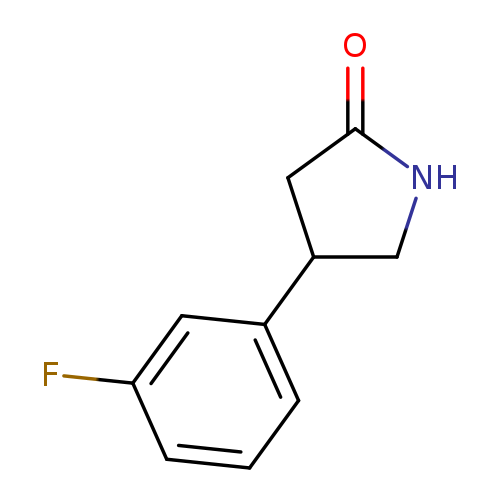
4-(3-Fluorophenyl)pyrrolidin-2-oneCatalog No.:AA00H9ML CAS No.:1019650-87-3 MDL No.:MFCD09955048 MF:C10H10FNO MW:179.1909 |
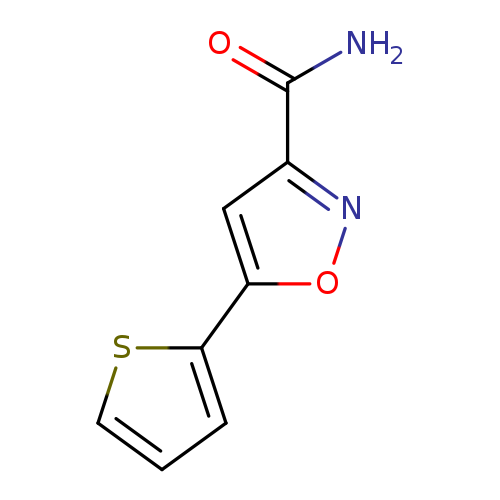
5-(Thiophen-2-yl)-1,2-oxazole-3-carboxamideCatalog No.:AA01A7V1 CAS No.:1019656-18-8 MDL No.:MFCD11643942 MF:C8H6N2O2S MW:194.2104 |

4-(2,5-Dimethylphenyl)thiazol-2-ylamineCatalog No.:AA000619 CAS No.:101967-39-9 MDL No.:MFCD00985673 MF:C11H12N2S MW:204.2914 |
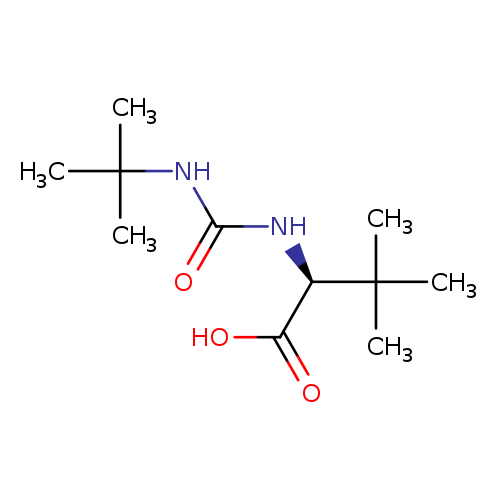
(S)-2-(3-(tert-Butyl)ureido)-3,3-dimethylbutanoic acidCatalog No.:AA000613 CAS No.:101968-85-8 MDL No.:MFCD12796012 MF:C11H22N2O3 MW:230.3040 |
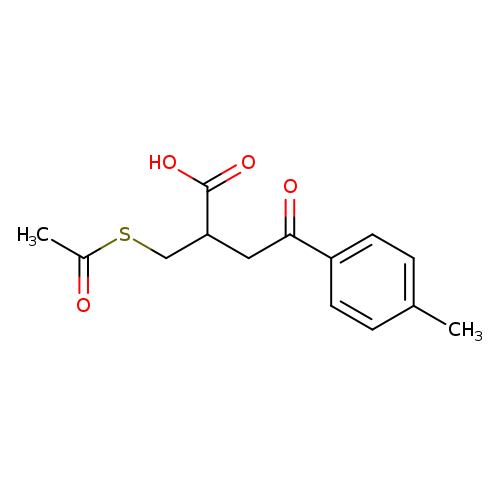
2-acetylthiomethyl-3-(4-methylbenzoyl)propionic acidCatalog No.:AA008UW9 CAS No.:101973-77-7 MDL No.:MFCD00872973 MF:C14H16O4S MW:280.3394 |
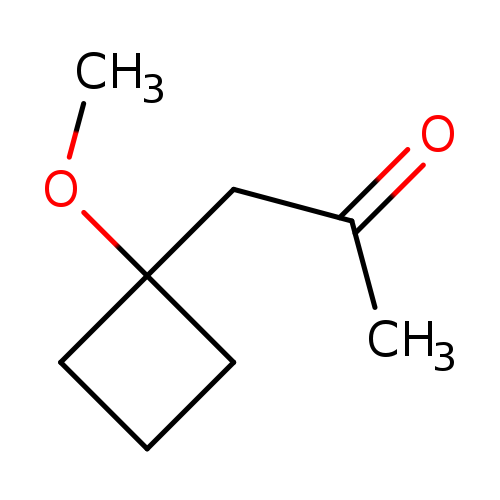
1-(1-methoxycyclobutyl)propan-2-oneCatalog No.:AA01BDXI CAS No.:101974-56-5 MDL No.:MFCD24691523 MF:C8H14O2 MW:142.1956 |
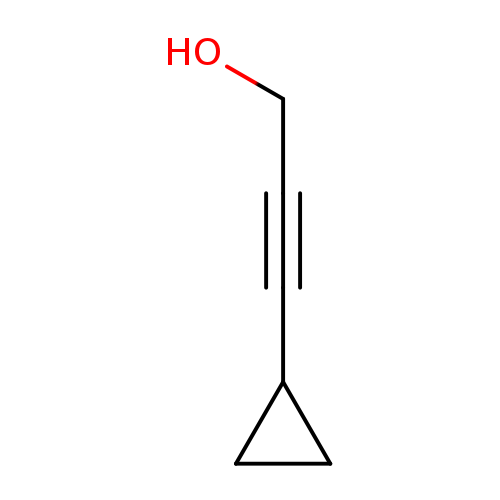
3-Cyclopropylprop-2-yn-1-olCatalog No.:AA00061S CAS No.:101974-69-0 MDL No.:MFCD06654125 MF:C6H8O MW:96.1271 |
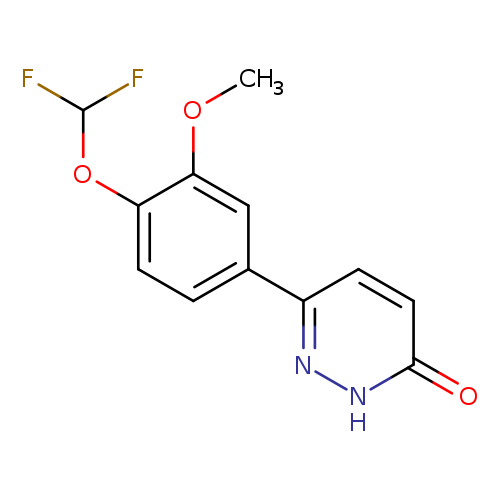
3(2H)-Pyridazinone, 6-[4-(difluoromethoxy)-3-methoxyphenyl]-Catalog No.:AA00061Q CAS No.:101975-10-4 MDL No.:MFCD00867059 MF:C12H10F2N2O3 MW:268.2162 |
1-[4-(difluoromethoxy)-3-methoxyphenyl]ethan-1-oneCatalog No.:AA0095BU CAS No.:101975-20-6 MDL No.:MFCD04621491 MF:C10H10F2O3 MW:216.1814 |
3'-(Difluoromethoxy)acetophenoneCatalog No.:AA00061O CAS No.:101975-23-9 MDL No.:MFCD00236220 MF:C9H8F2O2 MW:186.1554 |
4-(Benzyloxy)-3-bromopyridineCatalog No.:AA00062A CAS No.:1019767-63-5 MDL No.:MFCD11226996 MF:C12H10BrNO MW:264.1179 |
Sodium benzo[d]oxazole-2-carboxylateCatalog No.:AA000629 CAS No.:1019770-99-0 MDL No.:MFCD17676126 MF:C8H4NNaO3 MW:185.1120 |
(2,2-dimethyl-2H-1,3-benzodioxol-5-yl)methanamineCatalog No.:AA01A18A CAS No.:1019776-84-1 MDL No.:MFCD20726280 MF:C10H13NO2 MW:179.2157 |
Acetamide, 2-[(3,4-difluorophenyl)amino]-N-[2-methyl-5-(1-piperazinylmethyl)phenyl]-, hydrochloride (1:2)Catalog No.:AA000627 CAS No.:1019779-04-4 MDL No.:MFCD12828767 MF:C20H26Cl2F2N4O MW:447.3494 |
3-Methoxy-estra-2,5(10)-dien-17-olCatalog No.:AA0096JY CAS No.:101978-01-2 MDL No.:MFCD11111820 MF:C19H28O2 MW:288.4244 |
6-hydroxy-5-methyl-2-(pyridin-2-yl)-3,4-dihydropyrimidin-4-oneCatalog No.:AA01BVJD CAS No.:10198-75-1 MDL No.:MFCD18843876 MF:C10H9N3O2 MW:203.1974 |
4,6-dichloro-5-methyl-2-(pyridin-2-yl)pyrimidineCatalog No.:AA00063K CAS No.:10198-77-3 MDL No.:MFCD18844051 MF:C10H7Cl2N3 MW:240.0887 |
1,3-Di(2-pyridyl)-1,3-propanedioneCatalog No.:AA00063G CAS No.:10198-89-7 MDL No.:MFCD01321157 MF:C13H10N2O2 MW:226.2307 |
2-Bromo-1-isopropyl-4-nitrobenzeneCatalog No.:AA00063B CAS No.:101980-41-0 MDL No.:MFCD12024309 MF:C9H10BrNO2 MW:244.0852 |
(3aR,8aR)-(-)-4,4,8,8-Tetrakis (3,5-diMethylphenyl)tetrahydro-2,2-diMethyl-6-phenyl-1,3-dioxolo[4,5-e]dioxaphosphepinCatalog No.:AA008X1G CAS No.:1019840-96-0 MDL No.:MFCD23704798 MF:C45H49O4P MW:684.8419 |
Methyl 3-(3-oxocyclobutyl)propanoateCatalog No.:AA000631 CAS No.:1019842-24-0 MDL No.:MFCD19441111 MF:C8H12O3 MW:156.1791 |
N-(propan-2-yl)piperidine-4-carboxamide hydrochlorideCatalog No.:AA01A8B7 CAS No.:1019851-92-3 MDL No.:MFCD11615741 MF:C9H19ClN2O MW:206.7130 |
N-butylpiperidine-4-carboxamide, HClCatalog No.:AA01AAY3 CAS No.:1019851-94-5 MDL No.:MFCD11615740 MF:C10H21ClN2O MW:220.7395 |
N-(2-(dimethylamino)ethyl)piperidine-4-carboxamide dihydrochlorideCatalog No.:AA018MP8 CAS No.:1019851-96-7 MDL No.:MFCD10686831 MF:C10H21N3O MW:199.2932 |
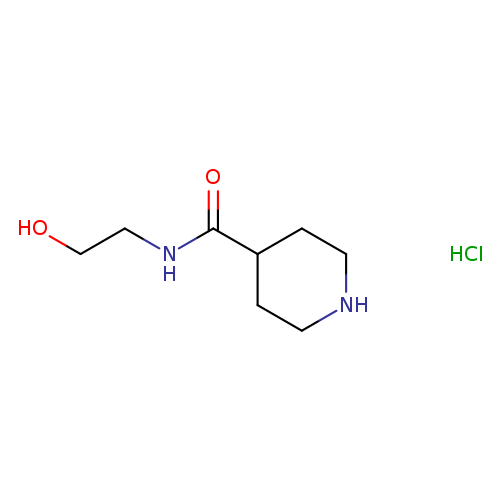
N-(2-Hydroxyethyl)piperidine-4-carboxamide hydrochlorideCatalog No.:AA01BRSN CAS No.:1019851-97-8 MDL No.:MFCD11615739 MF:C8H17ClN2O2 MW:208.6858 |
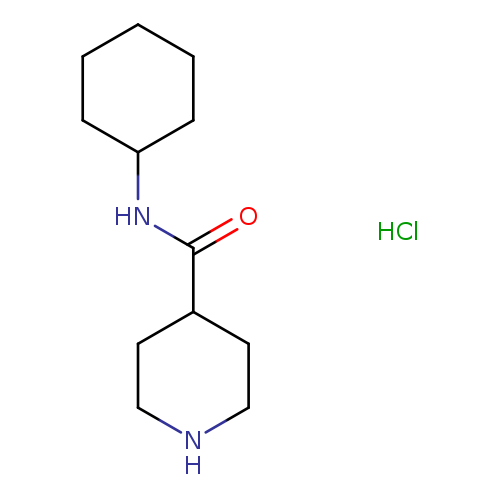
N-Cyclohexylpiperidine-4-carboxamideCatalog No.:AA00H9MP CAS No.:1019851-98-9 MDL No.:MFCD06739716 MF:C12H23ClN2O MW:246.7768 |
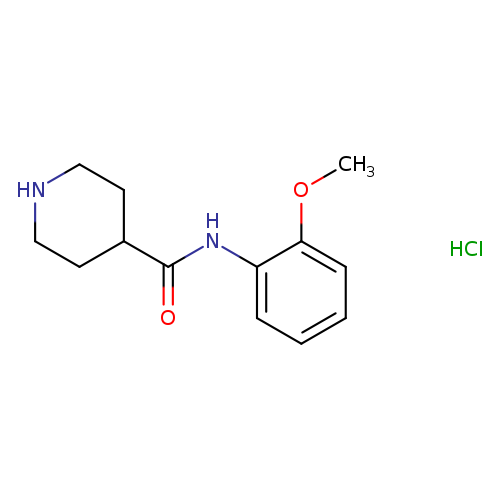
N-(2-methoxyphenyl)piperidine-4-carboxamide hydrochlorideCatalog No.:AA019MMT CAS No.:1019851-99-0 MDL No.:MFCD11099451 MF:C13H19ClN2O2 MW:270.7552 |
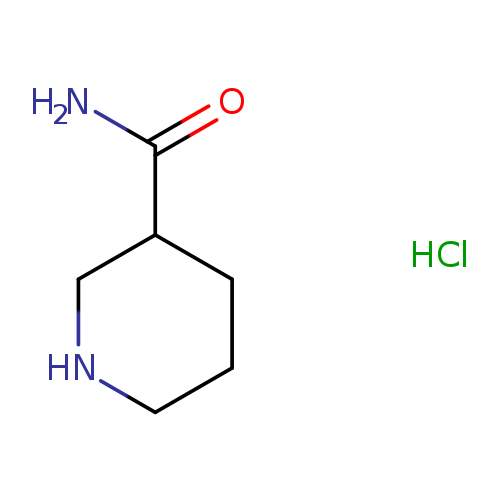
3-Piperidinecarboxamide, hydrochloride (1:1)Catalog No.:AA00062Y CAS No.:1019852-04-0 MDL No.:MFCD09037931 MF:C6H13ClN2O MW:164.6332 |
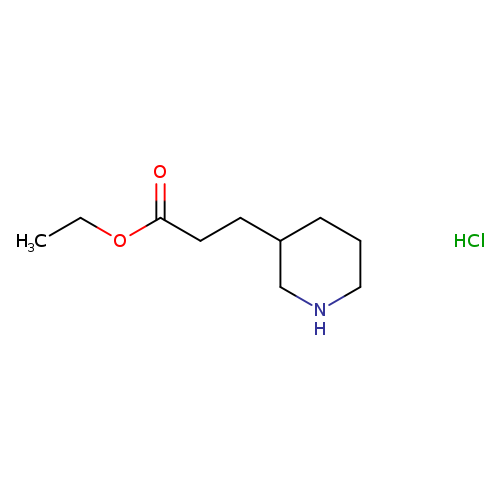
ethyl 3-(piperidin-3-yl)propanoate hydrochlorideCatalog No.:AA01A1Z6 CAS No.:1019852-05-1 MDL No.:MFCD22566022 MF:C10H20ClNO2 MW:221.7243 |
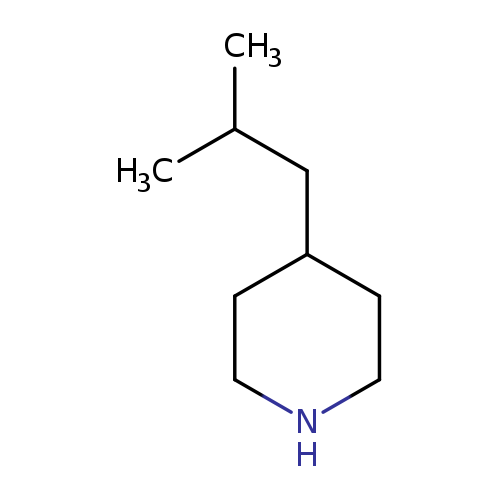
4-(2-methylpropyl)piperidine hydrochlorideCatalog No.:AA018O0T CAS No.:1019852-12-0 MDL No.:MFCD22375408 MF:C9H19N MW:141.2539 |
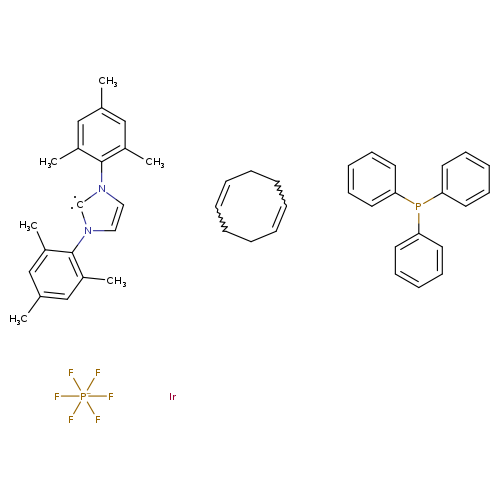
Triphenylphosphine(1,5-cyclooctadiene)[1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]iridium(I) hexafluorophosphateCatalog No.:AA01FR32 CAS No.:1019853-00-9 MDL No.:MFCD22666051 MF:C47H51F6IrN2P2- MW:1012.0762 |
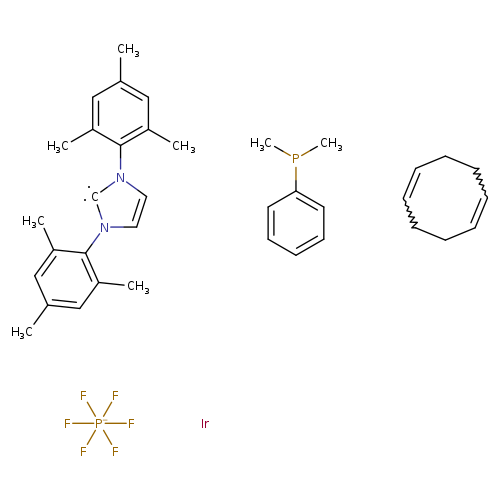
(Dimethylphenylphosphine)(1,5-cyclooctadiene)[1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]iridium(I)Catalog No.:AA008X4B CAS No.:1019853-03-2 MDL No.:MFCD22666052 MF:C37H47F6IrN2P2- MW:887.9374 |
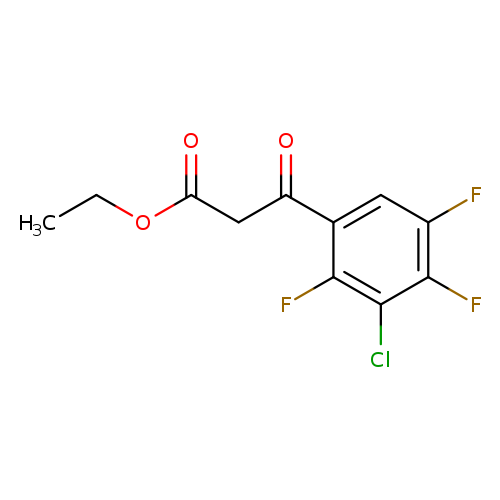
Ethyl 3-(3-chloro-2,4,5-trifluorophenyl)-3-oxopropanoateCatalog No.:AA000634 CAS No.:101987-86-4 MDL No.:MFCD09037898 MF:C11H8ClF3O3 MW:280.6276 |
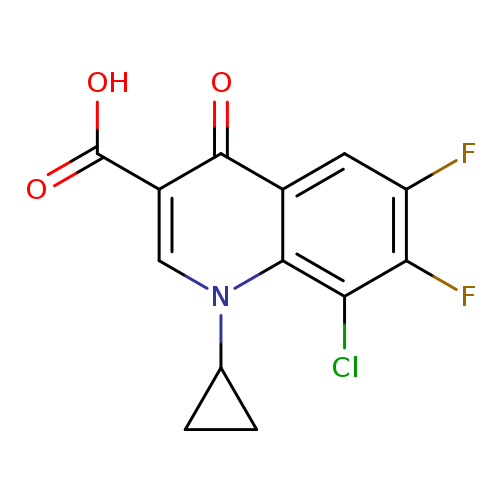
8-Chloro-1-cyclopropyl-6,7-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acidCatalog No.:AA000633 CAS No.:101987-89-7 MDL No.:MFCD08706388 MF:C13H8ClF2NO3 MW:299.6573 |
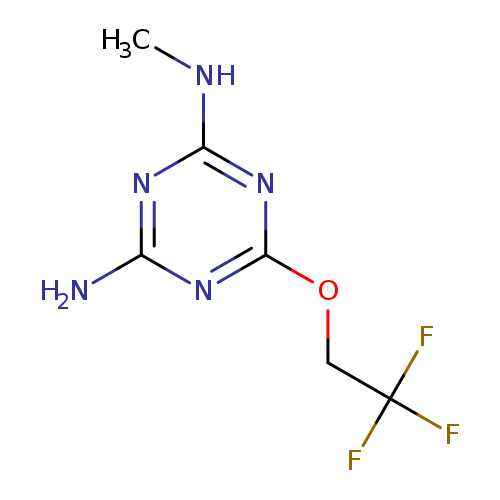
N-Methyl-6-(2,2,2-trifluoroethoxy)-1,3,5-triazine-2,4-diamineCatalog No.:AA000632 CAS No.:101988-70-9 MDL No.:MFCD00452913 MF:C6H8F3N5O MW:223.1558 |
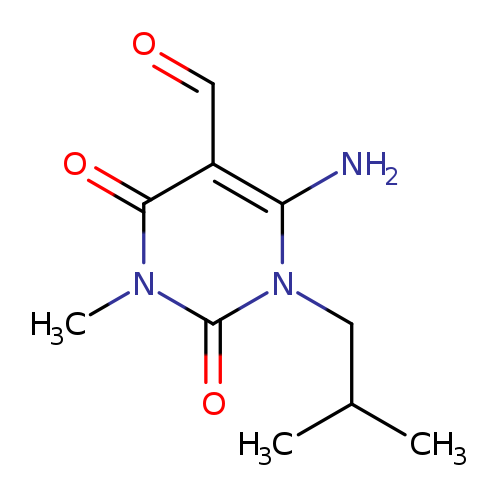
5-Pyrimidinecarboxaldehyde,6-amino-1,2,3,4-tetrahydro-3-methyl-1-(2-methylpropyl)-2,4-dioxo-Catalog No.:AA00H9MQ CAS No.:101989-80-4 MDL No.:MFCD04612985 MF:C10H15N3O3 MW:225.2444 |
2,2'-BipyrazineCatalog No.:AA00063E CAS No.:10199-00-5 MDL No.:MFCD00010288 MF:C8H6N4 MW:158.1600 |
2-methyl-5-phenylpyrazol-3-amineCatalog No.:AA00063D CAS No.:10199-50-5 MDL No.:MFCD00067874 MF:C10H11N3 MW:173.2144 |
Ethyl 1-Methyl-5-phenylpyrazole-3-carboxylateCatalog No.:AA00063C CAS No.:10199-51-6 MDL No.:MFCD08060533 MF:C13H14N2O2 MW:230.2625 |
1-Methyl-5-phenylpyrazole-3-carboxylic acidCatalog No.:AA000649 CAS No.:10199-53-8 MDL No.:MFCD08271933 MF:C11H10N2O2 MW:202.2093 |
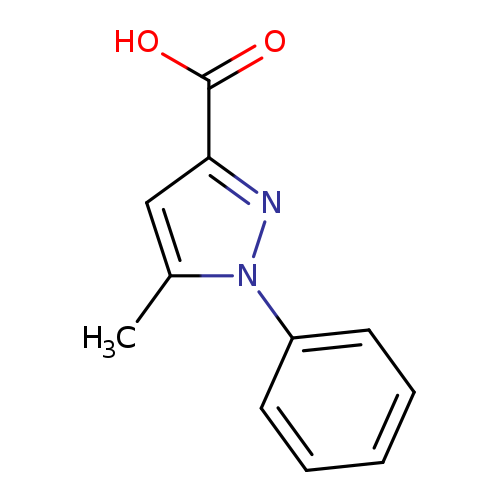
5-Methyl-1-phenylpyrazole-3-carboxylic acidCatalog No.:AA000648 CAS No.:10199-57-2 MDL No.:MFCD00124910 MF:C11H10N2O2 MW:202.2093 |
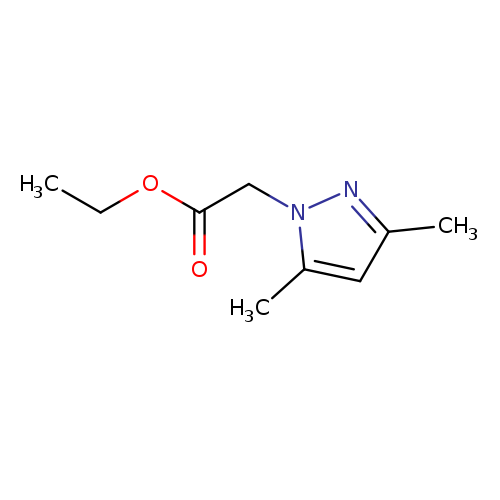
ethyl 2-(3,5-dimethyl-1H-pyrazol-1-yl)acetateCatalog No.:AA009NTX CAS No.:10199-60-7 MDL No.:MFCD00297277 MF:C9H14N2O2 MW:182.2197 |
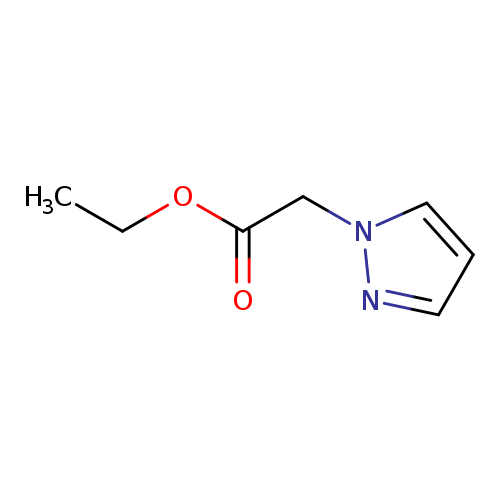
ethyl 2-(pyrazol-1-yl)acetateCatalog No.:AA000646 CAS No.:10199-61-8 MDL No.:MFCD00159620 MF:C7H10N2O2 MW:154.1665 |
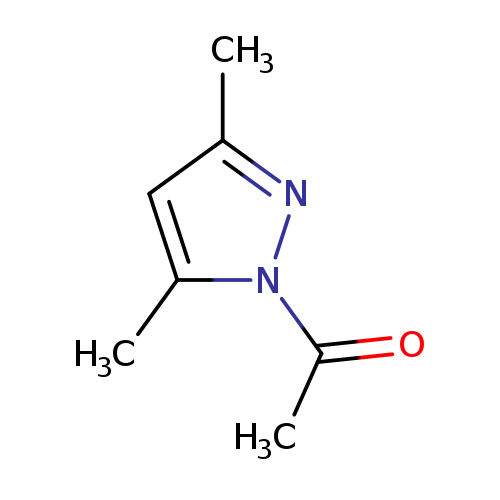
1-(3,5-Dimethyl-1h-pyrazol-1-yl)-1-ethanoneCatalog No.:AA000645 CAS No.:10199-63-0 MDL No.:MFCD00461792 MF:C7H10N2O MW:138.1671 |
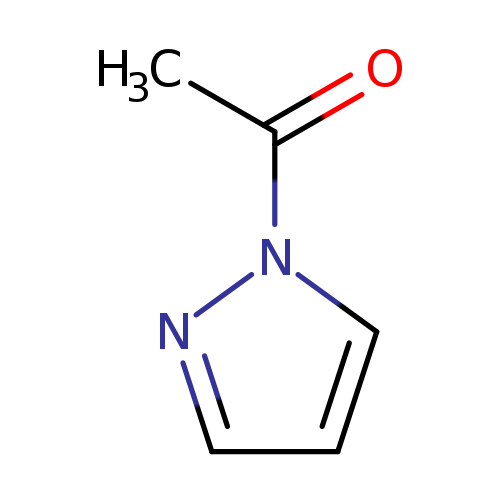
1-(1H-Pyrazol-1-yl)ethanoneCatalog No.:AA000644 CAS No.:10199-64-1 MDL No.:MFCD01336293 MF:C5H6N2O MW:110.1139 |
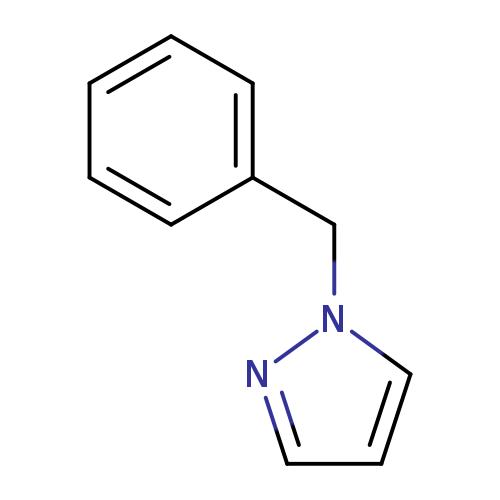
1-BenzylpyrazoleCatalog No.:AA000642 CAS No.:10199-67-4 MDL No.:MFCD00462231 MF:C10H10N2 MW:158.1998 |
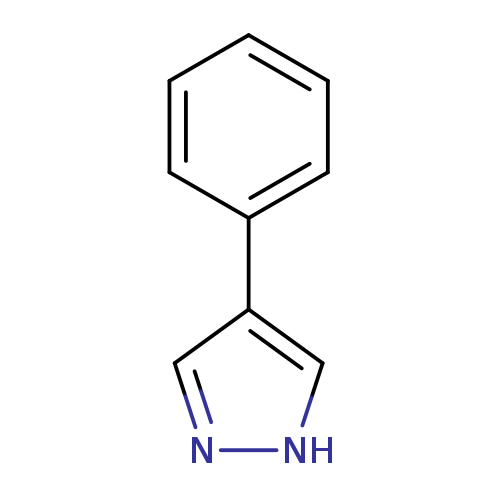
4-Phenyl-1h-pyrazoleCatalog No.:AA000641 CAS No.:10199-68-5 MDL No.:MFCD00462204 MF:C9H8N2 MW:144.1732 |
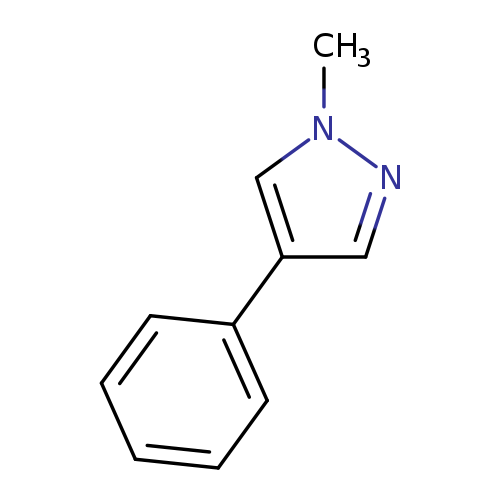
1-Methyl-4-phenyl-1H-pyrazoleCatalog No.:AA000640 CAS No.:10199-69-6 MDL No.:MFCD12964229 MF:C10H10N2 MW:158.1998 |
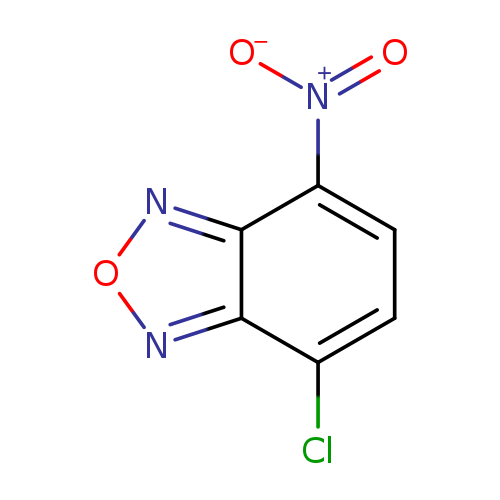
4-Chloro-7-nitrobenzofurazanCatalog No.:AA00063X CAS No.:10199-89-0 MDL No.:MFCD00005808 MF:C6H2ClN3O3 MW:199.5514 |
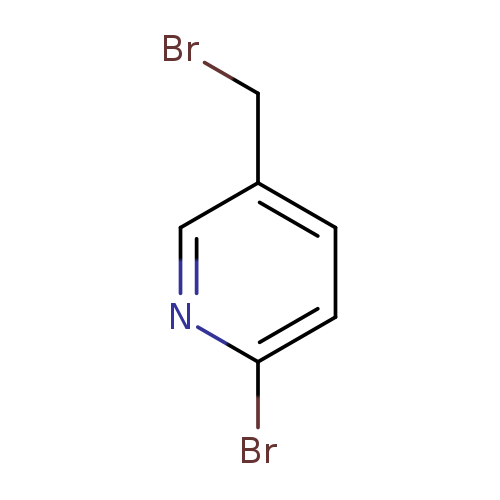
2-Bromo-5-(bromomethyl)pyridineCatalog No.:AA00063U CAS No.:101990-45-8 MDL No.:MFCD11656267 MF:C6H5Br2N MW:250.9186 |
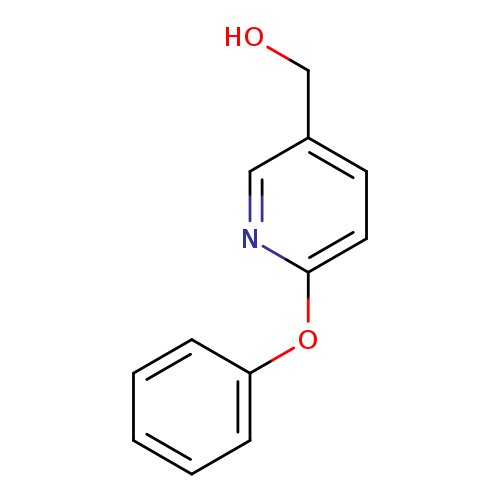
(6-Phenoxypyridin-3-yl)methanolCatalog No.:AA00063S CAS No.:101990-68-5 MDL No.:MFCD02682069 MF:C12H11NO2 MW:201.2212 |
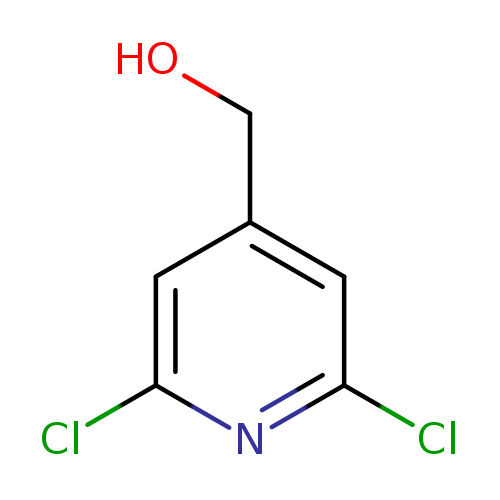
2,6-Dichloropyridine-4-methanolCatalog No.:AA00063R CAS No.:101990-69-6 MDL No.:MFCD00052638 MF:C6H5Cl2NO MW:178.0160 |
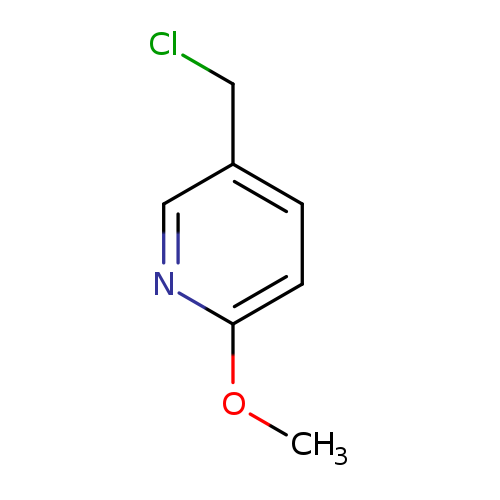
5-(Chloromethyl)-2-methoxypyridineCatalog No.:AA00063Q CAS No.:101990-70-9 MDL No.:MFCD10697570 MF:C7H8ClNO MW:157.5975 |
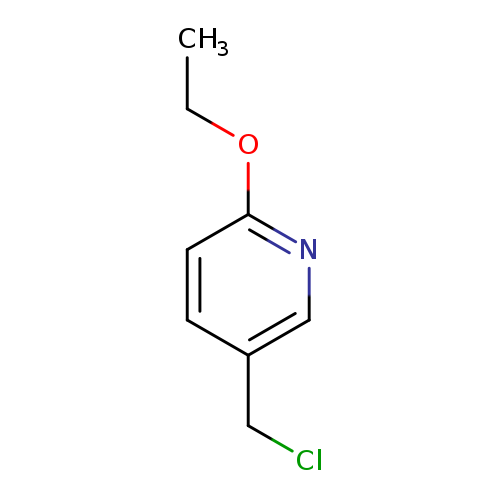
5-(Chloromethyl)-2-ethoxypyridineCatalog No.:AA009M1T CAS No.:101990-71-0 MDL No.:MFCD10697571 MF:C8H10ClNO MW:171.6241 |
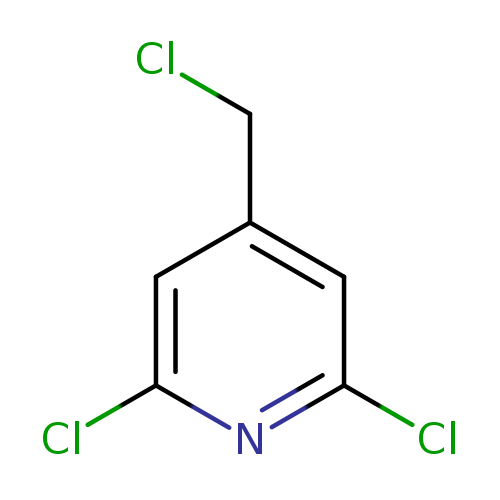
2,6-Dichloro-4-(chloromethyl)pyridineCatalog No.:AA00063P CAS No.:101990-72-1 MDL No.:MFCD03783562 MF:C6H4Cl3N MW:196.4617 |
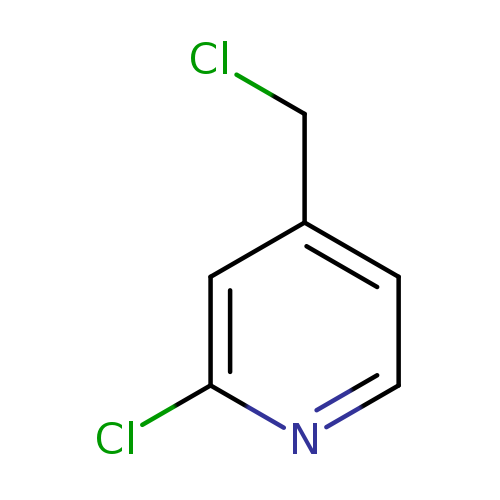
2-Chloro-4-(chloromethyl)pyridineCatalog No.:AA00063O CAS No.:101990-73-2 MDL No.:MFCD09991655 MF:C6H5Cl2N MW:162.0166 |
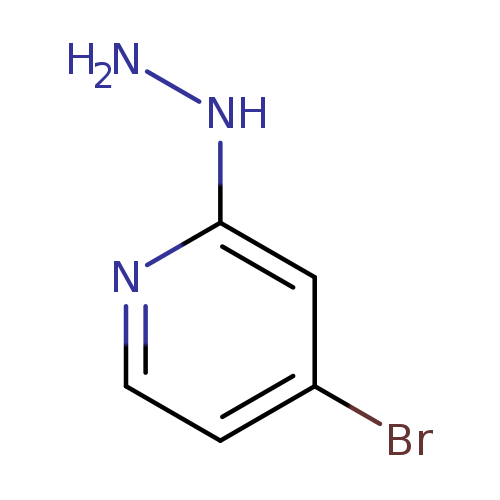
4-Bromo-2-hydrazinylpyridineCatalog No.:AA00063M CAS No.:1019918-39-8 MDL No.:MFCD00234091 MF:C5H6BrN3 MW:188.0252 |
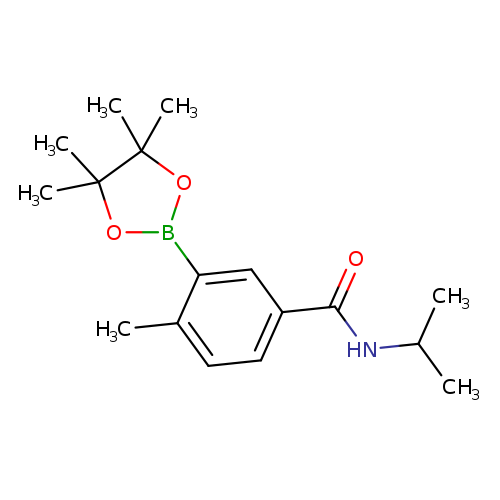
4-Methyl-N-(1-methylethyl)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamideCatalog No.:AA01FRFI CAS No.:1019918-74-1 MDL No.:MFCD22494193 MF:C17H26BNO3 MW:303.2042 |
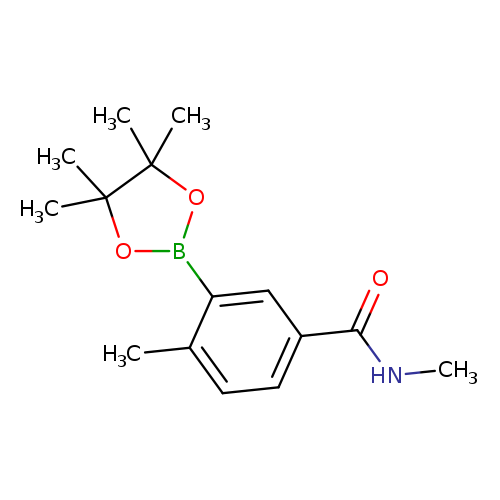
N,4-dimethyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamideCatalog No.:AA01FRFB CAS No.:1019918-76-3 MDL No.:MFCD18730378 MF:C15H22BNO3 MW:275.1511 |
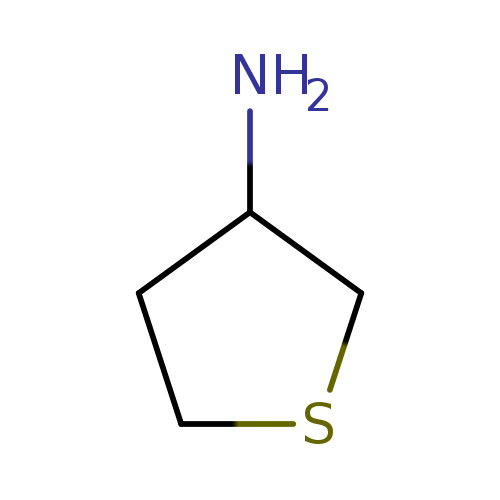
Tetrahydrothien-3-ylamineCatalog No.:AA008T5R CAS No.:101993-01-5 MDL No.:MFCD00120988 MF:C4H9NS MW:103.1860 |
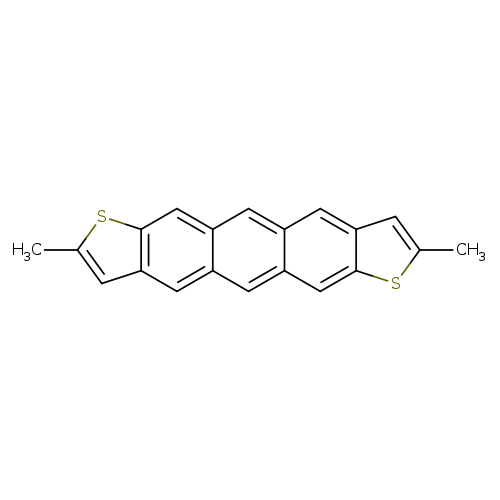
2,8-Dimethylanthra[2,3-b:6,7-b']dithiopheneCatalog No.:AA00IMD3 CAS No.:1019983-99-3 MDL No.:MFCD29905034 MF:C20H14S2 MW:318.4552 |

2-(7-Hydroxy-2-oxo-2H-chromen-4-yl)acetamideCatalog No.:AA00064N CAS No.:101999-45-5 MDL No.:MFCD06001840 MF:C11H9NO4 MW:219.1935 |
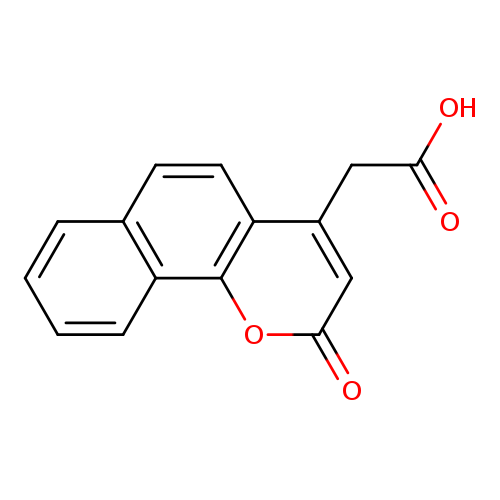
2H-Naphtho[1,2-b]pyran-4-acetic acid, 2-oxo-Catalog No.:AA00064M CAS No.:101999-46-6 MDL No.:MFCD00434035 MF:C15H10O4 MW:254.2375 |
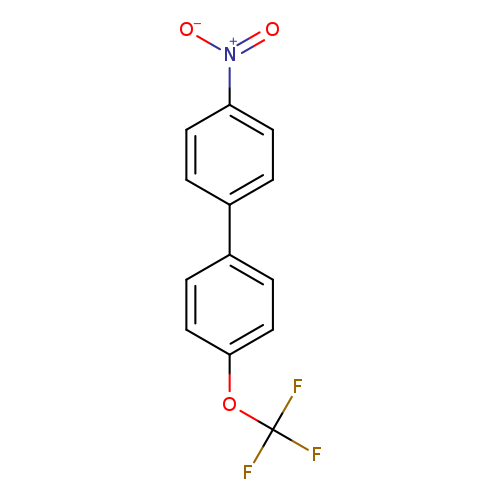
1-Nitro-4-[4-(trifluoromethoxy)phenyl]benzeneCatalog No.:AA00064Y CAS No.:1019996-86-1 MDL No.:MFCD21609608 MF:C13H8F3NO3 MW:283.2027 |
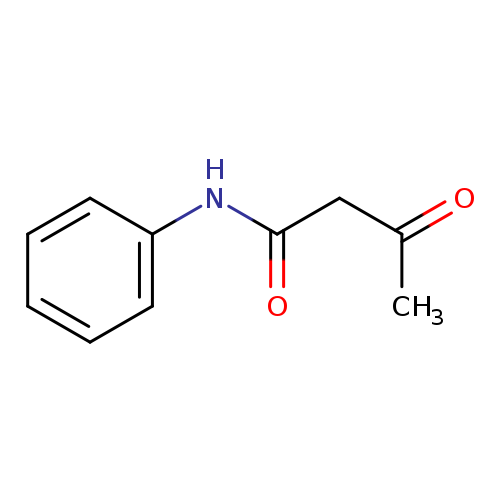
AcetoacetanilideCatalog No.:AA00065K CAS No.:102-01-2 MDL No.:MFCD00008780 MF:C10H11NO2 MW:177.1998 |
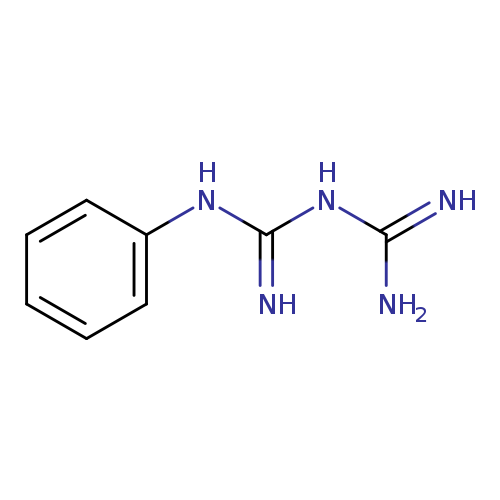
PhenylbiguanideCatalog No.:AA00065J CAS No.:102-02-3 MDL No.:MFCD01941521 MF:C8H11N5 MW:177.2064 |
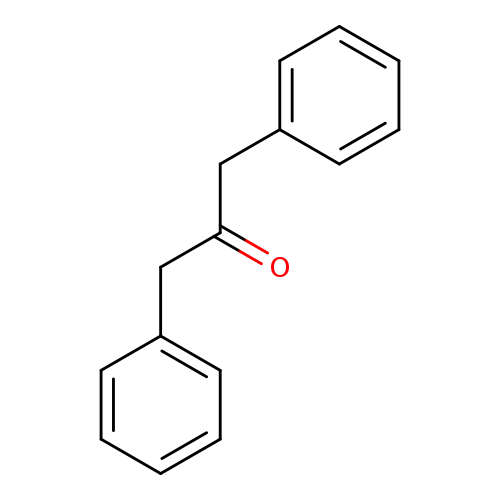
1,3-DiphenylacetoneCatalog No.:AA00065H CAS No.:102-04-5 MDL No.:MFCD00004795 MF:C15H14O MW:210.2711 |
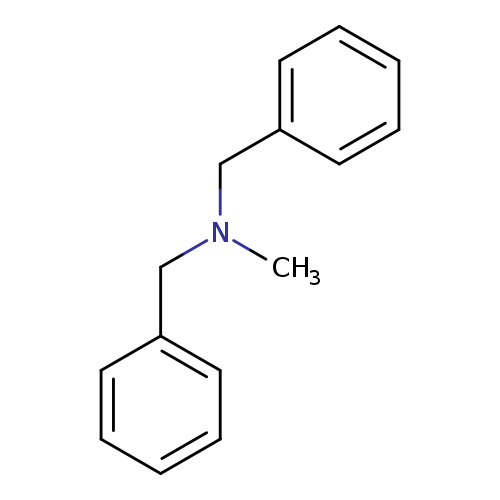
DibemethineCatalog No.:AA00065G CAS No.:102-05-6 MDL No.:MFCD00022018 MF:C15H17N MW:211.3022 |
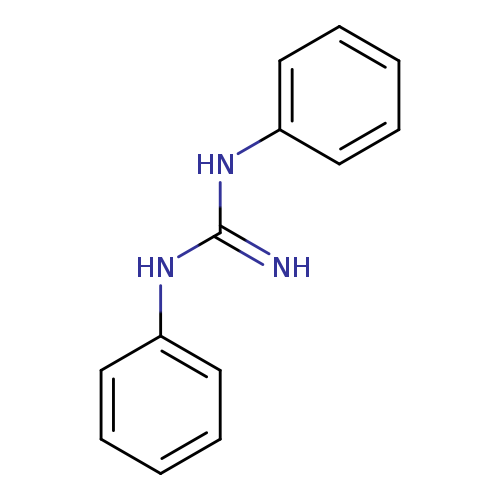
1,3-DiphenylguanidineCatalog No.:AA003DI7 CAS No.:102-06-7 MDL No.:MFCD00001758 MF:C13H13N3 MW:211.2624 |
1,3-DiphenylureaCatalog No.:AA00065F CAS No.:102-07-8 MDL No.:MFCD00003017 MF:C13H12N2O MW:212.2472 |
Thiourea, N,N'-diphenyl-Catalog No.:AA00065E CAS No.:102-08-9 MDL No.:MFCD00004921 MF:C13H12N2S MW:228.3128 |
Diphenyl carbonateCatalog No.:AA00065D CAS No.:102-09-0 MDL No.:MFCD00003037 MF:C13H10O3 MW:214.2167 |
N-Benzyl-n,n'-dimethylethylenediamineCatalog No.:AA00065B CAS No.:102-11-4 MDL No.:MFCD00014856 MF:C11H18N2 MW:178.2740 |
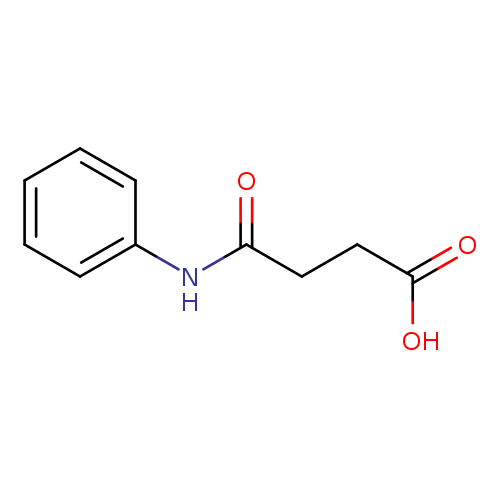
4-Anilino-4-oxobutanoic acidCatalog No.:AA000659 CAS No.:102-14-7 MDL No.:MFCD00029825 MF:C10H11NO3 MW:193.1992 |
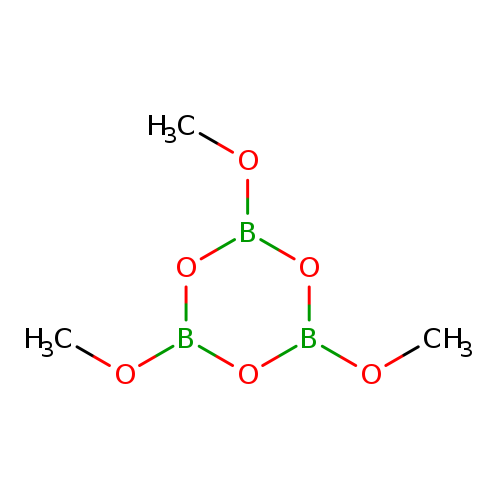
2,4,6-Trimethoxy-1,3,5,2,4,6-trioxatriborinaneCatalog No.:AA000652 CAS No.:102-24-9 MDL No.:MFCD00005946 MF:C3H9B3O6 MW:173.5330 |
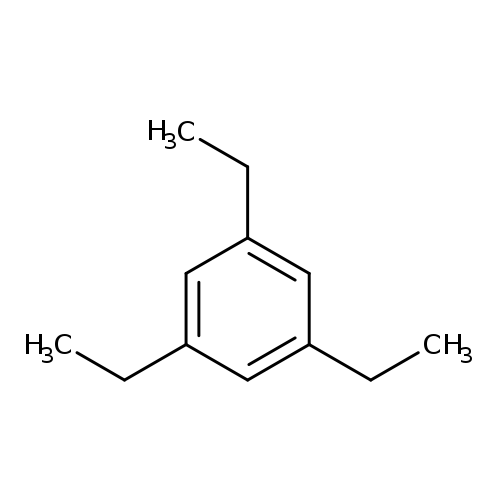
1,3,5-TriethylbenzeneCatalog No.:AA000651 CAS No.:102-25-0 MDL No.:MFCD00009261 MF:C12H18 MW:162.2713 |
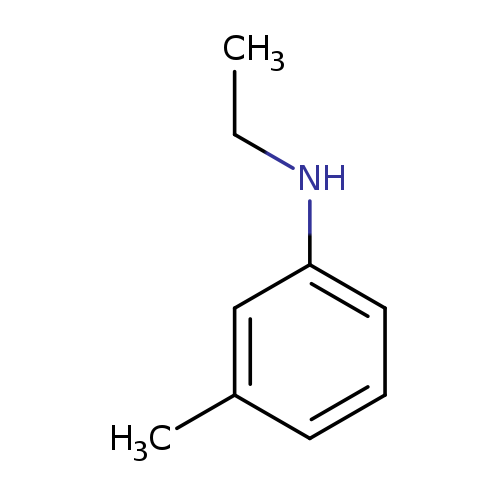
Benzenamine, N-ethyl-3-methyl-Catalog No.:AA000667 CAS No.:102-27-2 MDL No.:MFCD00009027 MF:C9H13N MW:135.2062 |

3'-AminoacetanilideCatalog No.:AA000666 CAS No.:102-28-3 MDL No.:MFCD02668755 MF:C8H10N2O MW:150.1778 |
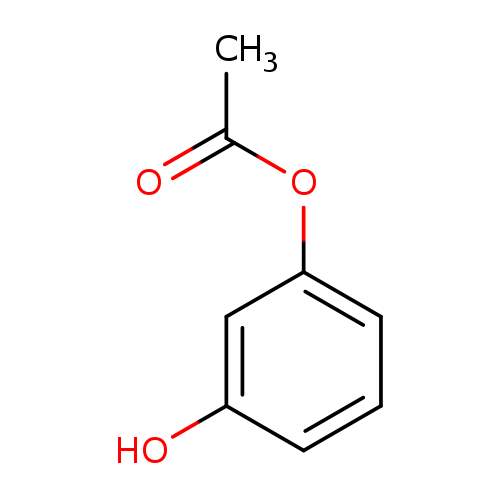
1,3-Benzenediol, 1-acetateCatalog No.:AA000665 CAS No.:102-29-4 MDL No.:MFCD00002266 MF:C8H8O3 MW:152.1473 |
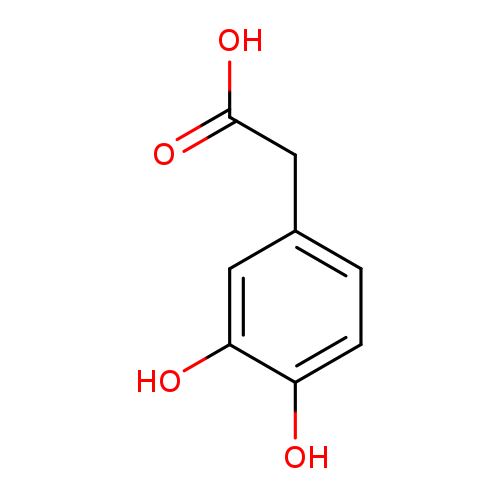
3,4-Dihydroxyphenylacetic acidCatalog No.:AA000664 CAS No.:102-32-9 MDL No.:MFCD00004338 MF:C8H8O4 MW:168.1467 |
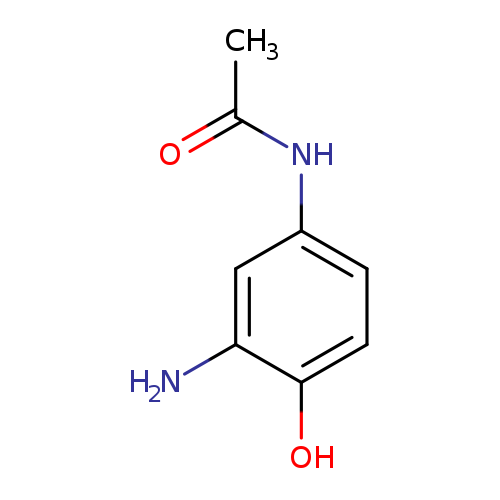
N-(3-Amino-4-hydroxyphenyl)acetamideCatalog No.:AA009QS3 CAS No.:102-33-0 MDL No.:MFCD20691146 MF:C8H10N2O2 MW:166.1772 |
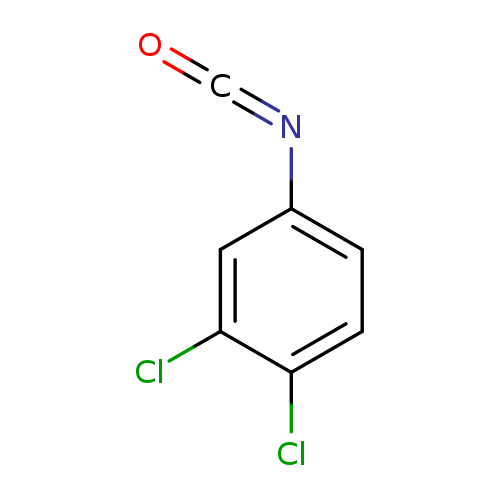
3,4-Dichlorophenyl isocyanateCatalog No.:AA000663 CAS No.:102-36-3 MDL No.:MFCD00002017 MF:C7H3Cl2NO MW:188.0108 |
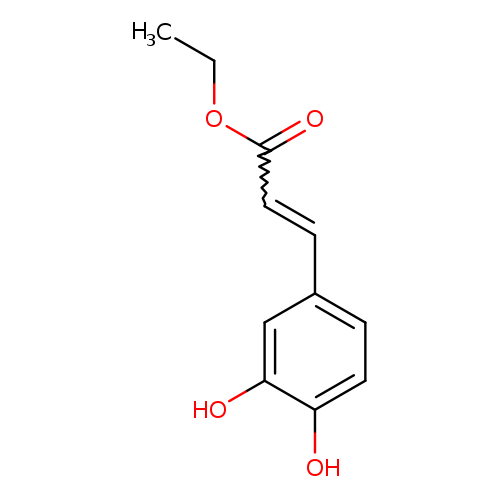
Ethyl CaffeateCatalog No.:AA000662 CAS No.:102-37-4 MDL No.:MFCD00045754 MF:C11H12O4 MW:208.2106 |
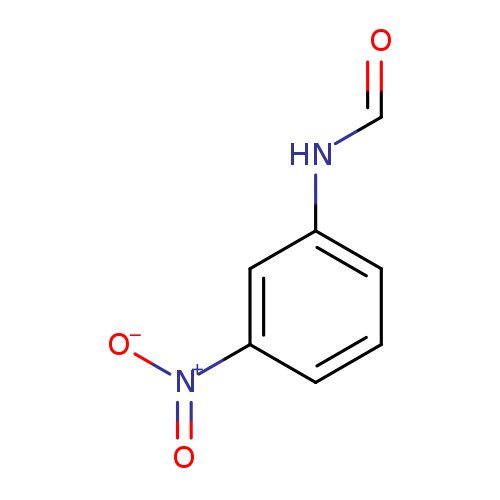
Formamide, N-(3-nitrophenyl)-Catalog No.:AA000661 CAS No.:102-38-5 MDL No.:MFCD00017014 MF:C7H6N2O3 MW:166.1341 |
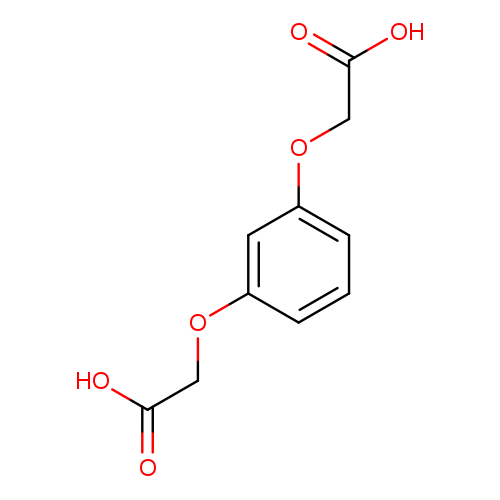
Acetic acid, 2,2'-[1,3-phenylenebis(oxy)]bis-Catalog No.:AA000660 CAS No.:102-39-6 MDL No.:MFCD00016696 MF:C10H10O6 MW:226.1828 |
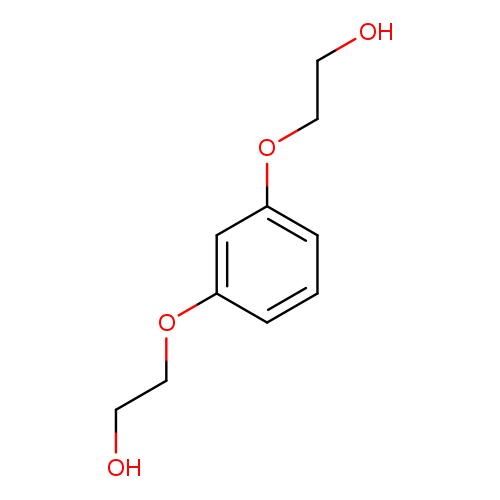
1,3-Bis(2-hydroxyethoxy)benzeneCatalog No.:AA003DEN CAS No.:102-40-9 MDL No.:MFCD00016566 MF:C10H14O4 MW:198.2158 |
1-Cyclopentyl-n-methylpropan-2-amineCatalog No.:AA00065Y CAS No.:102-45-4 MDL No.:MFCD00173872 MF:C9H19N MW:141.2539 |
4-(Chloromethyl)-1,2-dimethylbenzeneCatalog No.:AA00065X CAS No.:102-46-5 MDL No.:MFCD00000910 MF:C9H11Cl MW:154.6366 |
3,4-Dichlorobenzyl chlorideCatalog No.:AA00065W CAS No.:102-47-6 MDL No.:MFCD00000906 MF:C7H5Cl3 MW:195.4736 |
3,4-DimethylbenzylamineCatalog No.:AA00065V CAS No.:102-48-7 MDL No.:MFCD00025577 MF:C9H13N MW:135.2062 |
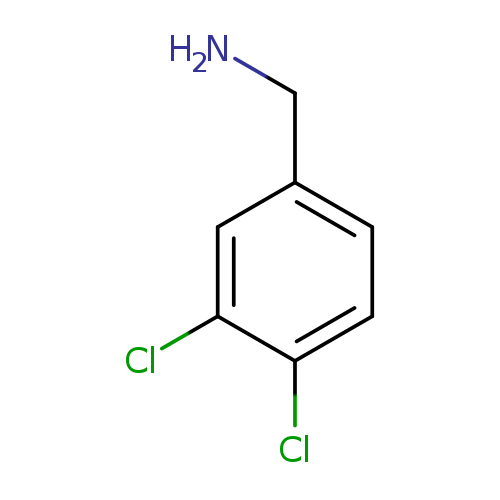
(3,4-Dichlorophenyl)methanamineCatalog No.:AA00065U CAS No.:102-49-8 MDL No.:MFCD00008114 MF:C7H7Cl2N MW:176.0432 |
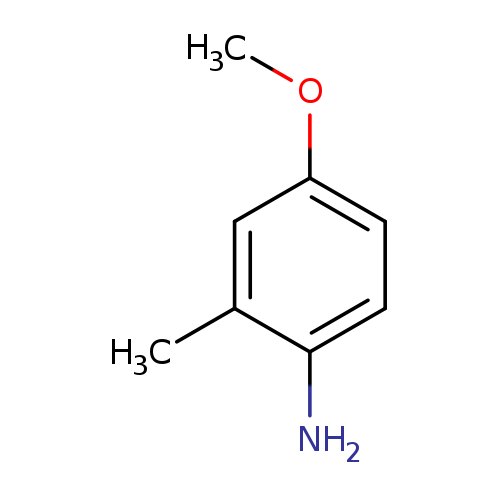
4-Methoxy-2-methylanilineCatalog No.:AA00065T CAS No.:102-50-1 MDL No.:MFCD00007735 MF:C8H11NO MW:137.1790 |
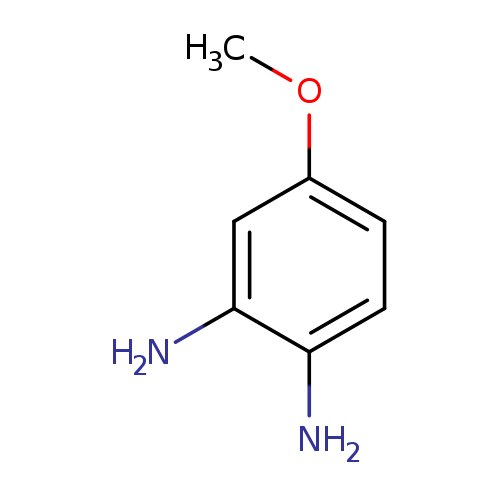
3,4-DiaminoanisoleCatalog No.:AA00065S CAS No.:102-51-2 MDL No.:MFCD00047837 MF:C7H10N2O MW:138.1671 |
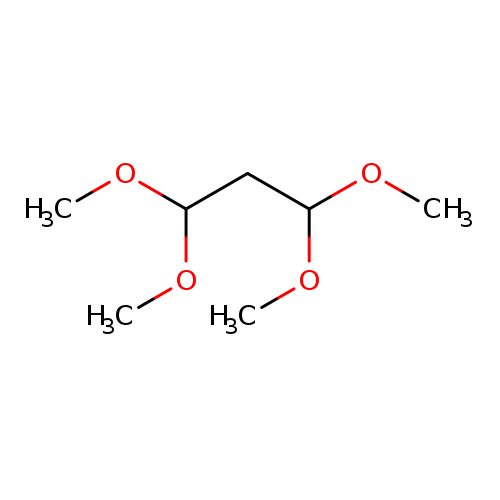
Propane, 1,1,3,3-tetramethoxy-Catalog No.:AA00065R CAS No.:102-52-3 MDL No.:MFCD00008488 MF:C7H16O4 MW:164.1995 |
Methanediamine, N,N,N',N'-tetraethyl-Catalog No.:AA00065Q CAS No.:102-53-4 MDL No.:MFCD00015115 MF:C9H22N2 MW:158.2844 |
FerroceneCatalog No.:AA00065P CAS No.:102-54-5 MDL No.:MFCD00001427 MF:C10H10Fe MW:186.0314 |

Benzenamine, 2,5-dimethoxy-Catalog No.:AA00065O CAS No.:102-56-7 MDL No.:MFCD00008368 MF:C8H11NO2 MW:153.1784 |
N,N,N',N'-Tetrakis(2-hydroxypropyl)ethylenediamineCatalog No.:AA003SDR CAS No.:102-60-3 MDL No.:MFCD00004534 MF:C14H32N2O4 MW:292.4149 |
3-Hydroxypropane-1,2-diyl diacetateCatalog No.:AA00065M CAS No.:102-62-5 MDL No.:MFCD00040498 MF:C7H12O5 MW:176.1672 |
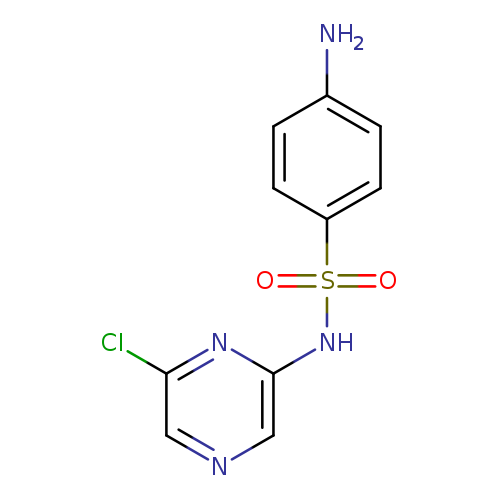
Sulfaclozine sodium monohydrateCatalog No.:AA00066T CAS No.:102-65-8 MDL No.:MFCD00868569 MF:C10H9ClN4O2S MW:284.7221 |
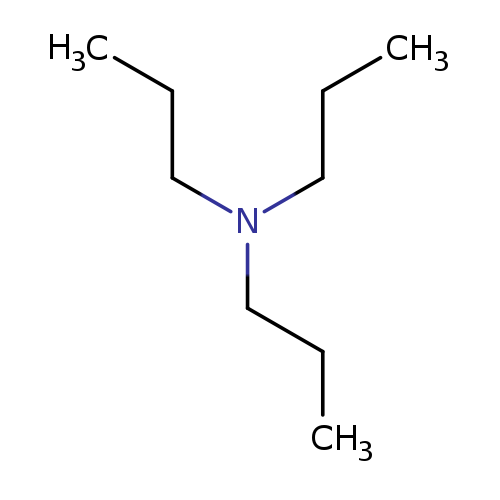
1-Propanamine, N,N-dipropyl-Catalog No.:AA00066S CAS No.:102-69-2 MDL No.:MFCD00009363 MF:C9H21N MW:143.2697 |
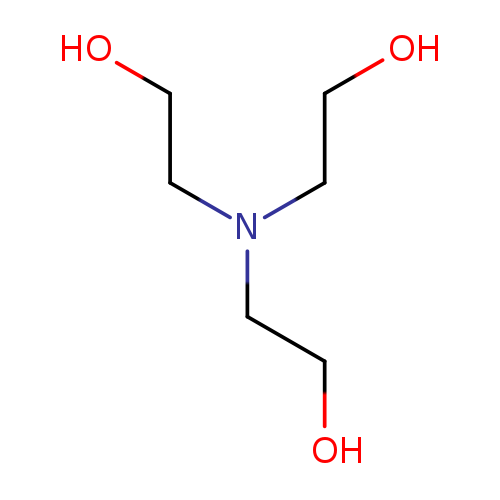
TriethanolamineCatalog No.:AA003UYI CAS No.:102-71-6 MDL No.:MFCD00002855 MF:C6H15NO3 MW:149.1882 |
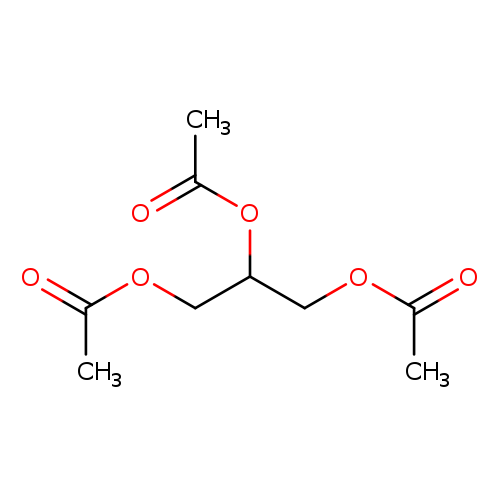
1,2,3-Propanetriol, 1,2,3-triacetateCatalog No.:AA00066Q CAS No.:102-76-1 MDL No.:MFCD00008716 MF:C9H14O6 MW:218.2039 |
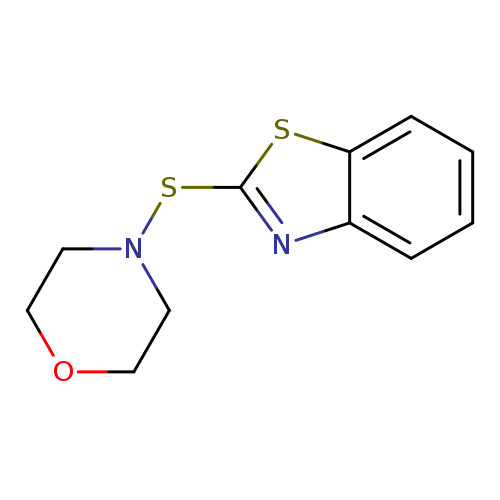
4-(Benzo[d]thiazol-2-ylthio)morpholineCatalog No.:AA003K35 CAS No.:102-77-2 MDL No.:MFCD00022870 MF:C11H12N2OS2 MW:252.3558 |
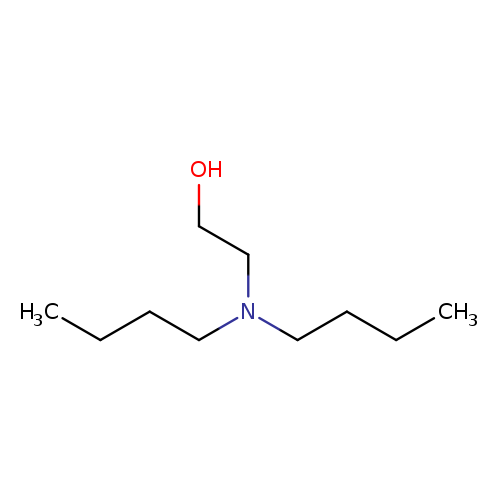
Ethanol, 2-(dibutylamino)-Catalog No.:AA00066P CAS No.:102-81-8 MDL No.:MFCD00014033 MF:C10H23NO MW:173.2957 |
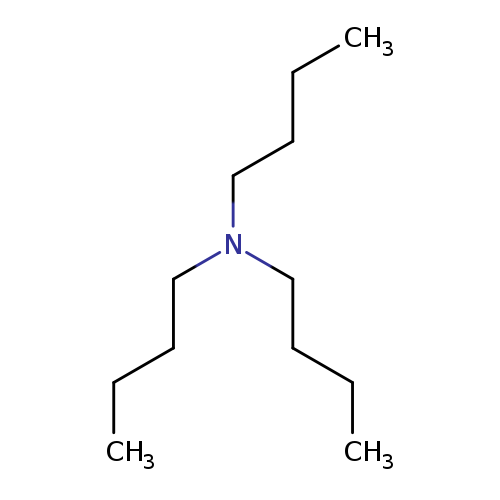
1-Butanamine, N,N-dibutyl-Catalog No.:AA00066O CAS No.:102-82-9 MDL No.:MFCD00009431 MF:C12H27N MW:185.3495 |
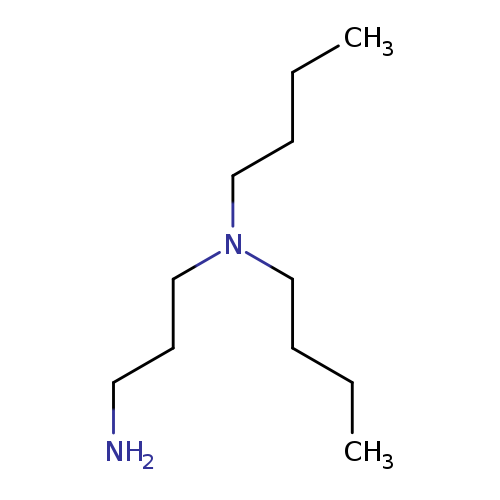
1,3-Propanediamine, N1,N1-dibutyl-Catalog No.:AA00066N CAS No.:102-83-0 MDL No.:MFCD00008219 MF:C11H26N2 MW:186.3375 |
Phosphorous acid, tributyl esterCatalog No.:AA00066L CAS No.:102-85-2 MDL No.:MFCD00009437 MF:C12H27O3P MW:250.3147 |
1-Hexanamine, N,N-dihexyl-Catalog No.:AA00066K CAS No.:102-86-3 MDL No.:MFCD00009523 MF:C18H39N MW:269.5090 |
TridodecylamineCatalog No.:AA00066J CAS No.:102-87-4 MDL No.:MFCD00008971 MF:C36H75N MW:521.9874 |
2-Propenoyl chloride, 3-phenyl-Catalog No.:AA00066H CAS No.:102-92-1 MDL No.:MFCD00000732 MF:C9H7ClO MW:166.6043 |
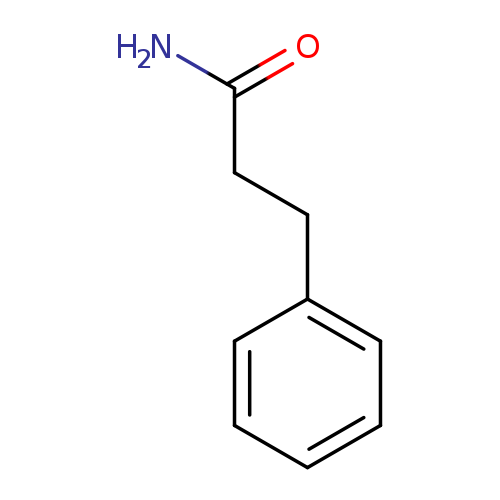
3-Phenyl-propionamideCatalog No.:AA00066G CAS No.:102-93-2 MDL No.:MFCD00025535 MF:C9H11NO MW:149.1897 |
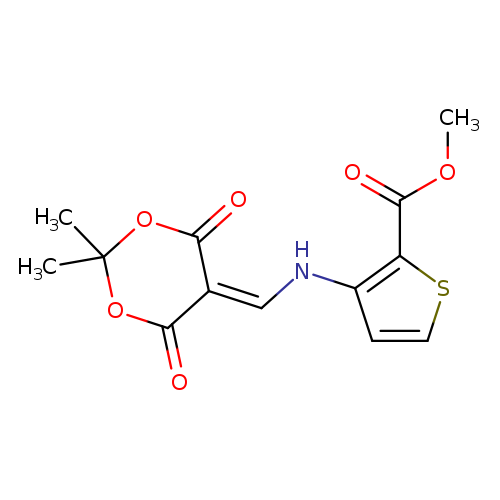
methyl 3-{[(2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ylidene)methyl]amino}thiophene-2-carboxylateCatalog No.:AA00IX53 CAS No.:1020252-21-4 MDL No.:MFCD00170561 MF:C13H13NO6S MW:311.3104 |
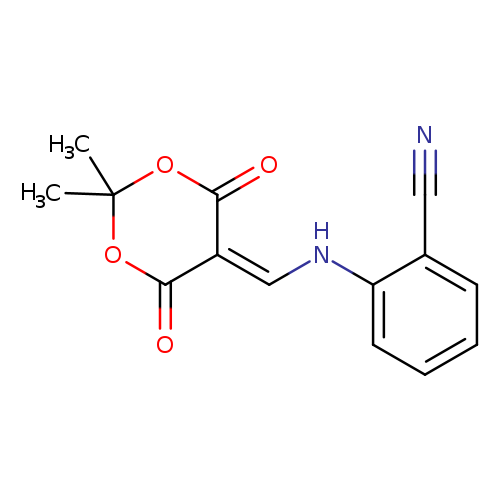
2-(((2,2-Dimethyl-4,6-dioxo-1,3-dioxan-5-ylidene)methyl)amino)benzonitrileCatalog No.:AA0093IE CAS No.:1020252-22-5 MDL No.:MFCD00170562 MF:C14H12N2O4 MW:272.2561 |
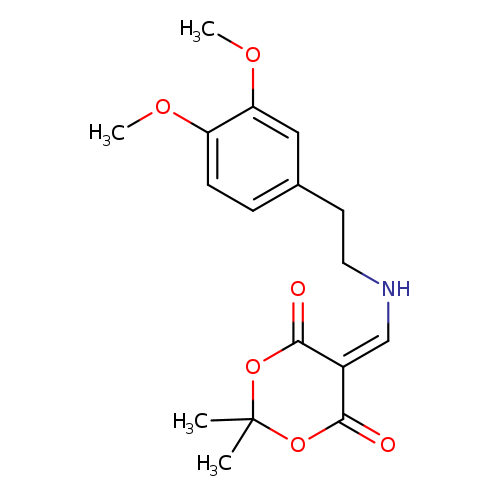
5-({[2-(3,4-dimethoxyphenyl)ethyl]amino}methylidene)-2,2-dimethyl-1,3-dioxane-4,6-dioneCatalog No.:AA00IX55 CAS No.:1020252-23-6 MDL No.:MFCD00129472 MF:C17H21NO6 MW:335.3517 |
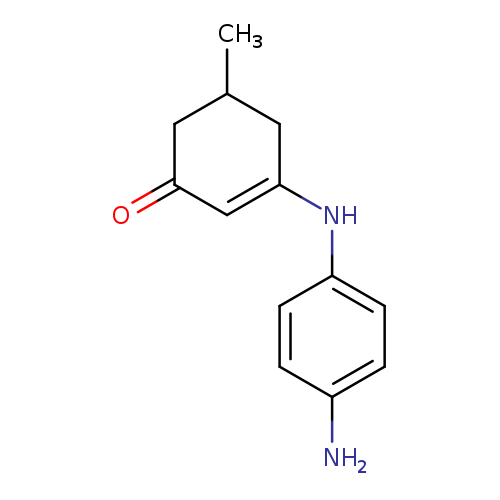
3-[(4-aminophenyl)amino]-5-methylcyclohex-2-en-1-oneCatalog No.:AA00IX56 CAS No.:1020252-24-7 MDL No.:MFCD00129488 MF:C13H16N2O MW:216.2789 |
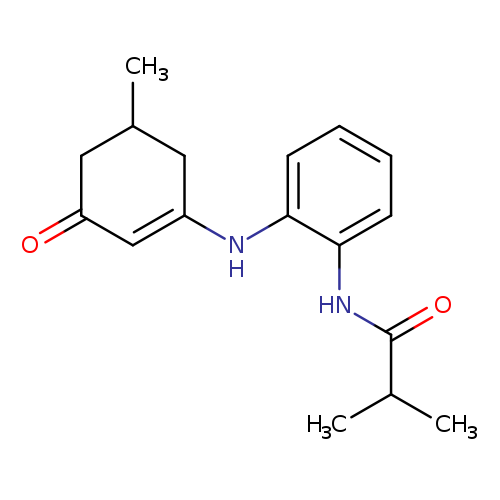
2-methyl-N-{2-[(5-methyl-3-oxocyclohex-1-en-1-yl)amino]phenyl}propanamideCatalog No.:AA00IT9W CAS No.:1020252-25-8 MDL No.:MFCD00137589 MF:C17H22N2O2 MW:286.3688 |
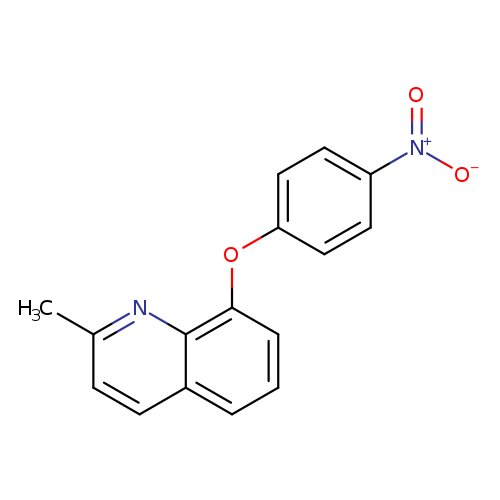
2-methyl-8-(4-nitrophenoxy)quinolineCatalog No.:AA00IT9Y CAS No.:1020252-26-9 MDL No.:MFCD00170581 MF:C16H12N2O3 MW:280.2781 |
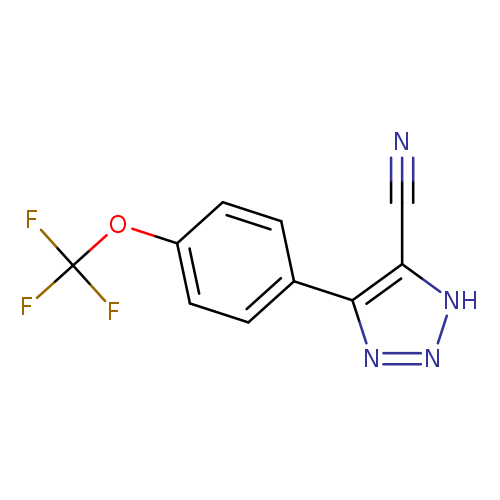
4-[4-(trifluoromethoxy)phenyl]-1H-1,2,3-triazole-5-carbonitrileCatalog No.:AA00IZWO CAS No.:1020252-27-0 MDL No.:MFCD00170589 MF:C10H5F3N4O MW:254.1681 |
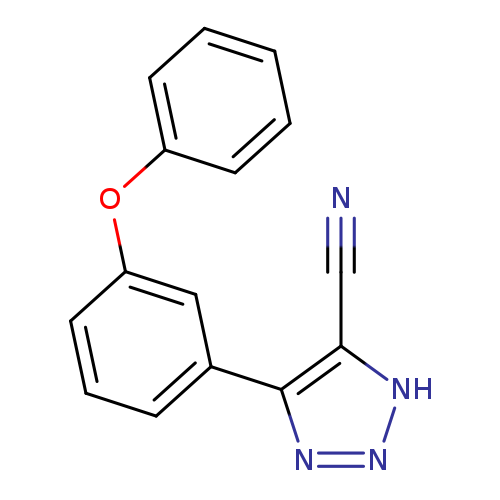
4-(3-Phenoxyphenyl)-1H-1,2,3-triazole-5-carbonitrileCatalog No.:AA00IX58 CAS No.:1020252-28-1 MDL No.:MFCD00170590 MF:C15H10N4O MW:262.2661 |
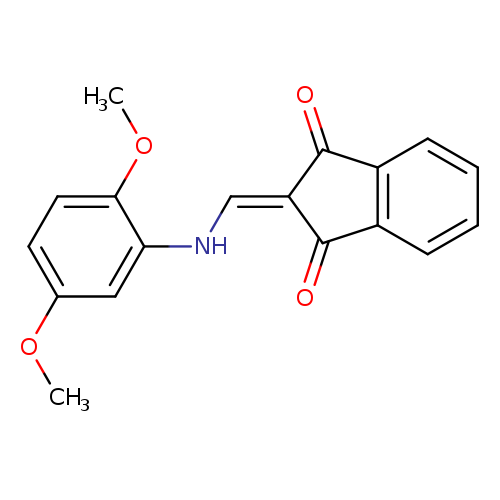
2-{[(2,5-dimethoxyphenyl)amino]methylidene}-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IV38 CAS No.:1020252-29-2 MDL No.:MFCD00170603 MF:C18H15NO4 MW:309.3160 |
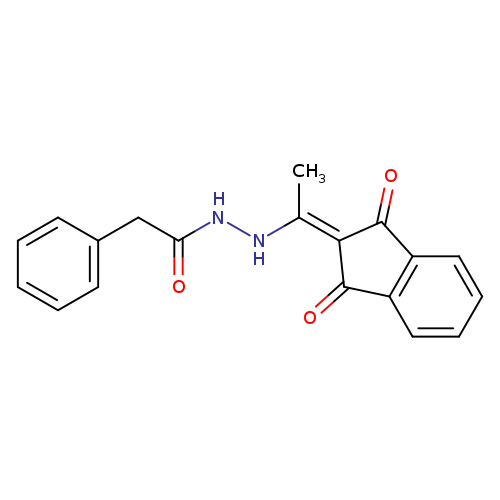
N'-[1-(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)ethyl]-2-phenylacetohydrazideCatalog No.:AA00ITA1 CAS No.:1020252-30-5 MDL No.:MFCD00170609 MF:C19H16N2O3 MW:320.3419 |
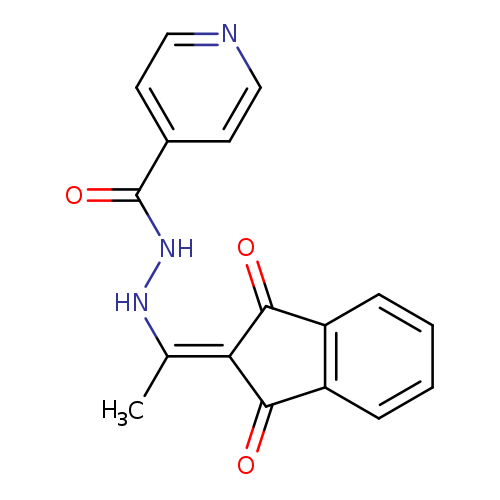
N'-[1-(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)ethyl]pyridine-4-carbohydrazideCatalog No.:AA00IV39 CAS No.:1020252-31-6 MDL No.:MFCD00170610 MF:C17H13N3O3 MW:307.3034 |
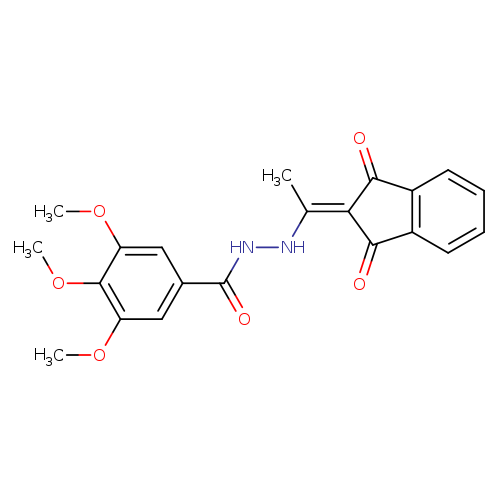
N'-[1-(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)ethyl]-3,4,5-trimethoxybenzohydrazideCatalog No.:AA00IX5B CAS No.:1020252-34-9 MDL No.:MFCD00170616 MF:C21H20N2O6 MW:396.3933 |
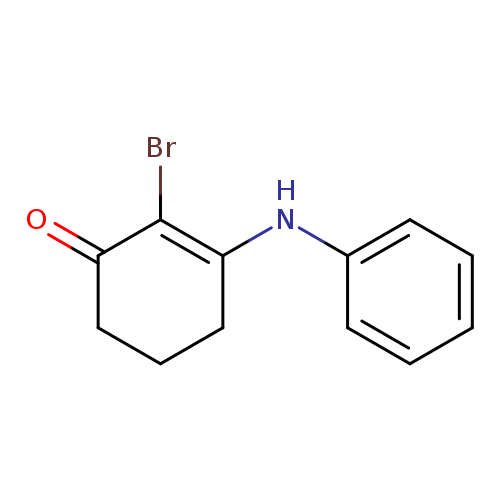
2-bromo-3-(phenylamino)cyclohex-2-en-1-oneCatalog No.:AA00IX5C CAS No.:1020252-35-0 MDL No.:MFCD00129273 MF:C12H12BrNO MW:266.1338 |
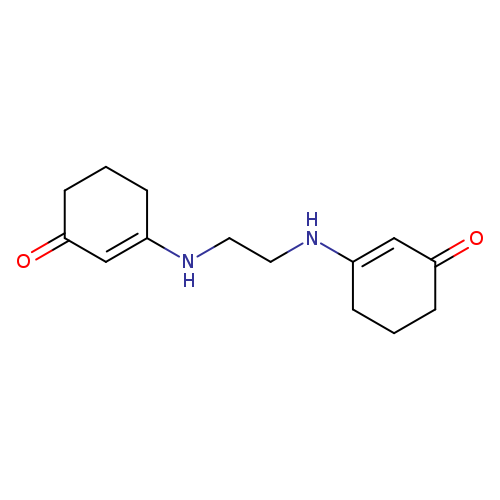
3-({2-[(3-oxocyclohex-1-en-1-yl)amino]ethyl}amino)cyclohex-2-en-1-oneCatalog No.:AA00IZWQ CAS No.:1020252-36-1 MDL No.:MFCD00129274 MF:C14H20N2O2 MW:248.3208 |
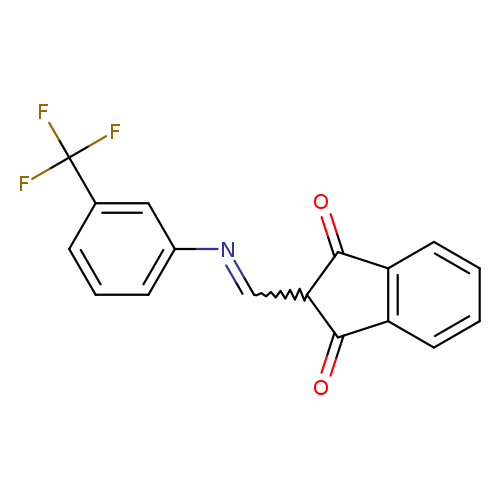
2-({[3-(trifluoromethyl)phenyl]imino}methyl)-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZWR CAS No.:1020252-37-2 MDL No.:MFCD00129310 MF:C17H10F3NO2 MW:317.2620 |
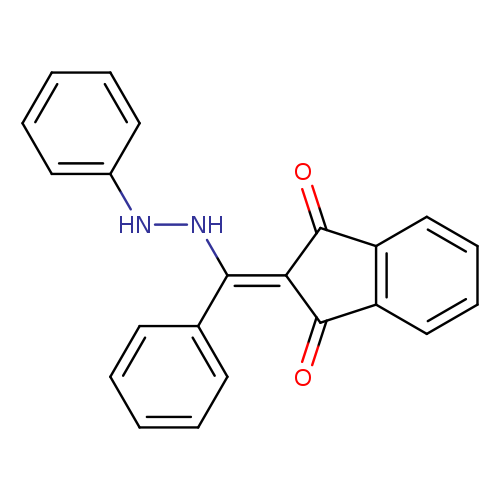
2-[phenyl(2-phenylhydrazin-1-yl)methylidene]-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZWT CAS No.:1020252-38-3 MDL No.:MFCD00129342 MF:C22H16N2O2 MW:340.3746 |
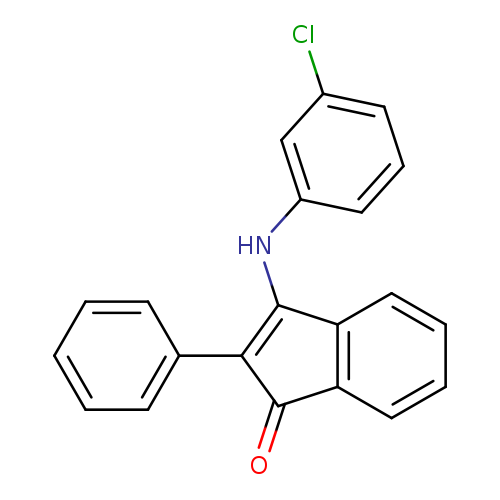
3-[(3-chlorophenyl)amino]-2-phenyl-1H-inden-1-oneCatalog No.:AA00IV3E CAS No.:1020252-39-4 MDL No.:MFCD00129561 MF:C21H14ClNO MW:331.7950 |
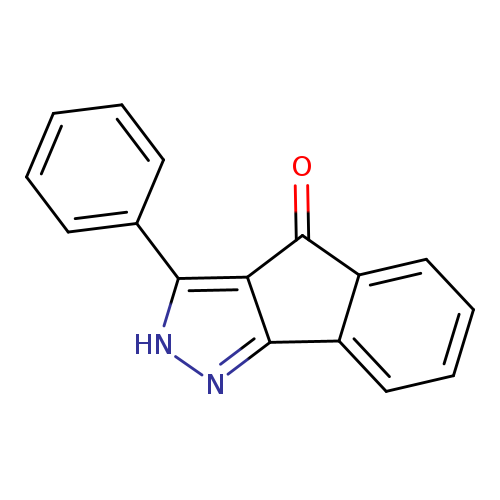
3-phenyl-2H,4H-indeno[1,2-c]pyrazol-4-oneCatalog No.:AA00IX5D CAS No.:1020252-40-7 MDL No.:MFCD00170634 MF:C16H10N2O MW:246.2634 |
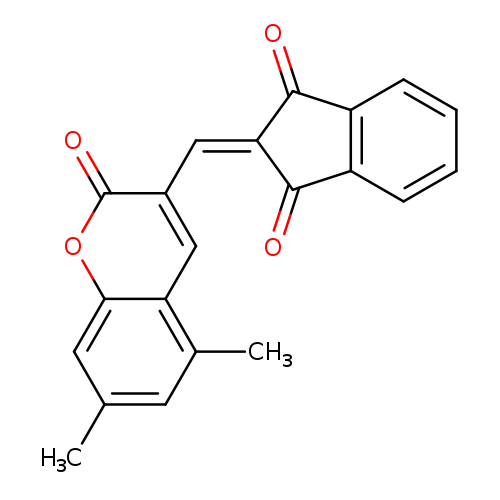
2-[(5,7-dimethyl-2-oxo-2H-chromen-3-yl)methylidene]-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZWV CAS No.:1020252-45-2 MDL No.:MFCD00170651 MF:C21H14O4 MW:330.3335 |
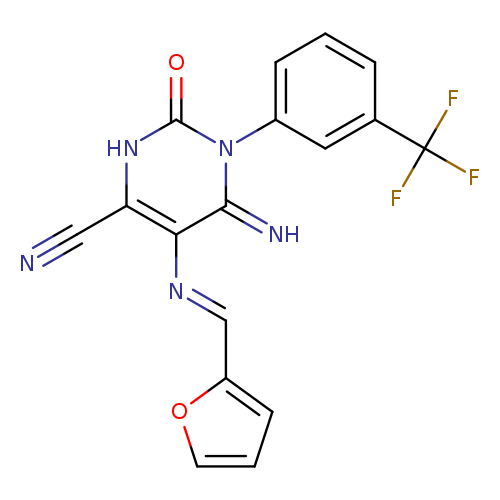
5-[(E)-[(furan-2-yl)methylidene]amino]-6-imino-2-oxo-1-[3-(trifluoromethyl)phenyl]-1,2,3,6-tetrahydropyrimidine-4-carbonitrileCatalog No.:AA00IX5E CAS No.:1020252-46-3 MDL No.:MFCD00170656 MF:C17H10F3N5O2 MW:373.2888 |
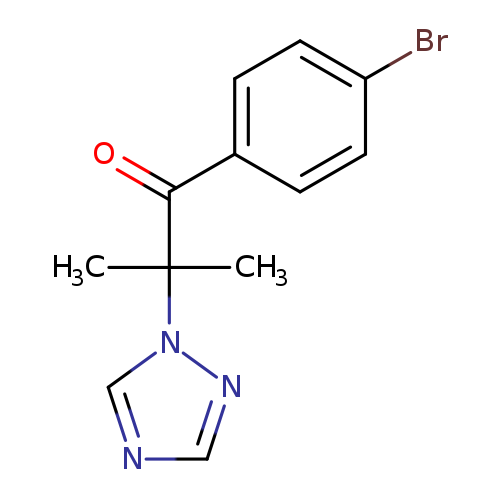
1-(4-bromophenyl)-2-methyl-2-(1H-1,2,4-triazol-1-yl)propan-1-oneCatalog No.:AA00IX5F CAS No.:1020252-47-4 MDL No.:MFCD00170657 MF:C12H12BrN3O MW:294.1472 |
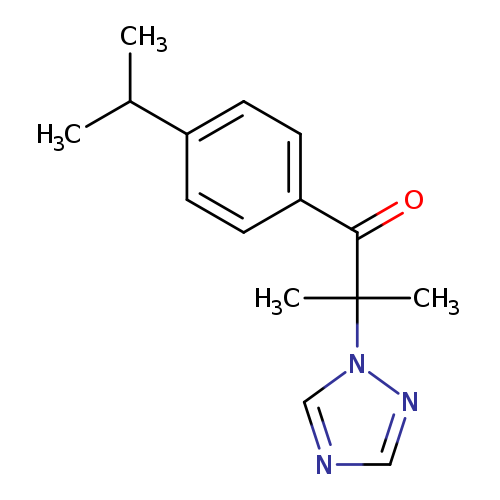
2-methyl-1-[4-(propan-2-yl)phenyl]-2-(1H-1,2,4-triazol-1-yl)propan-1-oneCatalog No.:AA00IX5G CAS No.:1020252-48-5 MDL No.:MFCD00170667 MF:C15H19N3O MW:257.3309 |
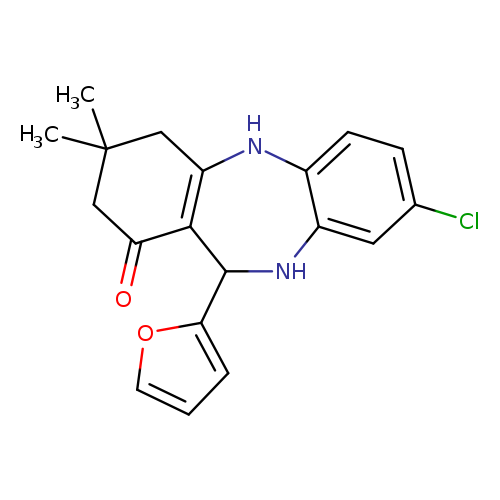
6-chloro-10-(furan-2-yl)-14,14-dimethyl-2,9-diazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6-tetraen-12-oneCatalog No.:AA00IV3G CAS No.:1020252-51-0 MDL No.:MFCD00170679 MF:C19H19ClN2O2 MW:342.8194 |
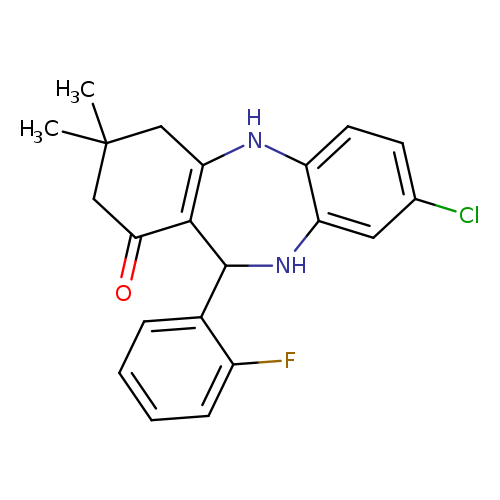
6-chloro-10-(2-fluorophenyl)-14,14-dimethyl-2,9-diazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6-tetraen-12-oneCatalog No.:AA00IV3H CAS No.:1020252-52-1 MDL No.:MFCD00170680 MF:C21H20ClFN2O MW:370.8477 |
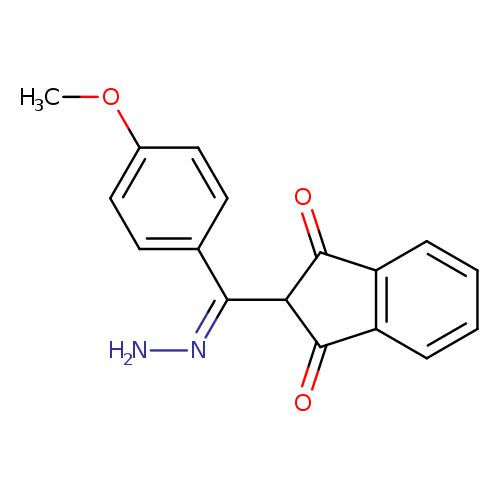
2-[(4-methoxyphenyl)methanehydrazonoyl]-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00ITA4 CAS No.:1020252-54-3 MDL No.:MFCD03839366 MF:C17H14N2O3 MW:294.3047 |
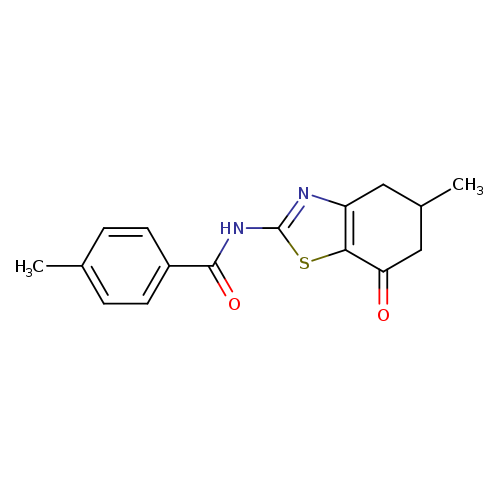
4-methyl-N-(5-methyl-7-oxo-4,5,6,7-tetrahydro-1,3-benzothiazol-2-yl)benzamideCatalog No.:AA00ITA5 CAS No.:1020252-55-4 MDL No.:MFCD00170702 MF:C16H16N2O2S MW:300.3754 |
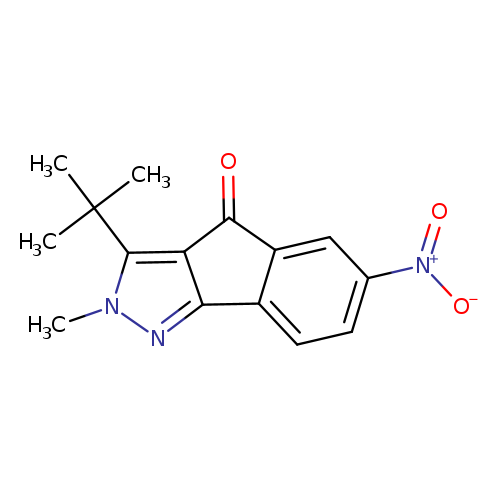
3-tert-butyl-2-methyl-6-nitro-2H,4H-indeno[1,2-c]pyrazol-4-oneCatalog No.:AA00IX5J CAS No.:1020252-56-5 MDL No.:MFCD00129304 MF:C15H15N3O3 MW:285.2979 |
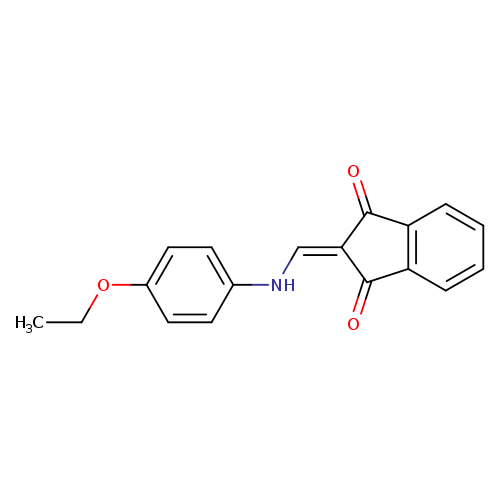
2-{[(4-ethoxyphenyl)amino]methylidene}-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IV3I CAS No.:1020252-57-6 MDL No.:MFCD00129323 MF:C18H15NO3 MW:293.3166 |
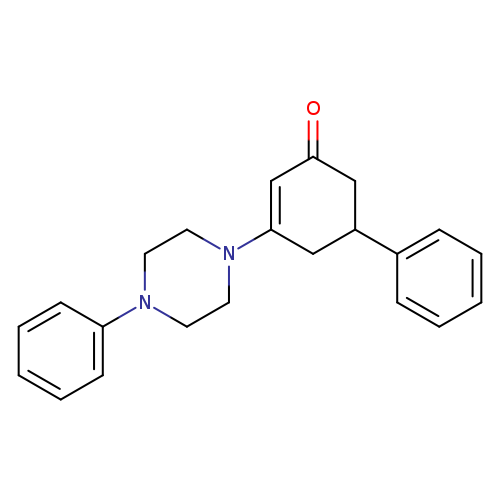
5-phenyl-3-(4-phenylpiperazin-1-yl)cyclohex-2-en-1-oneCatalog No.:AA00IV3J CAS No.:1020252-58-7 MDL No.:MFCD00137588 MF:C22H24N2O MW:332.4388 |
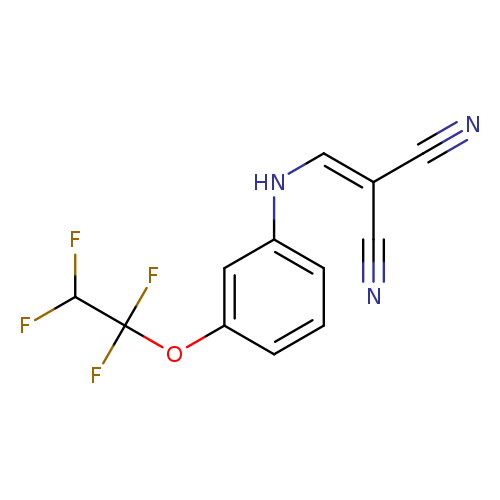
2-({[3-(1,1,2,2-tetrafluoroethoxy)phenyl]amino}methylidene)propanedinitrileCatalog No.:AA00IV3K CAS No.:1020252-59-8 MDL No.:MFCD00129615 MF:C12H7F4N3O MW:285.1971 |
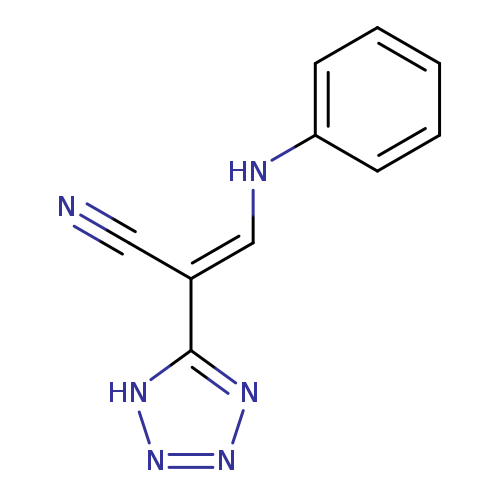
(2E)-3-(phenylamino)-2-(1H-1,2,3,4-tetrazol-5-yl)prop-2-enenitrileCatalog No.:AA00IV3L CAS No.:1020252-60-1 MDL No.:MFCD00129614 MF:C10H8N6 MW:212.2107 |
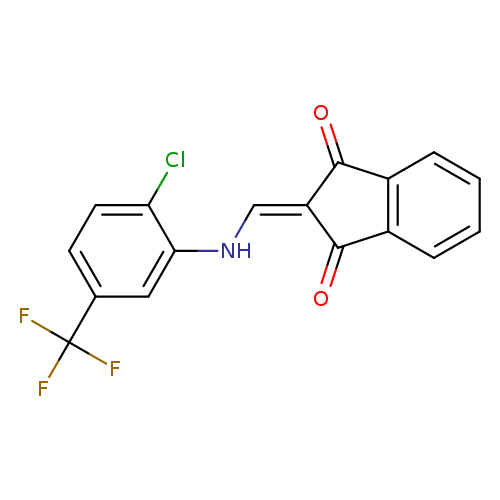
2-({[2-chloro-5-(trifluoromethyl)phenyl]amino}methylidene)-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZWY CAS No.:1020252-62-3 MDL No.:MFCD00129462 MF:C17H9ClF3NO2 MW:351.7071 |
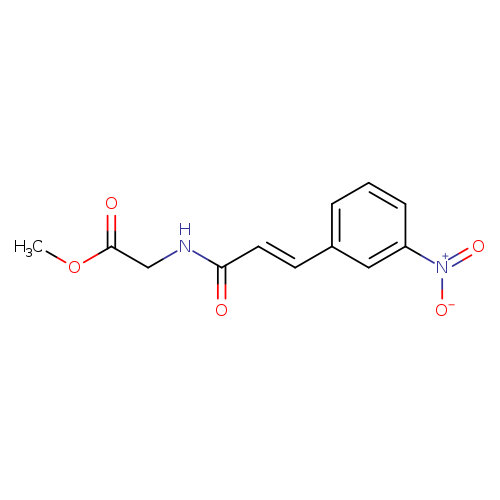
methyl 2-[(2E)-3-(3-nitrophenyl)prop-2-enamido]acetateCatalog No.:AA00ITA8 CAS No.:1020252-63-4 MDL No.:MFCD00170734 MF:C12H12N2O5 MW:264.2341 |
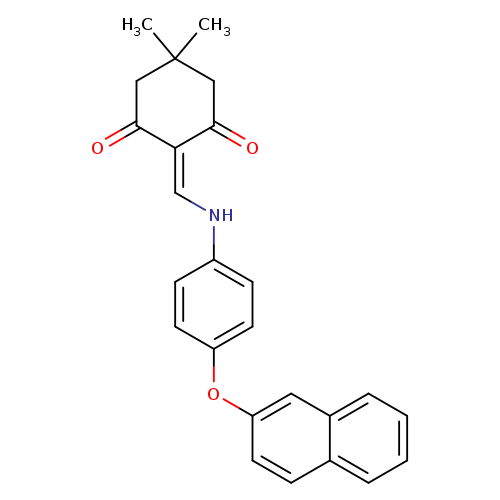
5,5-dimethyl-2-({[4-(naphthalen-2-yloxy)phenyl]amino}methylidene)cyclohexane-1,3-dioneCatalog No.:AA00IX5M CAS No.:1020252-64-5 MDL No.:MFCD00170738 MF:C25H23NO3 MW:385.4550 |
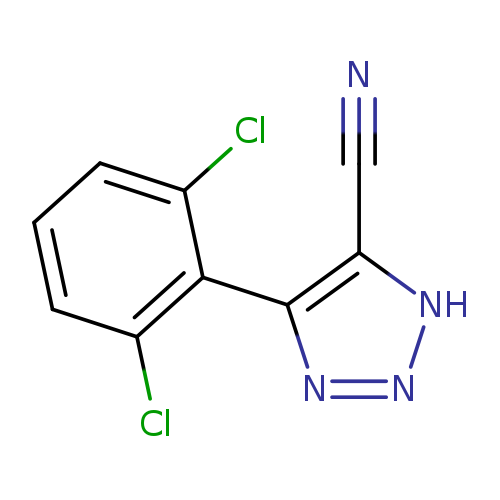
4-(2,6-dichlorophenyl)-1H-1,2,3-triazole-5-carbonitrileCatalog No.:AA00IV3M CAS No.:1020252-66-7 MDL No.:MFCD00170744 MF:C9H4Cl2N4 MW:239.0609 |
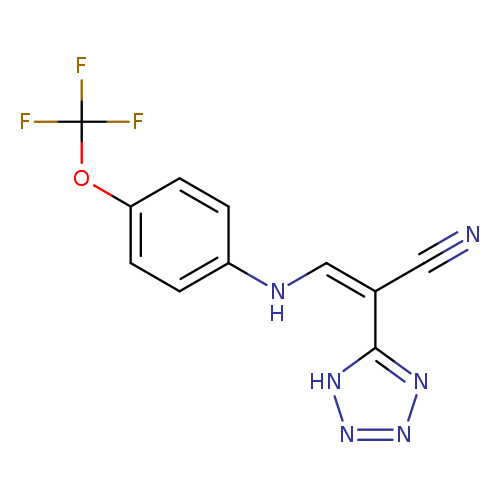
(2Z)-2-(1H-1,2,3,4-tetrazol-5-yl)-3-{[4-(trifluoromethoxy)phenyl]amino}prop-2-enenitrileCatalog No.:AA00ITA9 CAS No.:1020252-67-8 MDL No.:MFCD00170751 MF:C11H7F3N6O MW:296.2081 |
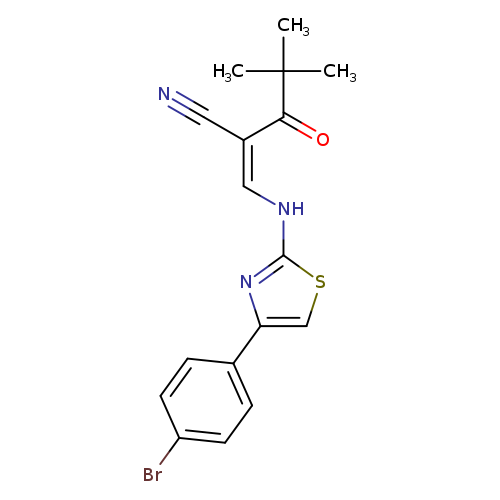
(2Z)-2-({[4-(4-bromophenyl)-1,3-thiazol-2-yl]amino}methylidene)-4,4-dimethyl-3-oxopentanenitrileCatalog No.:AA00IX5N CAS No.:1020252-68-9 MDL No.:MFCD00170753 MF:C17H16BrN3OS MW:390.2974 |
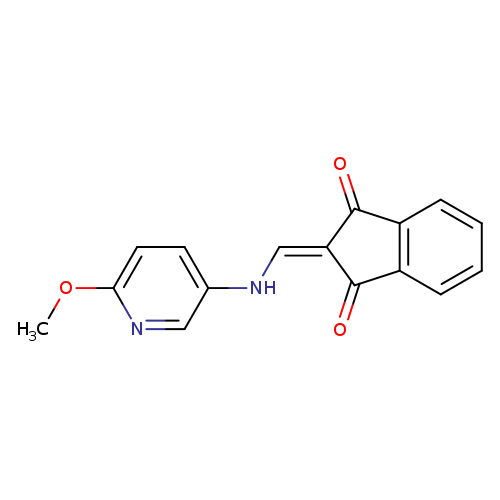
2-{[(6-methoxypyridin-3-yl)amino]methylidene}-2,3-dihydro-1H-indene-1,3-dioneCatalog No.:AA00IZX3 CAS No.:1020252-69-0 MDL No.:MFCD00170764 MF:C16H12N2O3 MW:280.2781 |
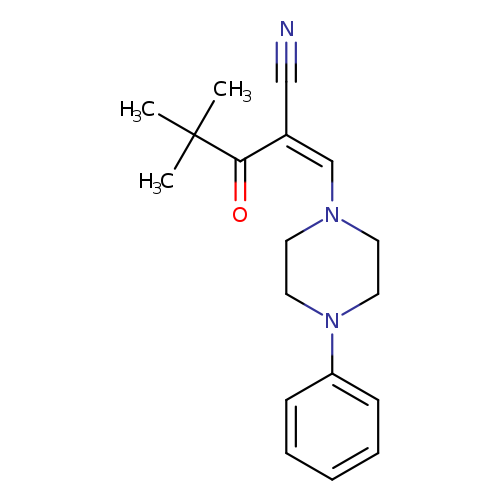
(2Z)-4,4-dimethyl-3-oxo-2-[(4-phenylpiperazin-1-yl)methylidene]pentanenitrileCatalog No.:AA00IZX5 CAS No.:1020252-72-5 MDL No.:MFCD00170779 MF:C18H23N3O MW:297.3947 |