2019-12-08 09:19:52
Xiao-Yu Liu and Yong Qin
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Sichuan Engineering Laboratory for
Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy,
Sichuan University, Chengdu 610041, China
1. INTRODUCTION
Indole alkaloids belong to one of the largest families of secondary metabolites created by nature.1 As a unique category of these molecules, the monoterpene indole alkaloids biogenetically originate from tryptamine and secologanin (Figure 1).2 Condensation of these two fragments via an enzymatic Pictet−Spengler reaction affords strictosidine; diversification of the latter common intermediate results in
over 40 structural types and more than 3000 members of monoterpene indole alkaloids.1 Of particular note, many of these compounds exhibit a wide range of bioactivities, and molecules such as reserpine (antihypertensive), vincamine (nootropic), quinine (antimalaria), and vinblastine/vincristine (antitumor) have been used as important drugs.
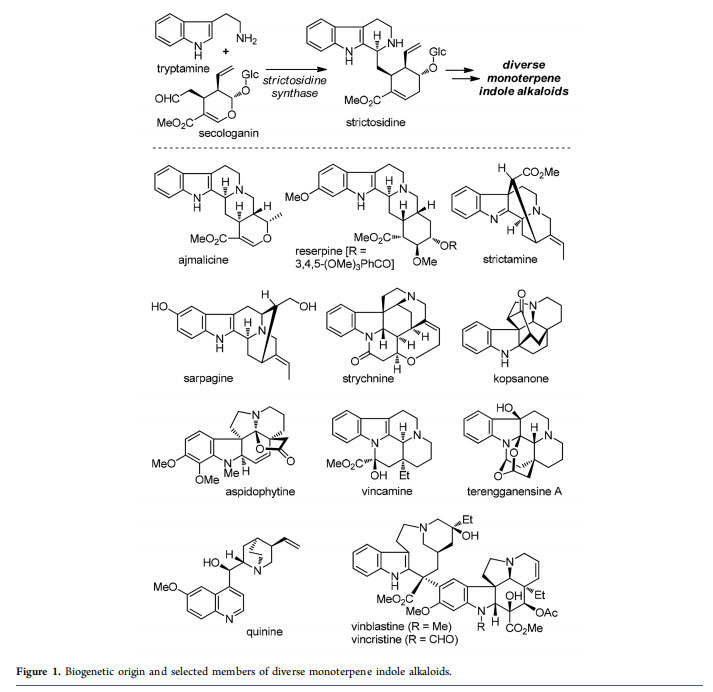
Due to their significant biological profiles and intricate structures, various monoterpene indole alkaloids have long challenged synthetic chemists. Such synthetic campaigns could be traced back to as early as the 1950s, when Woodward and co-workers first conquered strychnine4 and reserpine. Since then, the structural diversity and complexity of monoterpene indole alkaloids have made them important testing grounds for synthetic methodologies and tactics.6 Traditionally, many elegant approaches were documented but were only suitable for the synthesis of individual molecules or small groups of molecules from this natural product family. However, efficient access to large collections of complex indole alkaloids is always desirable and would call for a breakthrough in developing innovative synthetic methods and strategies.
Visible-light-mediated photoredox chemistry has been an active research field in the most recent decade and offers chemists new opportunities to form chemical bonds.7 Nevertheless, these single electron transfer (SET) processes have only begun to benefit natural product total synthesis,8,9 and they were mostly employed to realize single transformations. Design of visible light photoinduced radical cascade reactions to facilitate multiple bond-forming events in one pot would allow chemists to assemble the cores of natural products as well as to achieve their total synthesis in highly efficient fashion.
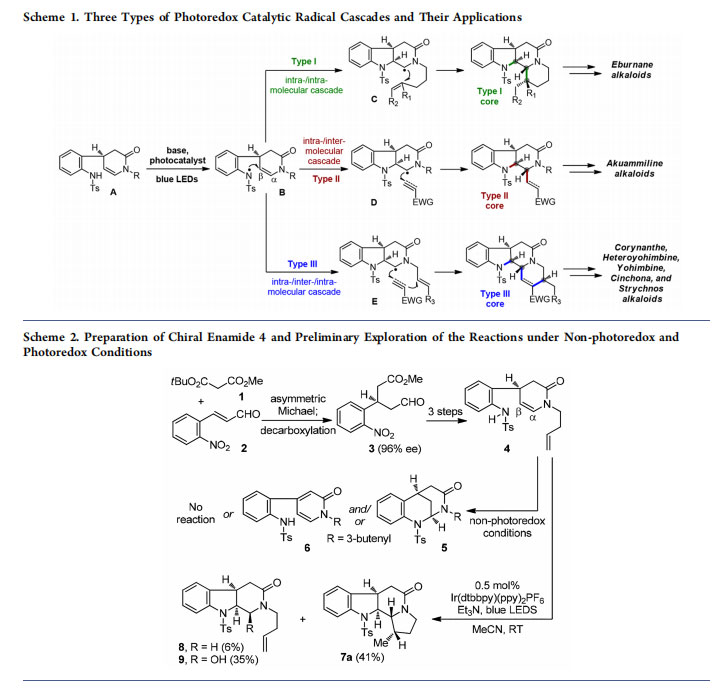
Stemming from a long-standing interest in the synthesis of intriguing indole alkaloids,10,11 our group recently developed a new photoredox catalytic radical cascade strategy that led to the asymmetric total synthesis of a collection of monoterpene indole alkaloids.12−16 As illustrated in Scheme 1, treating the sulfonamide substrate A with base and photocatalyst under the irradiation of blue light-emitting diodes (LEDs) generated a key nitrogen-centered radical B, which could be subsequently involved in further bond-forming events. Specifically, we designed three types of radical cascade pathways [intra-/ intramolecular, intra-/intermolecular, and intra-/inter-/intramolecular] and realized facile construction of the aspidosperma (type I, via C), tetrahydrocarbolinone (type II, via D), and corynanthe (type III, via E) core structures. While some of the obtained compounds already possessed the frameworks of indole alkaloids, the versatile functionalities in these cores could be manipulated to access more alkaloid types. As a result, harnessing the three types of photocatalytic radical cascades as key reactions allowed the asymmetric total synthesis of 42 monoterpene indole alkaloids belonging to 7 structural types.12−16 This Account summarizes the radical cascade methodologies developed in our laboratory and their applications in natural product total synthesis.
2. TYPE I RADICAL CASCADE AND ITS APPLICATIONS IN TOTAL SYNTHESIS
2.1. Development of the Type I Radical Cascade Reaction
Bearing in mind the idea of developing a radical cascade approach to indole-alkaloid skeletons, we initiated our studies with the preparation of chiral substrate 4 (Scheme 2) from tertbutyl methyl malonate (1) and o-nitrocinnamic aldehyde (2). The organocatalytic asymmetric Michael addition of commercially available 1 to 2 followed by decarboxylation yielded chiral aldehyde ester 3 (96% ee),17 which was readily conducted on a 500 g scale in our laboratory. Compound 3 was then converted to 4 in three steps. By subjecting sulfonamide 4 to various non-photoredox conditions, we observed the cyclization product (tetrahydroquinoline 5) or oxidation product (pyridinone 6) or no reaction. Generation of tetrahydroquinoline 5 was, not surprisingly, the result of a C−N bond formation between the aniline nitrogen and the electron-deficient α-carbon of the enamide functionality. To reach the desired indoline product, a C−N bond formation at the β-position of the enamide was required. Classical methods
for the generation of nitrogen-centered radicals18 from N− heteroatom bonds or directly from N−H bonds under harsh oxidative conditions were neither economic nor environmentally benign, which also proved to be unsuitable for our substrate. After much experimentation, we were delighted to isolate the tetracyclic product 7a (41% yield) along with tricycles 8 (6% yield) and 9 (35% yield) in the reaction of 4 with Ir(dtbbpy)(ppy)2PF6 and Et3N under the irradiation of blue LEDs.
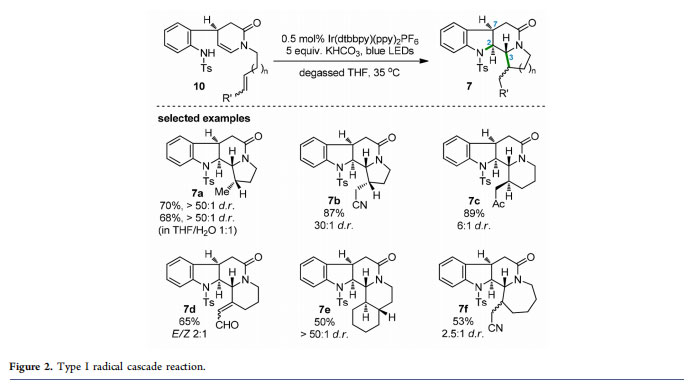

Obviously, not only an unusual reactivity between two nucleophilic amine and enamine groups (in 4) but also an impressive radical cascade reaction (product 7a) was achieved in the aforementioned photocatalytic transformation. With the initial results, we next screened the reaction conditions and found that the radical cascade of 4 proceeded smoothly to produce 7a in 70% yield (>50:1 dr) using Ir(dtbbpy)-(ppy)2PF6 (0.5 mol %; dtbbpy = 4,4′-bis(di-t-butyl)-2,2′-bipyridine; ppy = 2-phenylpyridine) and KHCO3 in degassed THF with irradiation of 5 W blue LEDs. In addition to 4, a series of substrates were designed and synthesized to examine the generality of this intra-/intramolecular radical cascade reaction. As depicted in Figure 2, 12 substrates possessing either electron-rich or electron-deficient double/triple bonds tethered to the enamide nitrogen atom were suitable for the cascade reaction, furnishing the corresponding products (e.g., selected examples 7b−e) with fused pyrrolidine or piperidine
rings in moderate to high yields and high to excellent dr values.
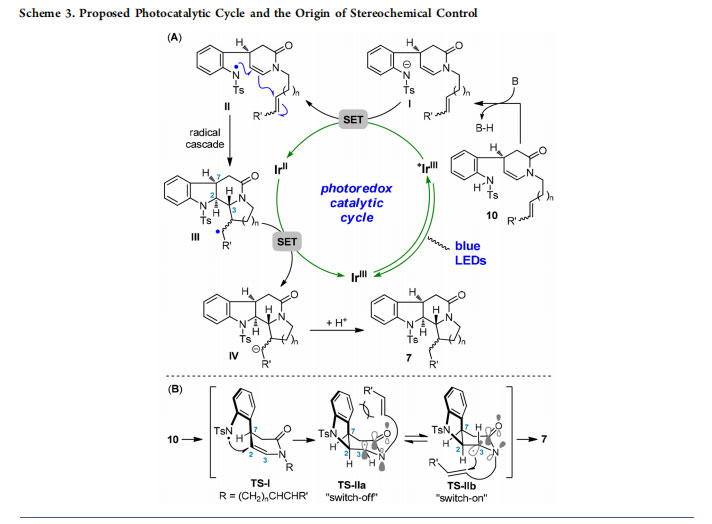
Exceptionally, the adduct 7f with a more flexible azepane unit was generated with a lower dr value (2.5:1). Of note, this cascade reaction proved to be robust and worked without loss of yield and diastereoselectivity in aqueous THF (product 7a). A proposed catalytic cycle for the photoredox radical cascade reaction is shown in Scheme 3A. Initially, deprotonation of the N−H bond in 10 took place in the presence of base to generate anion I. The photoexcited IrIII complex then oxidized I (via SET) and produced nitrogen-centered radical II, triggering subsequent cascade cyclization to yield carbon radical III. After SET reduction of radical III with the reductive state IrII complex, the resultant anion IV could be converted into product 7 via protonation. Regeneration of the ground-state IrIII photocatalyst completed the catalytic cycle.
Concerning the observed stereoselectivity of the radical cascade, formation of a cis relationship between H2 and H7 via transition state TS-I was realized in a substrate-controlled fashion (Scheme 3B).12 The resulting amide nitrogenassociated carbon radical might adopt two transition states, TS-IIa and TS-IIb, which acted as controllable switches for the subsequent reactions. The N-atom in TS-IIa had no
contribution to enhancing the nucleophilicity of the adjacent carbon radical due to donation of the nitrogen lone pair electrons to the carbonyl moiety in the characteristic amide functionality. On the other hand, unfavorable steric repulsion between the Ts-protected indoline group and the radical acceptor in TS-IIa also inhibited occurrence of the ensuing reaction. By contrast, the key two-center, three-electron interaction in TS-IIb greatly contributed to enhancing the radical stability, nucleophilicity, and selectivity.19 First, because of the greater stability, the sufficient lifetime of the carbon radical enabled subsequent bond formations especially in intermolecular manners (type II and III cascades). Second, twisting of the amide bond in TS-IIb restored the electrondonating ability of the N-atom to the carbon radical, thus increasing the radical nucleophilicity. Furthermore, attack of the carbon radical to the intramolecular acceptors (or intermolecular ones in type II and III cascades) exclusively from the bottom face of the piperidinone ring led to complete stereocontrol at C3.
2.2. Total Synthesis of the Eburnane Alkaloids
Inspired by the development of an intra-/intramolecular radical cascade for preparing indole-alkaloid-like skeletons, we sought to investigate its applications in natural product total synthesis. The eburnane indole alkaloids (Figure 3) are a group of structurally diverse molecules featuring a common pentacyclic core (e.g., 11−13) and an all-carbon quaternary center at C20.20,21 Functionalizations at the terminal C18 as well as further cyclizations may result in more variants of these natural products (e.g., 14−17). In this context, the C18 unfunctionalized eburnane alkaloids were envisaged to be synthesized from 18a (R = H), while various C18 functionalized counterparts could be derived from 18b (R = OPG). In turn, the versatile tetracyclic intermediate 18a,b with a crucial C20 quaternary stereogenic center would be accessible by the photocatalytic type I radical cascade of precursor 19a,b.
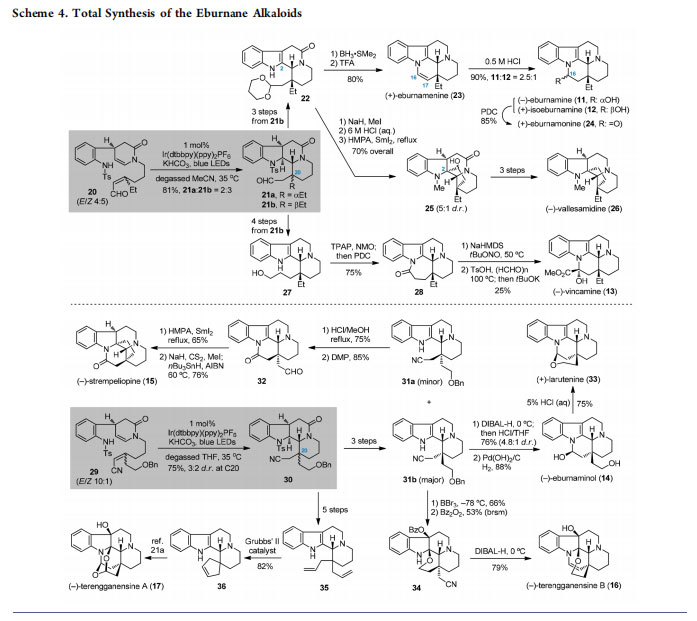
According to the synthetic plan, preparation of the C18 unfunctionalized eburnane alkaloids was examined using sulfonamide 20 as a starting material (Scheme 4).12 To evaluate the potential applications of a new synthetic methodology, especially in natural product total synthesis, an important criterion is whether it could be scaled up without loss of efficacy. Gratifyingly, the designed radical cascade reaction of 20 was easily performed on a decagram scale under photocatalytic conditions,12 where the solvent MeCN was found to be superior to THF, giving the bis-cyclization products 21a and 21b (81% overall yield, 2:3 dr). As most C18 unfunctionalized eburnane alkaloids possess a β-Et substituent at C20, the corresponding intermediate 21b with a β-Et group was used for further manipulations. On one hand, three steps of functional group transformations converted 21b into acetal 22. Reduction of the amide group in 22 to the corresponding amine followed by treatment with TFA afforded (+)-eburnamenine (23) in 80% overall yield. Subsequent hydration of the C16−C17 double bond delivered (−)-eburnamine (11) and (+)-isoeburnamine (12); oxidation of the resultant hydroxyl group with pyridinium dichromate (PDC) gave (+)-eburnamonine (24). The intermediate 22 was also used for the synthesis of (−)-vallesamidine (26). Establishing the remaining five-membered carbocycle in 26 relied on a SmI2-mediated reductive cyclization of the aldehyde to the C2 of indole. On the other hand, subjecting 21b to a four-step sequence produced amino alcohol 27. The latter underwent oxidation/ cyclization/oxidation to generate lactam 28, which was finally converted into (−)-vincamine (13) according to a literature method.22
Employing precursor 29 with a C18-OBn group for the radical cascade reaction under the standard photoredox conditions smoothly furnished the tetracyclic product 30 as a pair of inseparable diastereomers (Scheme 4).14 Both isomers were used for accessing eburnane indole alkaloids with C18 functionalities. Specifically, the diastereomeric mixture of 30 was converted to separable 31a and 31b by three steps.
Treatment of the minor 31a with HCl/MeOH at reflux forged a new lactam ring with simultaneous removal of the benzyl group, which was followed by Dess−Martin periodinane (DMP) oxidation of the resultant primary alcohol, leading to aldehyde 32. A SmI2-mediated radical cyclization of 32 with subsequent Barton deoxygenation generated (−)-strempeliopine (15). Meanwhile, starting from 31b, formation of a hemiaminal with ensuing deprotection of the benzyl group yielded (−)-eburnaminol (14); the latter was treated with HCl to give (+)-larutenine (33). On the other hand, first removal of
the benzyl group in 31b, followed by oxidation and cyclization in the presence of Bz2O2, furnished tetrahydropyran 34.
Exposure of 34 to di-isobutyl aluminum hydride (DIBAL-H) at 0 °C completed the synthesis of (−)-terengganensine B (16). Moreover, the radical cascade product (30) underwent a five-step transformation to provide access to the diallyl product 35. Ring-closing metathesis (RCM) of 35 with Grubbs’ II catalyst delivered 36 (82% yield); the latter was further converted to (−)-terengganensine A (17) based on the
method developed by Zhu and colleagues.
1-(Cyclopropylsulfonyl)piperidine-4-carboxylic acidCatalog No.:AA01AQHH CAS No.:1036738-97-2 MDL No.:MFCD16653434 MF:C9H15NO4S MW:233.2847 |
Methyl 2-chloroquinazoline-6-carboxylateCatalog No.:AA008TU2 CAS No.:1036755-96-0 MDL No.:MFCD17016011 MF:C10H7ClN2O2 MW:222.6278 |
6-Amino-3-bromo-2-fluorobenzoic acidCatalog No.:AA0096WA CAS No.:1036756-03-2 MDL No.:MFCD20482512 MF:C7H5BrFNO2 MW:234.0225 |
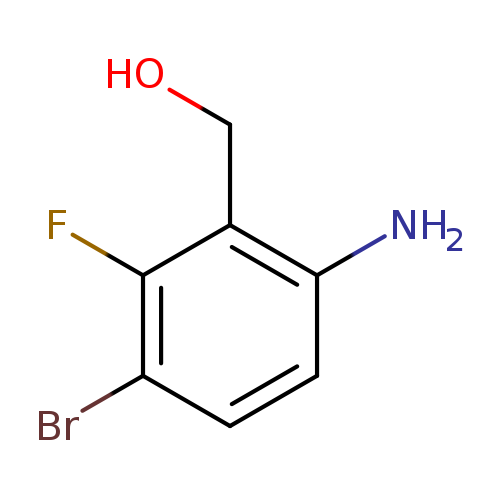
(6-Amino-3-bromo-2-fluoro-phenyl)-methanolCatalog No.:AA01FR30 CAS No.:1036756-04-3 MDL No.:MFCD30723666 MF:C7H7BrFNO MW:220.0390 |
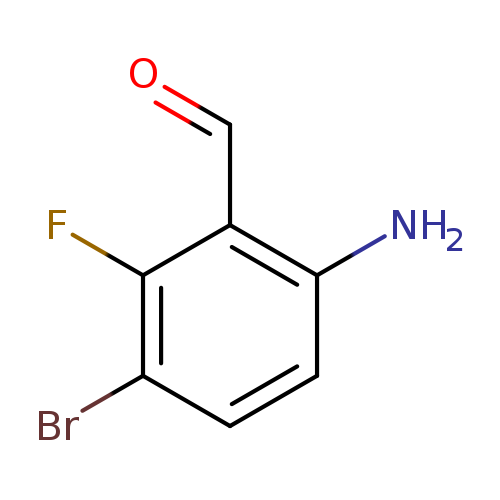
6-Amino-3-bromo-2-fluorobenzaldehydeCatalog No.:AA01FR4F CAS No.:1036756-05-4 MDL No.:MFCD30471489 MF:C7H5BrFNO MW:218.0231 |
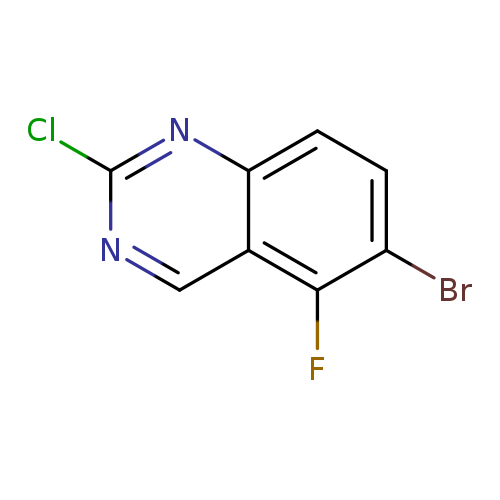
6-Bromo-2-chloro-5-fluoro-quinazolineCatalog No.:AA009LQZ CAS No.:1036756-07-6 MDL No.:MFCD16250427 MF:C8H3BrClFN2 MW:261.4782 |
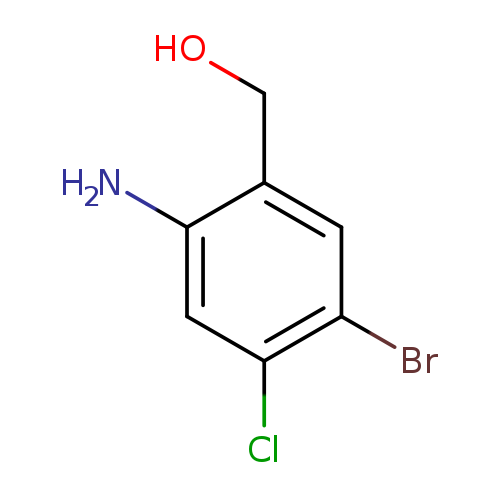
(2-Amino-5-bromo-4-chlorophenyl)methanolCatalog No.:AA0093IX CAS No.:1036757-10-4 MDL No.:MFCD20037891 MF:C7H7BrClNO MW:236.4936 |
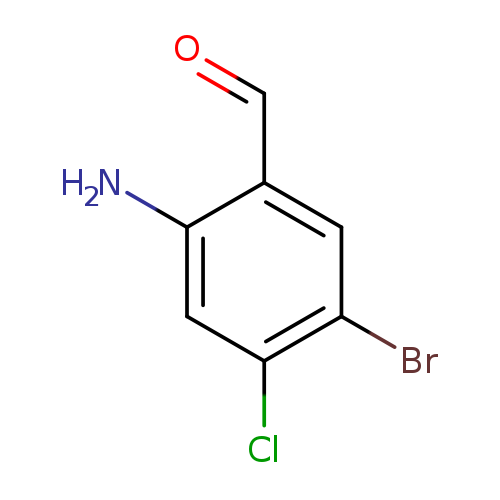
2-Amino-5-bromo-4-chlorobenzaldehydeCatalog No.:AA0096US CAS No.:1036757-11-5 MDL No.:MFCD20037890 MF:C7H5BrClNO MW:234.4777 |
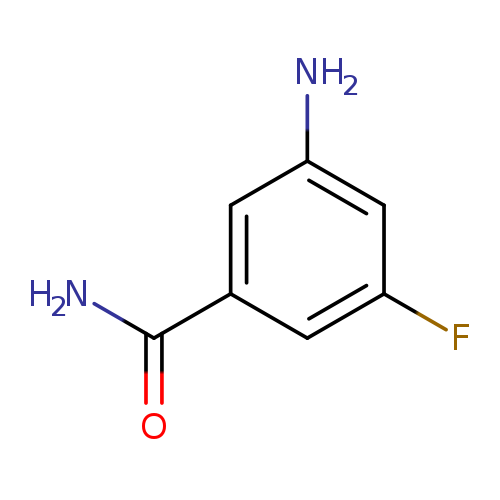
3-Amino-5-fluorobenzamideCatalog No.:AA003ITO CAS No.:1036757-40-0 MDL No.:MFCD08447276 MF:C7H7FN2O MW:154.1417 |
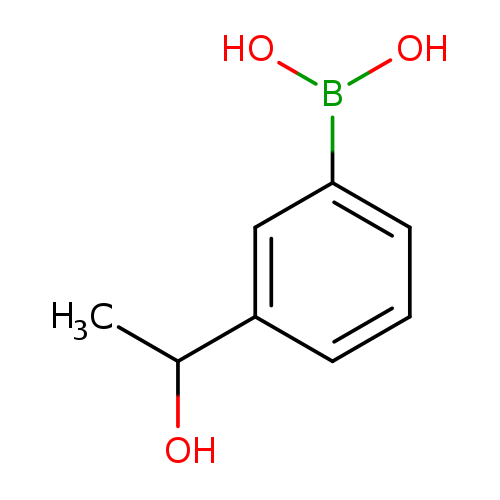
3-(1-Hydroxyethyl)phenylboronic acidCatalog No.:AA0085DU CAS No.:1036760-03-8 MDL No.:MFCD04112541 MF:C8H11BO3 MW:165.9821 |
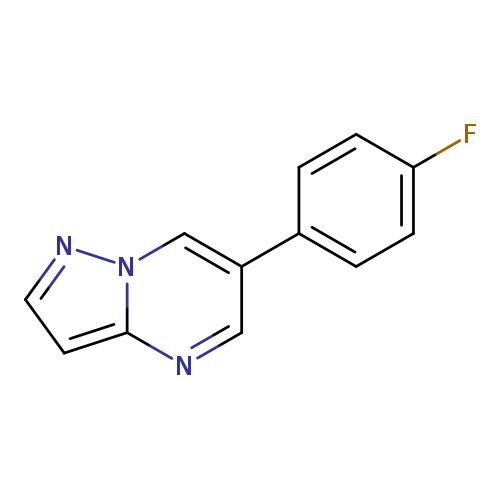
6-(4-Fluorophenyl)pyrazolo[1,5-a]pyrimidineCatalog No.:AA00HA2W CAS No.:1036762-04-5 MDL No.:MFCD28405285 MF:C12H8FN3 MW:213.2104 |
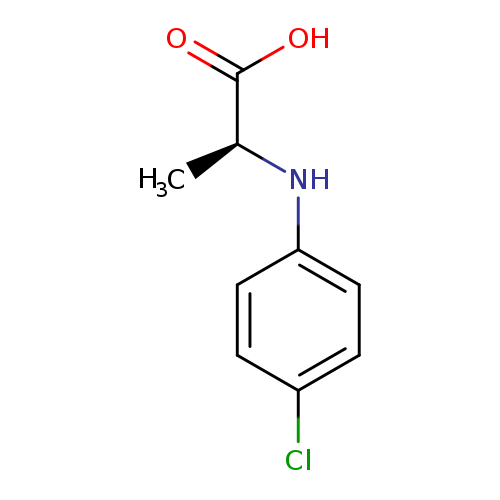
(2S)-2-[(4-chlorophenyl)amino]propanoic acidCatalog No.:AA01BGN4 CAS No.:103678-25-7 MDL No.:MFCD20641844 MF:C9H10ClNO2 MW:199.6342 |
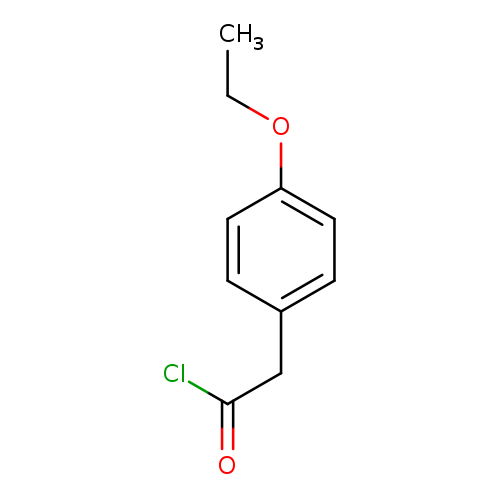
(4-ethoxyphenyl)acetyl chlorideCatalog No.:AA00J6U2 CAS No.:10368-35-1 MDL No.:MFCD00995155 MF:C10H11ClO2 MW:198.6461 |
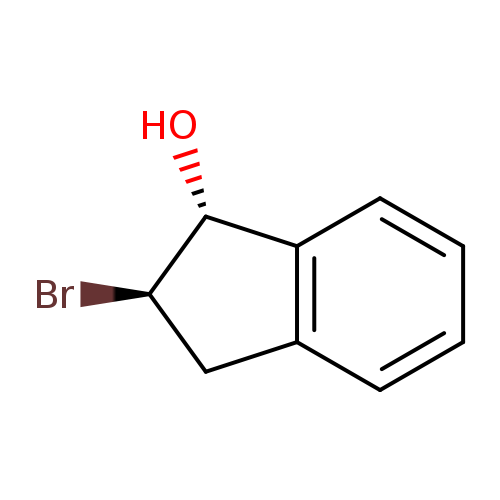
Trans-2-bromo-1-indanolCatalog No.:AA003UT6 CAS No.:10368-44-2 MDL No.:MFCD00151318 MF:C9H9BrO MW:213.0712 |
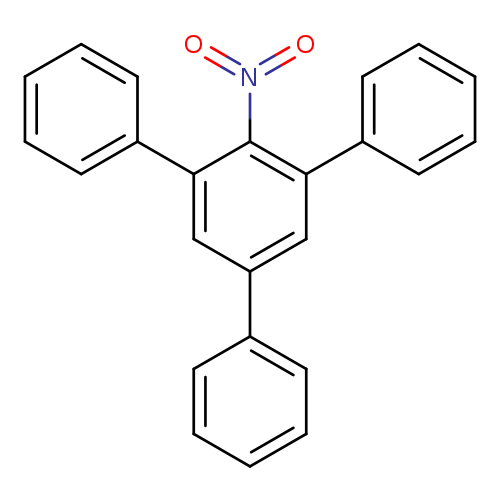
2,4,6-TriphenylnitrobenzeneCatalog No.:AA003FL9 CAS No.:10368-47-5 MDL No.:MFCD00060090 MF:C24H17NO2 MW:351.3973 |
2'-Bromo-5'-phenyl-1,1':3',1''-terphenylCatalog No.:AA009LVO CAS No.:10368-73-7 MDL No.:MFCD00092320 MF:C24H17Br MW:385.2958 |
3-aminopropylphosphinic acidCatalog No.:AA008ZYD CAS No.:103680-47-3 MDL No.:MFCD05662182 MF:C3H10NO2P MW:123.0908 |
2-(Diisopropylcarbanoyl) phenylboronic acidCatalog No.:AA008ROA CAS No.:103681-98-7 MDL No.:MFCD01318990 MF:C13H20BNO3 MW:249.1138 |
4-((2,5-Dioxo-2,5-dihydro-1h-pyrrol-1-yl)methyl)-n-(prop-2-yn-1-yl)cyclohexanecarboxamideCatalog No.:AA00HA2Y CAS No.:1036847-90-1 MDL No.:MFCD27956997 MF:C15H18N2O3 MW:274.3150 |
2-(3-propoxyphenyl)ethanamineCatalog No.:AA008V5C CAS No.:103686-13-1 MDL No.:MFCD08449852 MF:C11H17NO MW:179.2588 |
(2E)-3-(4-bromothiophen-2-yl)prop-2-enoic acidCatalog No.:AA003UU7 CAS No.:103686-16-4 MDL No.:MFCD00051662 MF:C7H5BrO2S MW:233.0824 |
Methanone, (4-chlorophenyl)-5-pyrimidinyl-Catalog No.:AA007X4J CAS No.:103686-55-1 MDL No.:MFCD13152954 MF:C11H7ClN2O MW:218.6391 |
2-Diethylaminoethyl hexanoateCatalog No.:AA0085DF CAS No.:10369-83-2 MDL No.:MFCD12827534 MF:C12H25NO2 MW:215.3324 |
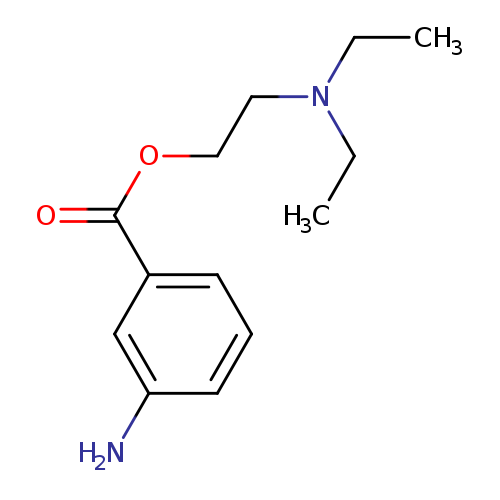
2-(diethylamino)ethyl 3-aminobenzoateCatalog No.:AA01B9QS CAS No.:10369-94-5 MDL No.:MFCD13425220 MF:C13H20N2O2 MW:236.3101 |
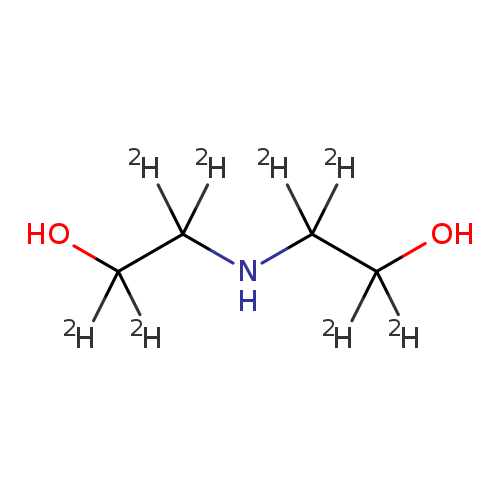
BIS(2-HYDROXYETHYL)-D8-AMINECatalog No.:AA008SXT CAS No.:103691-51-6 MDL No.:MFCD01317440 MF:C4H3D8NO2 MW:113.1849 |
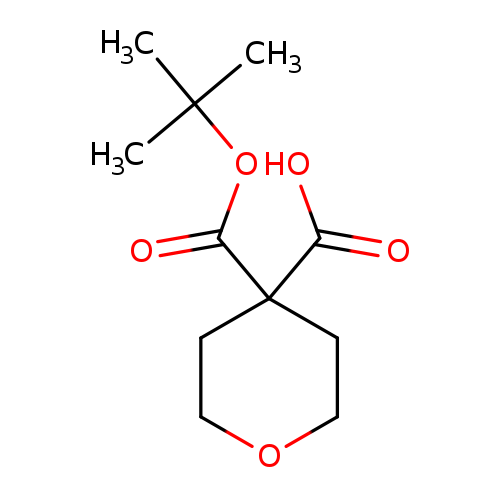
4-[(tert-butoxy)carbonyl]oxane-4-carboxylic acidCatalog No.:AA01C25E CAS No.:1036922-77-6 MDL No.:MFCD30342619 MF:C11H18O5 MW:230.2576 |
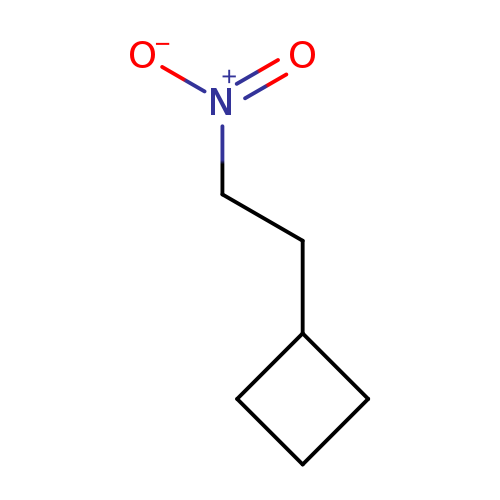
(2-Nitroethyl)cyclobutaneCatalog No.:AA0085DJ CAS No.:1036931-21-1 MDL No.:MFCD12828042 MF:C6H11NO2 MW:129.1570 |
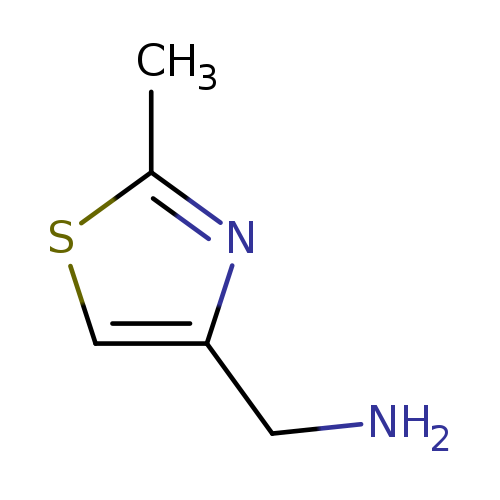
C-(2-Methyl-thiazol-4-yl)-methylamineCatalog No.:AA007FTC CAS No.:103694-26-4 MDL No.:MFCD06212804 MF:C5H8N2S MW:128.1954 |
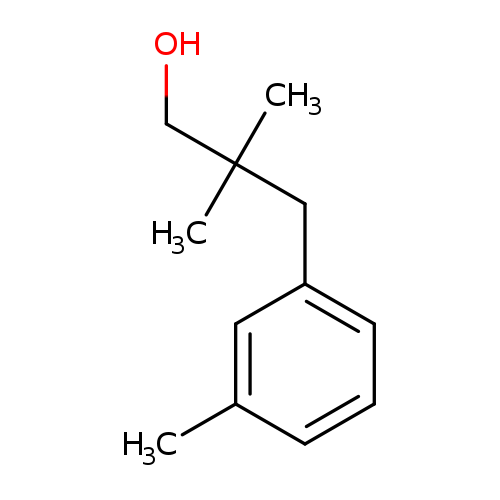
2,2-Dimethyl-3-(m-tolyl)propan-1-olCatalog No.:AA0085DH CAS No.:103694-68-4 MDL No.:MFCD03701092 MF:C12H18O MW:178.2707 |
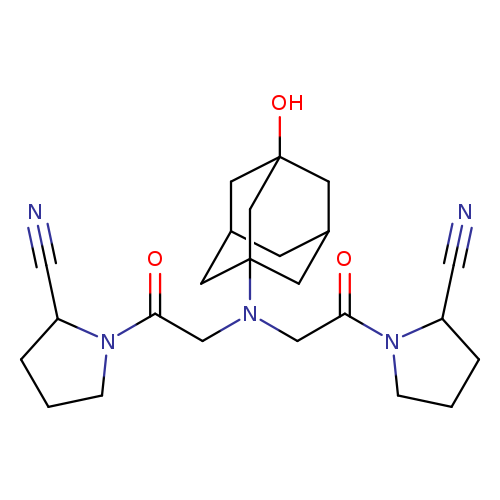
Vildagliptin IMpurity 2 (Mixture of DiastereoMers)Catalog No.:AA0091TZ CAS No.:1036959-23-5 MDL No.: MF:C24H33N5O3 MW:439.5505 |
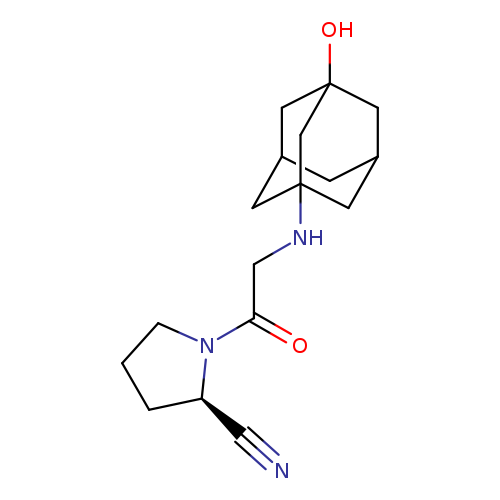
(2R)-1-[2-[(3-Hydroxytricyclo[3.3.1.1(3,7)]dec-1-yl)amino]acetyl]-2-pyrrolidinecarbonitrileCatalog No.:AA0095JE CAS No.:1036959-27-9 MDL No.:MFCD13250201 MF:C17H25N3O2 MW:303.3993 |
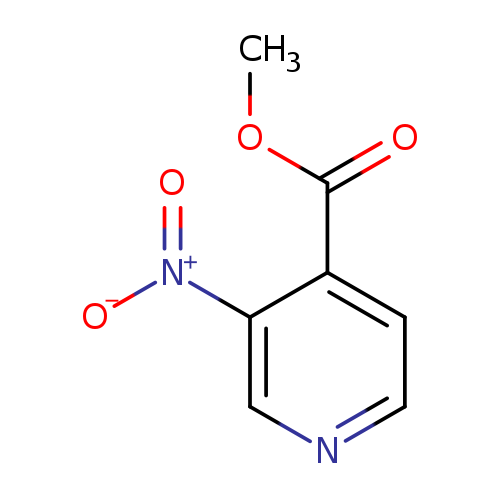
Methyl 3-nitroisonicotinateCatalog No.:AA003RQF CAS No.:103698-10-8 MDL No.:MFCD16657194 MF:C7H6N2O4 MW:182.1335 |
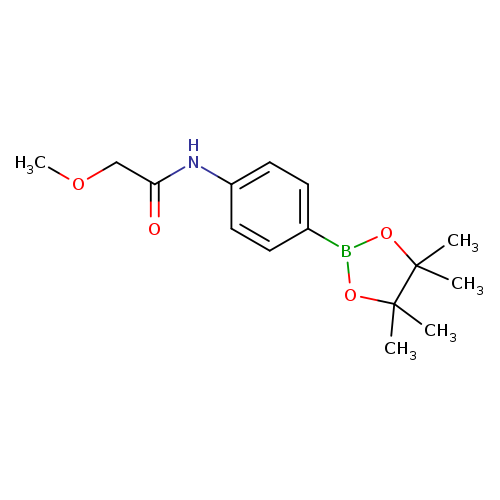
2-methoxy-N-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]acetamideCatalog No.:AA00QGGB CAS No.:1036990-20-1 MDL No.:MFCD22494280 MF:C15H22BNO4 MW:291.1505 |
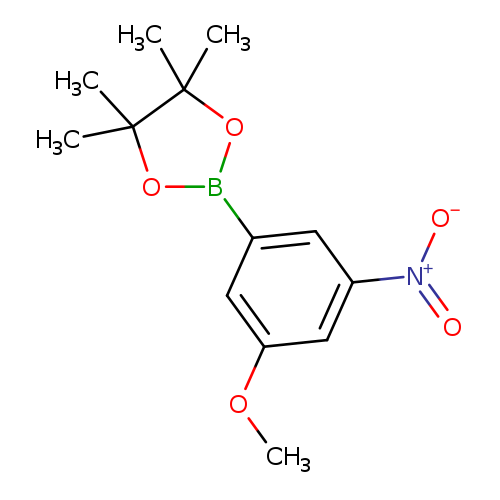
1,3,2-Dioxaborolane, 2-(3-methoxy-5-nitrophenyl)-4,4,5,5-tetramethyl-Catalog No.:AA00HA30 CAS No.:1036990-22-3 MDL No.:MFCD22493664 MF:C13H18BNO5 MW:279.0967 |
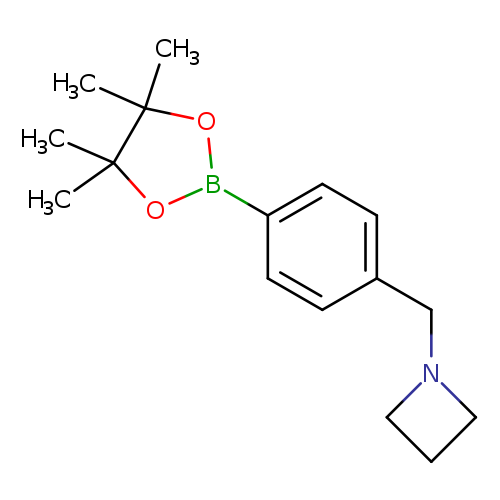
4-[(1-Azetidinyl)methyl]phenylboronic acid pinacol esterCatalog No.:AA00HA31 CAS No.:1036990-23-4 MDL No.:MFCD18735839 MF:C16H24BNO2 MW:273.1783 |
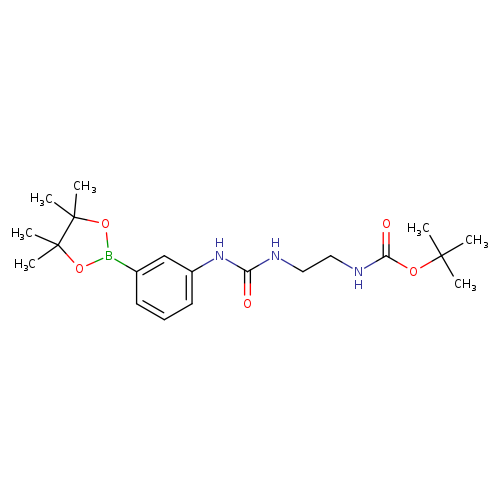
Carbamic acid,N-[2-[[[[3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]amino]carbonyl]amino]ethyl]-, 1,1-dimethylethyl esterCatalog No.:AA01FNQ4 CAS No.:1036990-28-9 MDL No.:MFCD32206586 MF:C20H32BN3O5 MW:405.2962 |
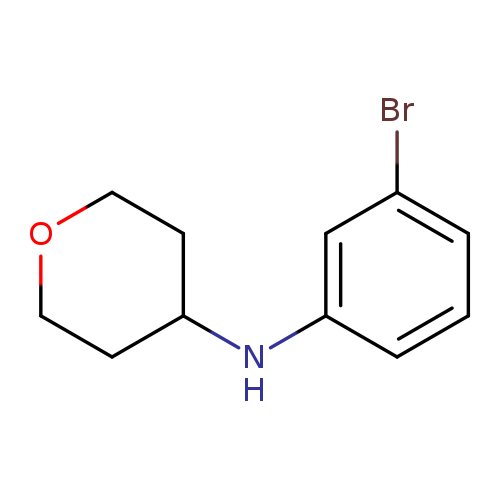
N-(3-Bromophenyl)oxan-4-amineCatalog No.:AA00ILLZ CAS No.:1036990-31-4 MDL No.:MFCD12087567 MF:C11H14BrNO MW:256.1390 |
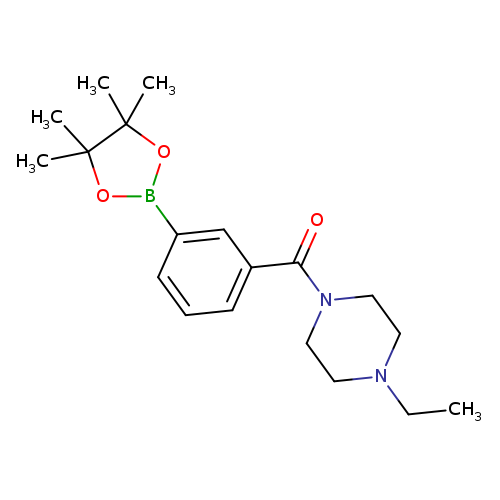
1-Ethyl-4-[3-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzoyl]piperazineCatalog No.:AA00HA33 CAS No.:1036990-36-9 MDL No.:MFCD22415367 MF:C19H29BN2O3 MW:344.2562 |
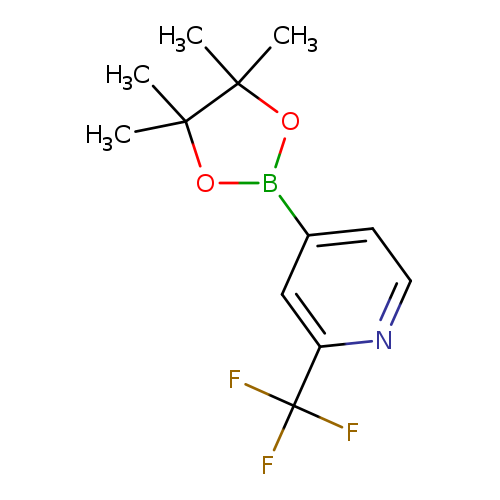
2-(Trifluoromethyl)pyridine-4-boronic acid pinacol esterCatalog No.:AA00365I CAS No.:1036990-42-7 MDL No.:MFCD09607678 MF:C12H15BF3NO2 MW:273.0592 |
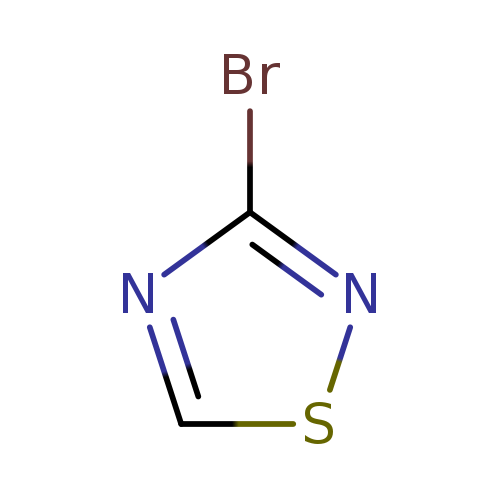
3-bromo-1,2,4-thiadiazoleCatalog No.:AA00J3BU CAS No.:1036990-54-1 MDL No.:MFCD11848380 MF:C2HBrN2S MW:165.0117 |
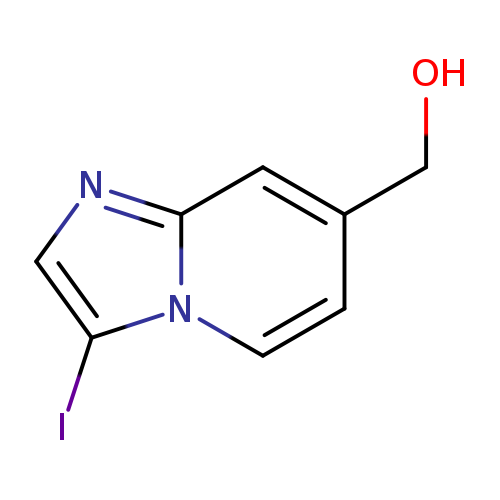
3-Iodo-imidazo[1,2-a]pyridine-7-methanolCatalog No.:AA0095ND CAS No.:1036990-66-5 MDL No.:MFCD12400829 MF:C8H7IN2O MW:274.0584 |
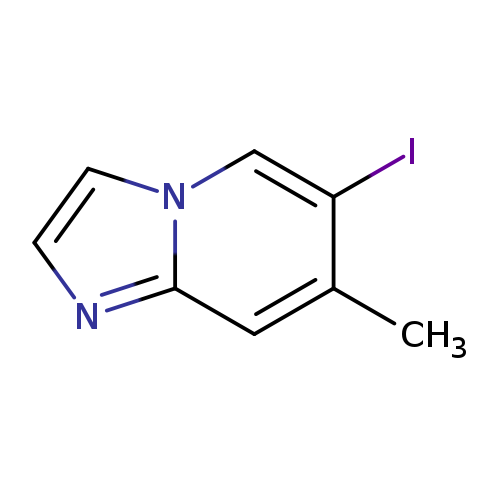
6-Iodo-7-methyl-imidazo[1,2-a]pyridineCatalog No.:AA0093HW CAS No.:1036991-04-4 MDL No.:MFCD23115412 MF:C8H7IN2 MW:258.0591 |
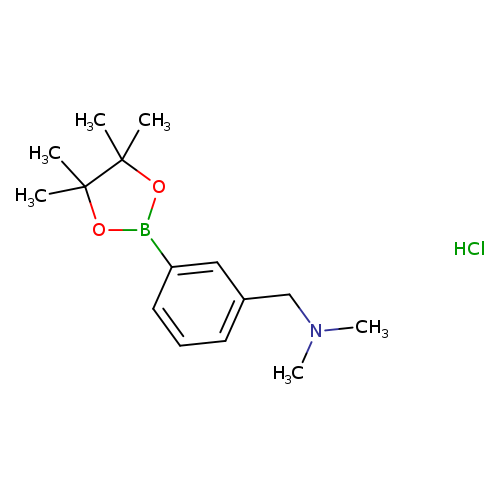
3-(N,N-Dimethylamino)methylphenylboronic acid, pinacol ester, HClCatalog No.:AA003SHK CAS No.:1036991-19-1 MDL No.:MFCD06657878 MF:C15H25BClNO2 MW:297.6285 |
4-(1-Pyrrolidinylmethyl)phenylboronic acidCatalog No.:AA0095HM CAS No.:1036991-20-4 MDL No.:MFCD06801708 MF:C11H16BNO2 MW:205.0612 |
6-(Dimethylamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA007X3Z CAS No.:1036991-24-8 MDL No.:MFCD11975406 MF:C13H21BN2O2 MW:248.1290 |
3-(4-Morpholinylcarbonyl)phenylboronic acid pinacol esterCatalog No.:AA00904Q CAS No.:1036991-25-9 MDL No.:MFCD09266172 MF:C17H24BNO4 MW:317.1878 |
3-Amino-5-(tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrileCatalog No.:AA01EHL3 CAS No.:1036991-35-1 MDL No.:MFCD22494384 MF:C13H17BN2O2 MW:244.0973 |
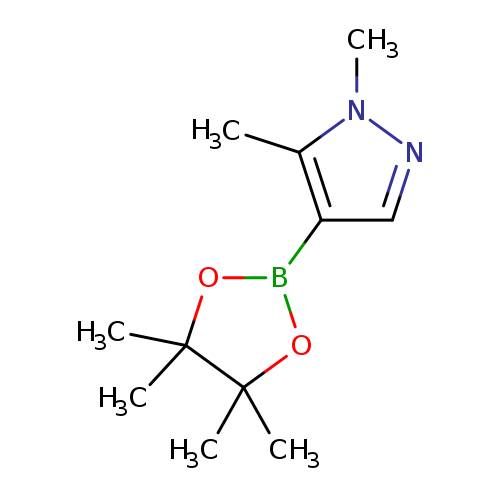
1,5-Dimethyl-1H-pyrazole-4-boronic acid,pinacol esterCatalog No.:AA00386M CAS No.:1036991-40-8 MDL No.:MFCD09864189 MF:C11H19BN2O2 MW:222.0918 |
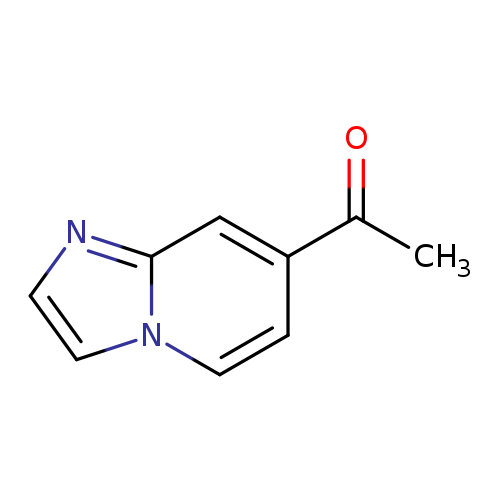
1-(Imidazo[1,2-a]pyridin-7-yl)ethanoneCatalog No.:AA0091DJ CAS No.:1036991-50-0 MDL No.:MFCD20527734 MF:C9H8N2O MW:160.1726 |
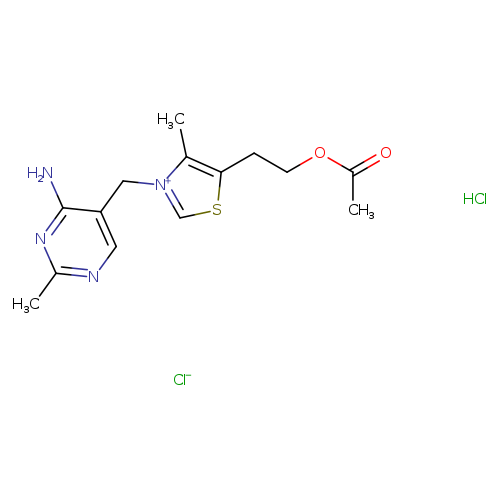
5-(2-Acetoxyethyl)-3-((4-amino-2-methylpyrimidin-5-yl)methyl)-4-methylthiazol-3-ium chlorideCatalog No.:AA008VPE CAS No.:1037-29-2 MDL No.:MFCD00210717 MF:C14H20Cl2N4O2S MW:379.3052 |
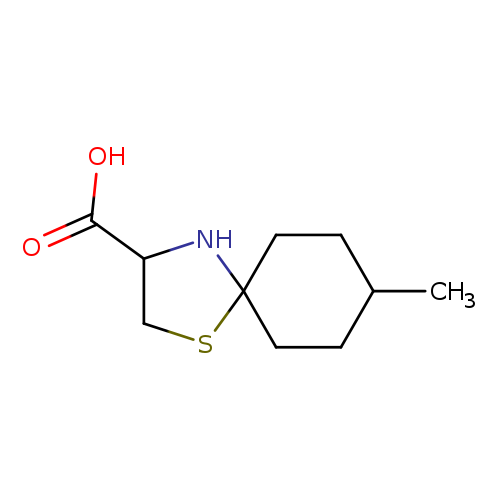
8-Methyl-1-thia-4-azaspiro[4.5]decane-3-carboxylic acidCatalog No.:AA00IW7B CAS No.:1037010-80-2 MDL No.:MFCD06633105 MF:C10H17NO2S MW:215.3125 |
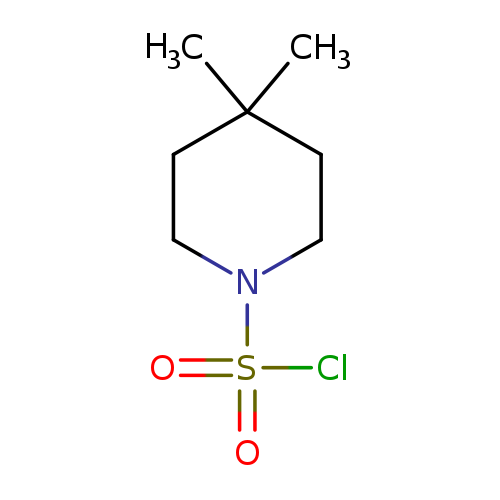
4,4-Dimethyl-1-piperidinesulfonyl chlorideCatalog No.:AA00J2AT CAS No.:1037041-50-1 MDL No.:MFCD17253007 MF:C7H14ClNO2S MW:211.7096 |
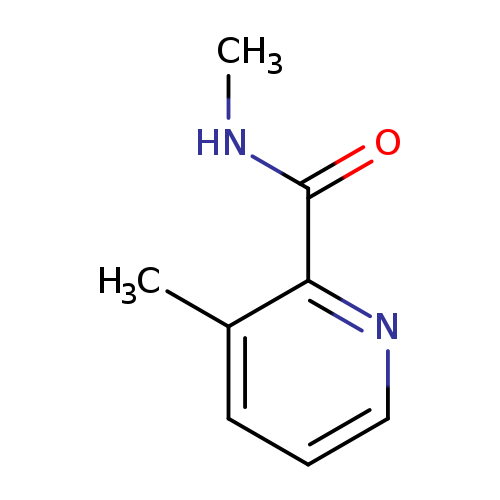
N,3-Dimethylpyridine-2-carboxamideCatalog No.:AA00HA37 CAS No.:1037045-67-2 MDL No.:MFCD19671808 MF:C8H10N2O MW:150.1778 |
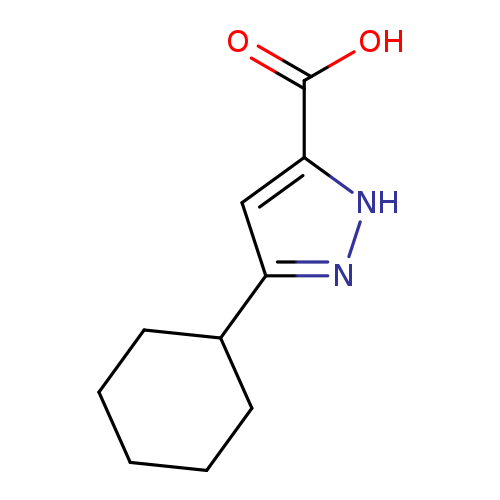
3-cyclohexyl-1H-pyrazole-5-carboxylic acidCatalog No.:AA01A2VE CAS No.:1037061-95-2 MDL No.:MFCD17193820 MF:C10H14N2O2 MW:194.2304 |
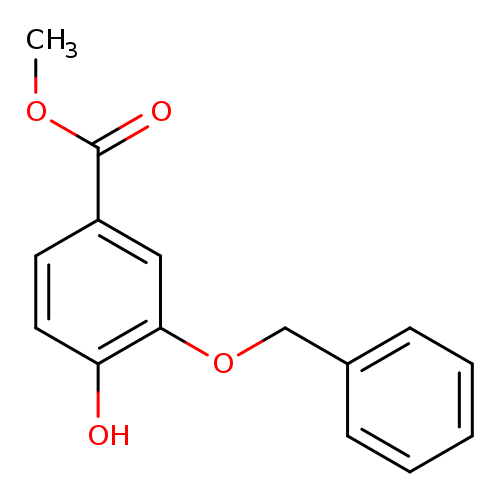
Methyl 3-(benzyloxy)-4-hydroxybenzoateCatalog No.:AA008WOO CAS No.:1037072-57-3 MDL No.:MFCD23110745 MF:C15H14O4 MW:258.2693 |
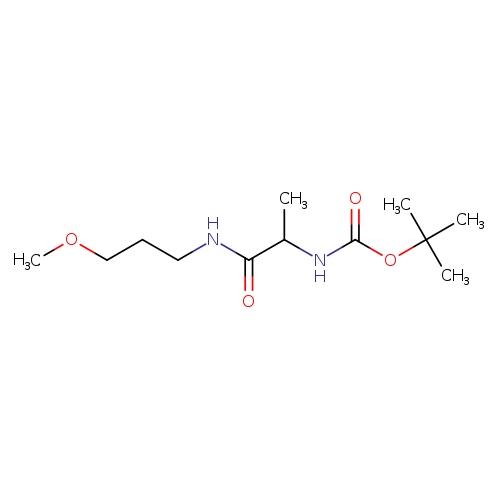
tert-Butyl N-{1-[(3-methoxypropyl)carbamoyl]ethyl}carbamateCatalog No.:AA009445 CAS No.:1037073-41-8 MDL No.:MFCD22549328 MF:C12H24N2O4 MW:260.3300 |
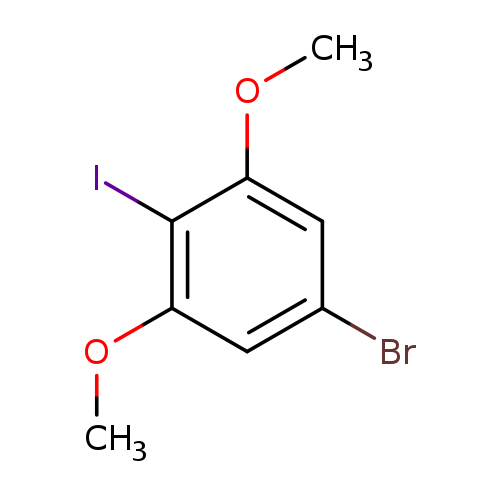
1-Bromo-3,5-dimethoxy-4-iodobenzeneCatalog No.:AA01ELD9 CAS No.:1037082-67-9 MDL No.:MFCD28738448 MF:C8H8BrIO2 MW:342.9564 |
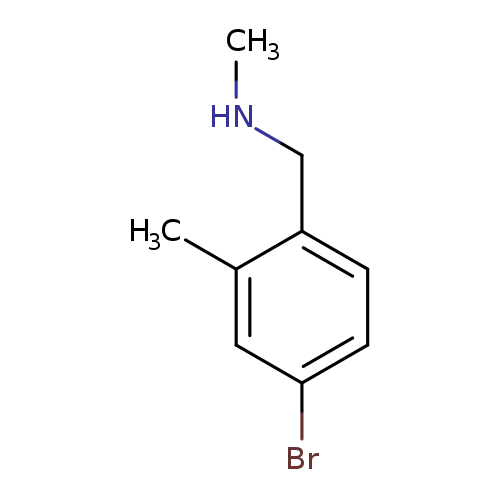
[(4-bromo-2-methylphenyl)methyl](methyl)amineCatalog No.:AA01BEBB CAS No.:1037090-12-2 MDL No.:MFCD12096719 MF:C9H12BrN MW:214.1023 |
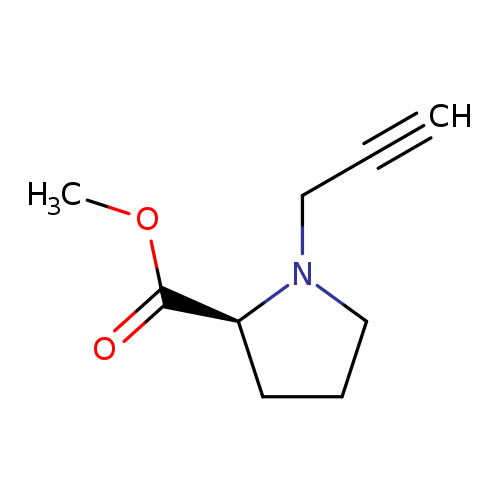
methyl (2s)-1-(prop-2-yn-1-yl)pyrrolidine-2-carboxylateCatalog No.:AA01B2HJ CAS No.:103711-16-6 MDL No.:MFCD29991645 MF:C9H13NO2 MW:167.2050 |
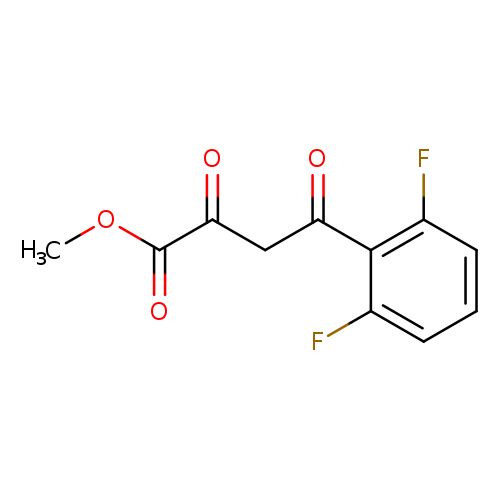
methyl 4-(2,6-difluorophenyl)-2,4-dioxobutanoateCatalog No.:AA019U9F CAS No.:1037130-74-7 MDL No.:MFCD11188865 MF:C11H8F2O4 MW:242.1756 |
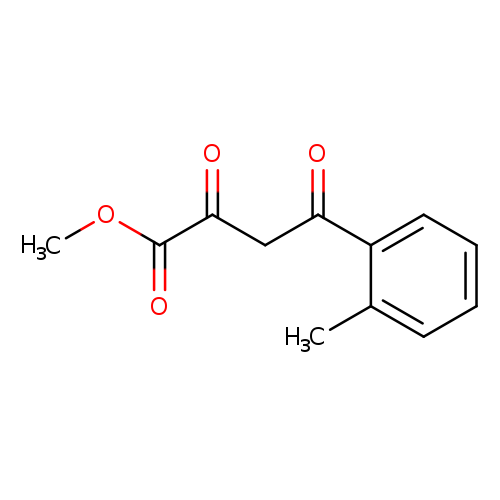
Methyl 2,4-dioxo-4-(o-tolyl)butanoateCatalog No.:AA008X8X CAS No.:1037130-77-0 MDL No.:MFCD11188866 MF:C12H12O4 MW:220.2213 |
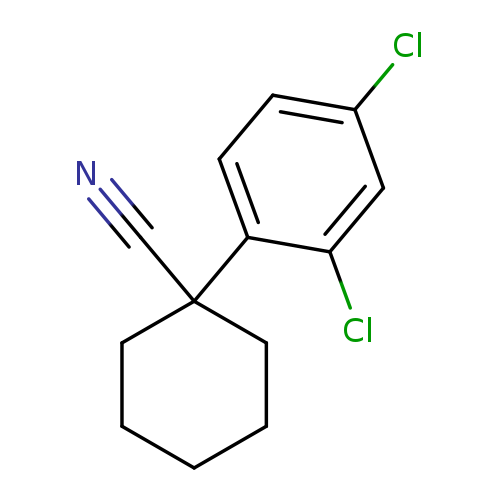
1-(2,4-dichlorophenyl)cyclohexane-1-carbonitrileCatalog No.:AA01C24S CAS No.:1037130-92-9 MDL No.:MFCD11036796 MF:C13H13Cl2N MW:254.1550 |
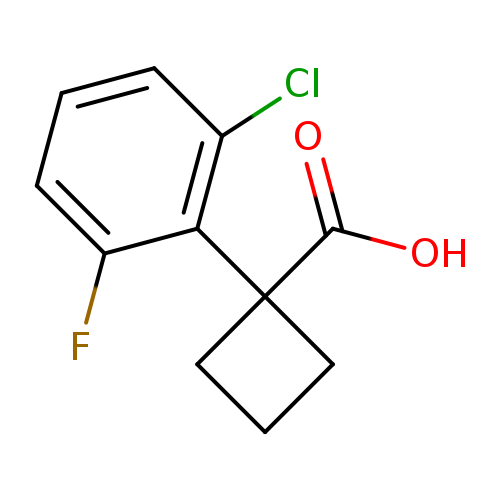
1-(2-chloro-6-fluorophenyl)cyclobutane-1-carboxylic acidCatalog No.:AA01DX4S CAS No.:1037131-07-9 MDL No.:MFCD11037026 MF:C11H10ClFO2 MW:228.6473 |
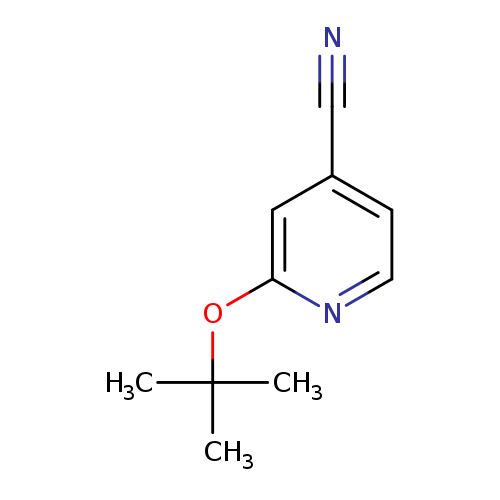
2-(tert-Butoxy)pyridine-4-carbonitrileCatalog No.:AA01A3R6 CAS No.:1037131-17-1 MDL No.:MFCD11196883 MF:C10H12N2O MW:176.2151 |
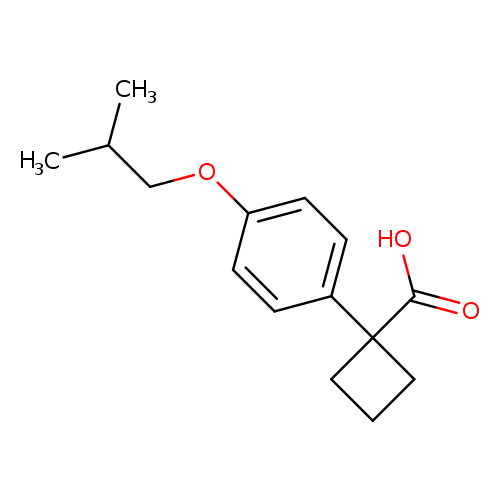
1-[4-(2-methylpropoxy)phenyl]cyclobutane-1-carboxylic acidCatalog No.:AA01AH1H CAS No.:1037131-39-7 MDL No.:MFCD11541476 MF:C15H20O3 MW:248.3175 |
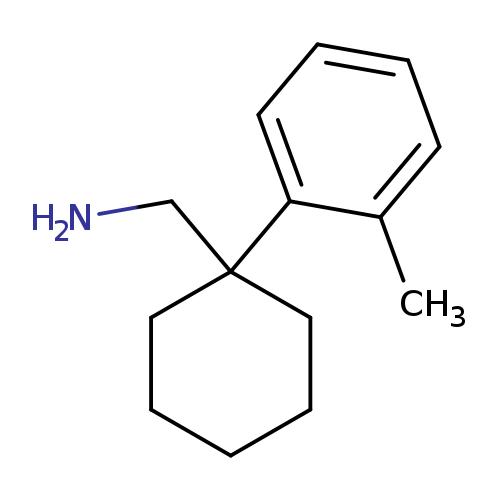
[1-(2-methylphenyl)cyclohexyl]methanamineCatalog No.:AA01A8Q6 CAS No.:1037131-52-4 MDL No.:MFCD11188923 MF:C14H21N MW:203.3232 |
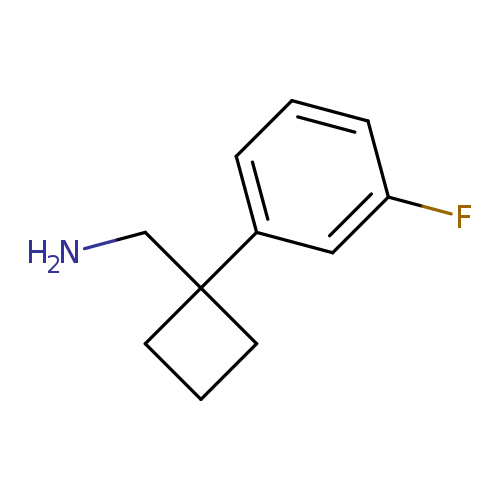
[1-(3-fluorophenyl)cyclobutyl]methanamineCatalog No.:AA019CYM CAS No.:1037131-77-3 MDL No.:MFCD11188946 MF:C11H14FN MW:179.2340 |
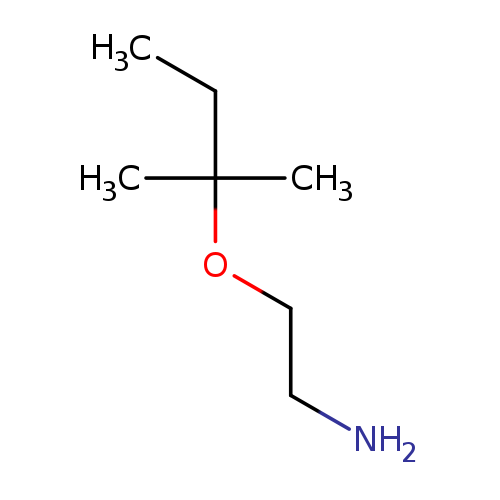
2-[(2-Methylbutan-2-yl)oxy]ethan-1-amineCatalog No.:AA01C22Y CAS No.:1037131-98-8 MDL No.:MFCD11196454 MF:C7H17NO MW:131.2160 |
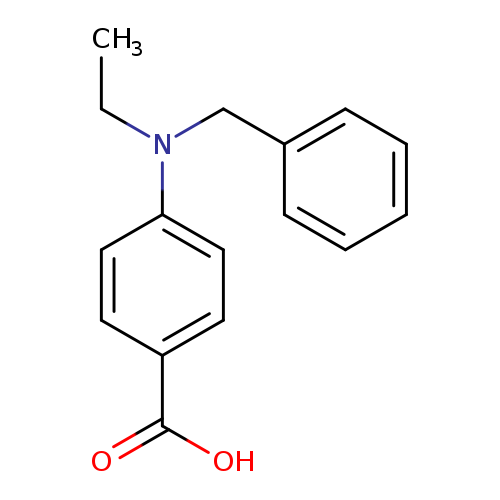
4-[benzyl(ethyl)amino]benzoic acidCatalog No.:AA01A8CB CAS No.:1037132-10-7 MDL No.:MFCD11923146 MF:C16H17NO2 MW:255.3117 |
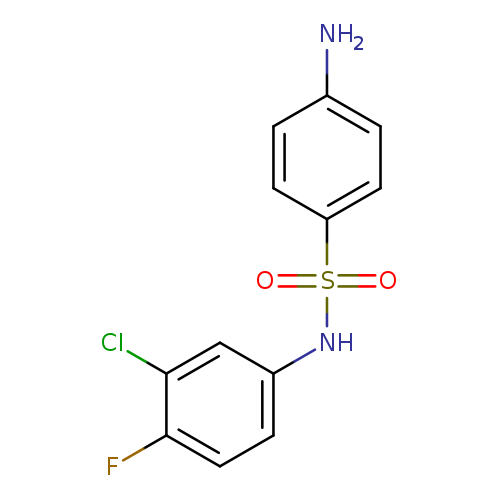
4-Amino-N-(3-chloro-4-fluorophenyl)benzenesulfonamideCatalog No.:AA019VSK CAS No.:1037132-84-5 MDL No.:MFCD12559329 MF:C12H10ClFN2O2S MW:300.7364 |
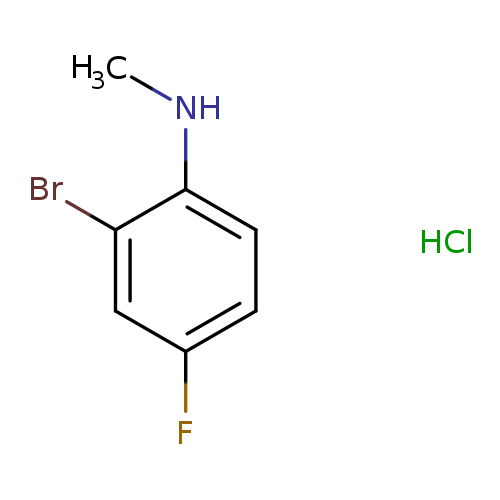
N-Methyl 2-bromo-4-fluoroanilineCatalog No.:AA003T2T CAS No.:1037138-94-5 MDL No.:MFCD13195687 MF:C7H8BrClFN MW:240.5005 |
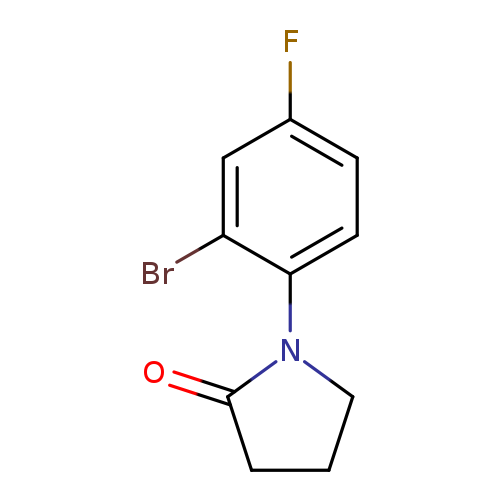
1-(2-Bromo-4-fluorophenyl)pyrrolidin-2-oneCatalog No.:AA007X3V CAS No.:1037150-18-7 MDL No.:MFCD11189340 MF:C10H9BrFNO MW:258.0870 |
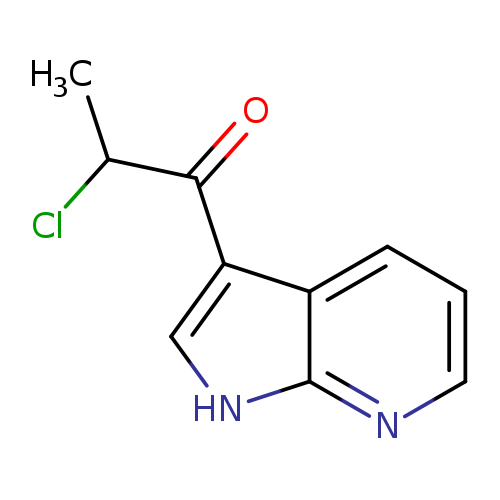
2-chloro-1-{1H-pyrrolo[2,3-b]pyridin-3-yl}propan-1-oneCatalog No.:AA01A3RD CAS No.:1037152-84-3 MDL No.:MFCD11189414 MF:C10H9ClN2O MW:208.6443 |
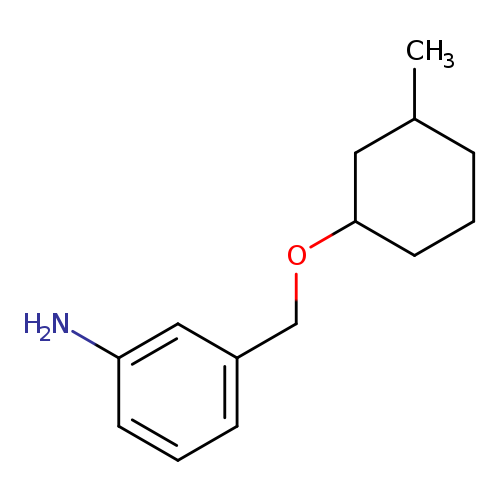
3-([(3-Methylcyclohexyl)oxy]methyl)anilineCatalog No.:AA01A7K8 CAS No.:1037157-93-9 MDL No.:MFCD11196406 MF:C14H21NO MW:219.3226 |
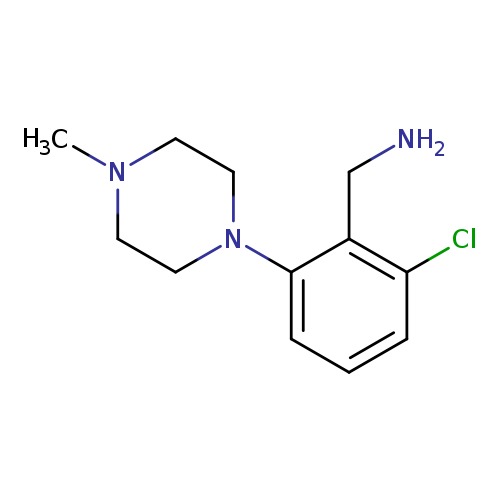
[2-chloro-6-(4-methylpiperazin-1-yl)phenyl]methanamineCatalog No.:AA019SL1 CAS No.:1037161-38-8 MDL No.:MFCD11191625 MF:C12H18ClN3 MW:239.7444 |
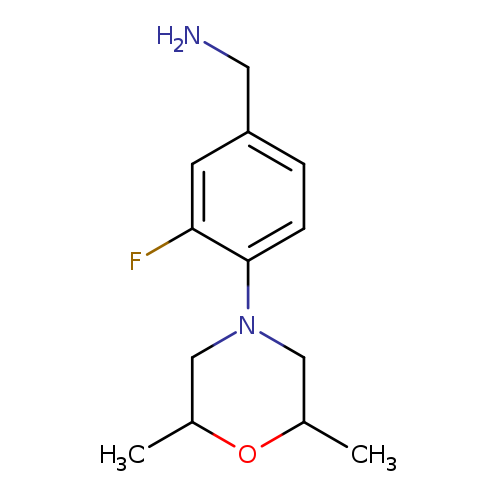
[4-(2,6-Dimethylmorpholin-4-yl)-3-fluorophenyl]methanamineCatalog No.:AA019VDY CAS No.:1037161-46-8 MDL No.:MFCD11191630 MF:C13H19FN2O MW:238.3012 |
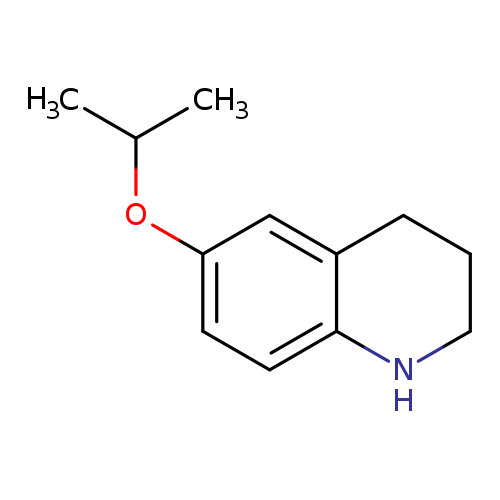
6-(PROPAN-2-YLOXY)-1,2,3,4-TETRAHYDROQUINOLINECatalog No.:AA01DX4T CAS No.:1037163-65-7 MDL No.:MFCD11192493 MF:C12H17NO MW:191.2695 |
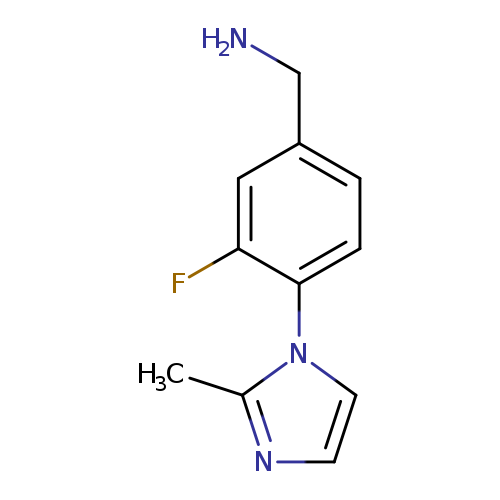
[3-fluoro-4-(2-methyl-1H-imidazol-1-yl)phenyl]methanamineCatalog No.:AA019XDR CAS No.:1037163-76-0 MDL No.:MFCD11191729 MF:C11H12FN3 MW:205.2315 |
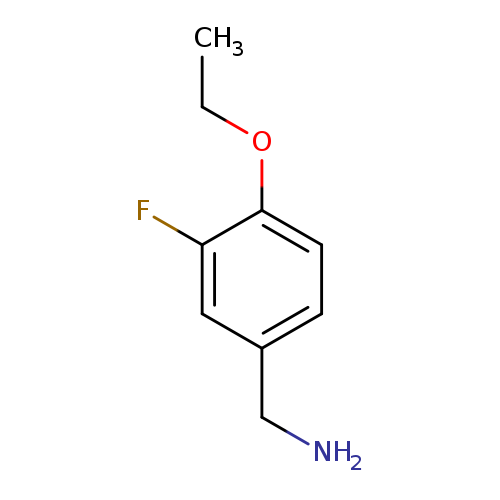
(4-Ethoxy-3-fluorophenyl)methanamineCatalog No.:AA01AB21 CAS No.:1037164-60-5 MDL No.:MFCD11191786 MF:C9H12FNO MW:169.1961 |
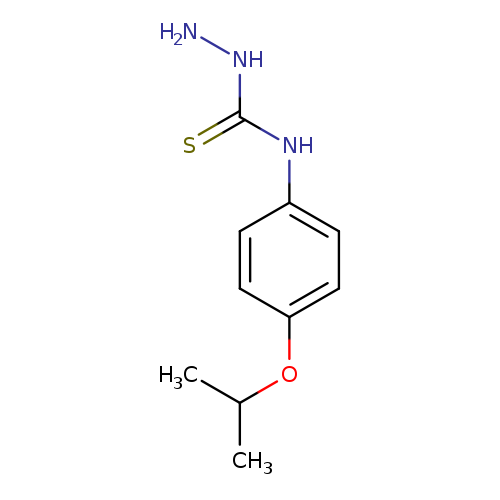
3-Amino-1-[4-(propan-2-yloxy)phenyl]thioureaCatalog No.:AA01A9QA CAS No.:1037165-03-9 MDL No.:MFCD11192533 MF:C10H15N3OS MW:225.3106 |
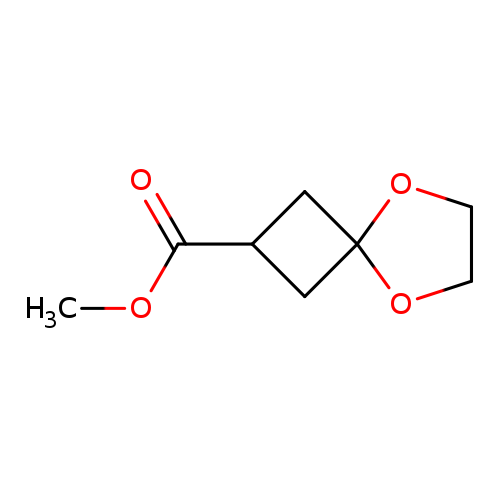
Methyl 5,8-dioxa-spiro[3.4]octane-2-carboxylateCatalog No.:AA00HA3E CAS No.:1037175-81-7 MDL No.:MFCD23106444 MF:C8H12O4 MW:172.1785 |
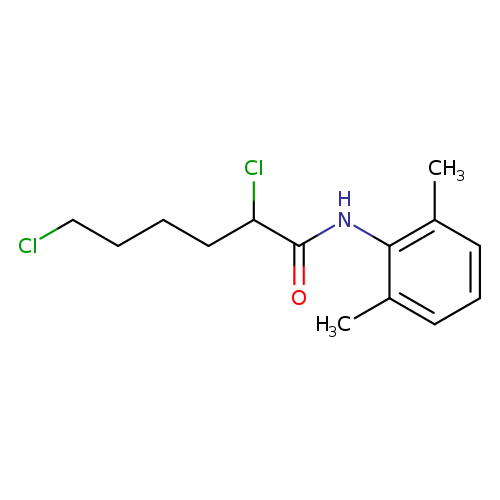
2,6-dichlorocapronic acid xylidideCatalog No.:AA008VNM CAS No.:1037184-07-8 MDL No.:MFCD25371999 MF:C14H19Cl2NO MW:288.2128 |
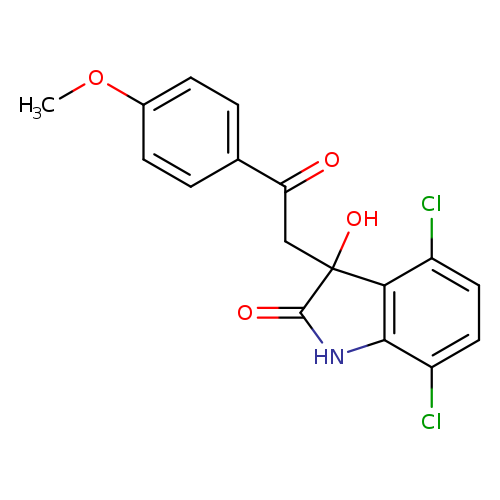
YK-4-279Catalog No.:AA008SO1 CAS No.:1037184-44-3 MDL No.:MFCD18382120 MF:C17H13Cl2NO4 MW:366.1954 |
N,N-Diethyl 3-aminobenzenesulfonamideCatalog No.:AA007X3T CAS No.:10372-41-5 MDL No.:MFCD02720457 MF:C10H16N2O2S MW:228.3112 |
2-Chloro-N,N-dimethyl-5-nitrobenzenesulfonamideCatalog No.:AA01DX4U CAS No.:10372-90-4 MDL No.:MFCD09699506 MF:C8H9ClN2O4S MW:264.6861 |
2-Amino-4-fluoro-5-methylbenzonitrileCatalog No.:AA0095UB CAS No.:1037206-84-0 MDL No.:MFCD16658717 MF:C8H7FN2 MW:150.1530 |
Methyl 2-amino-4-fluoro-5-methylbenzoateCatalog No.:AA0095BB CAS No.:1037206-86-2 MDL No.:MFCD20691241 MF:C9H10FNO2 MW:183.1796 |
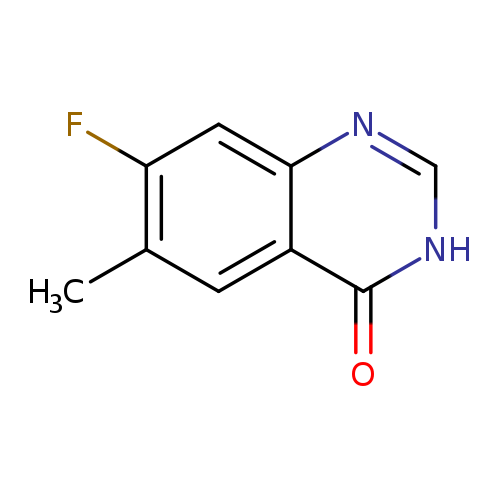
7-Fluoro-6-methyl-quinazolin-4-olCatalog No.:AA007FRN CAS No.:1037206-88-4 MDL No.:MFCD16658724 MF:C9H7FN2O MW:178.1631 |
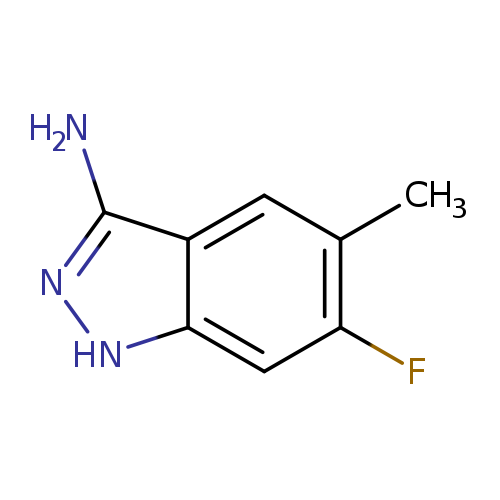
6-Fluoro-5-methyl-1h-indazol-3-amineCatalog No.:AA0096V1 CAS No.:1037206-99-7 MDL No.:MFCD16658719 MF:C8H8FN3 MW:165.1676 |
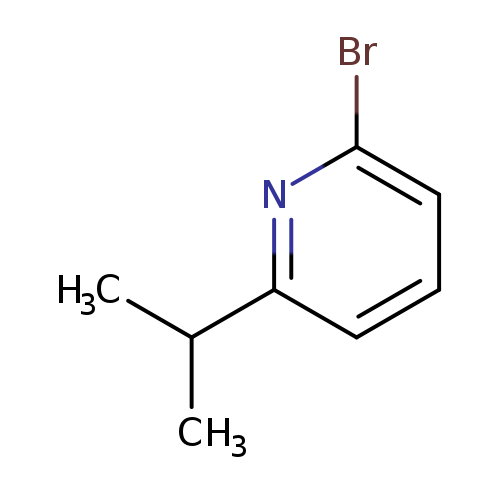
2-Bromo-6-isopropylpyridineCatalog No.:AA003GO6 CAS No.:1037223-35-0 MDL No.:MFCD11223220 MF:C8H10BrN MW:200.0757 |
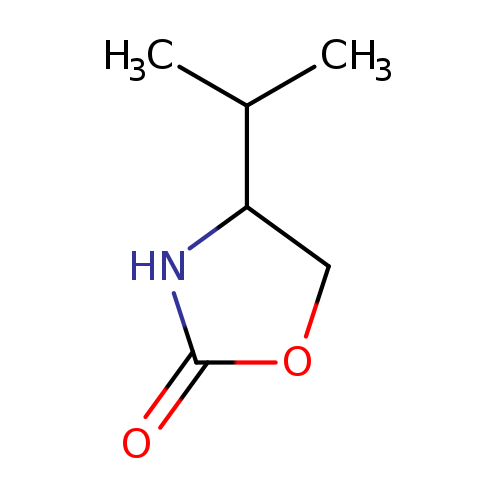
4-Isopropyloxazolidin-2-oneCatalog No.:AA018RZ2 CAS No.:103723-70-2 MDL No.:MFCD11041014 MF:C6H11NO2 MW:129.1570 |
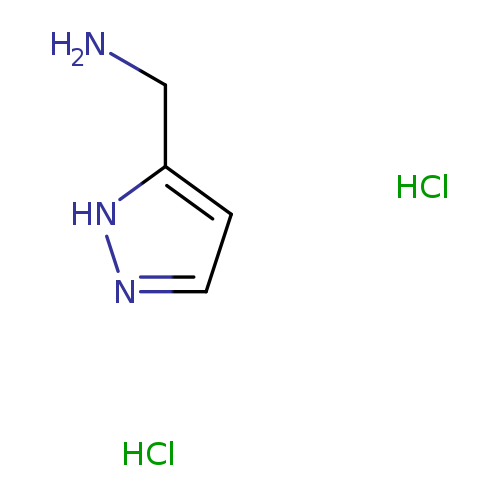
(1H-Pyrazol-3-yl)methanamine dihydrochlorideCatalog No.:AA008UBL CAS No.:1037237-32-3 MDL No.:MFCD12545920 MF:C4H9Cl2N3 MW:170.0404 |
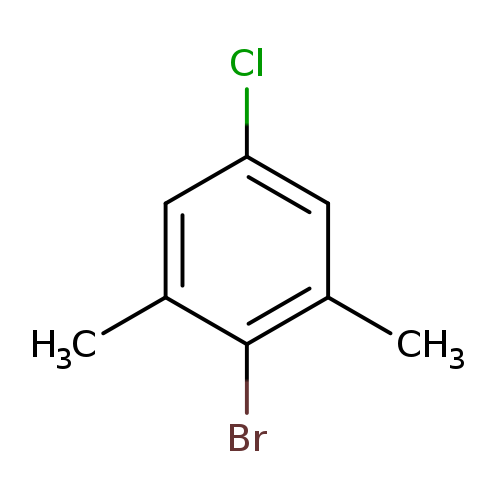
2-Bromo-5-chloro-1,3-dimethylbenzeneCatalog No.:AA008XMY CAS No.:103724-99-8 MDL No.:MFCD07780649 MF:C8H8BrCl MW:219.5061 |
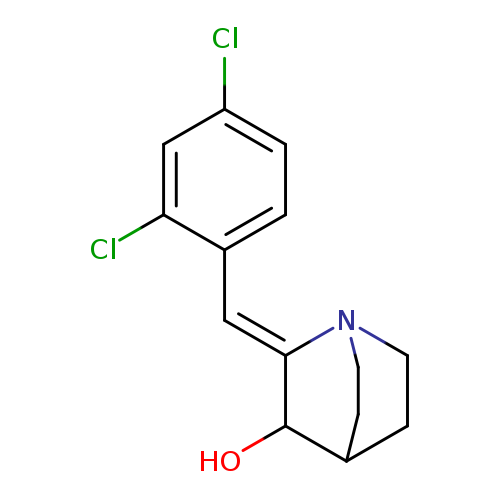
(2Z)-2-[(2,4-dichlorophenyl)methylidene]-1-azabicyclo[2.2.2]octan-3-olCatalog No.:AA00IYYV CAS No.:1037240-01-9 MDL No.:MFCD05256359 MF:C14H15Cl2NO MW:284.1810 |
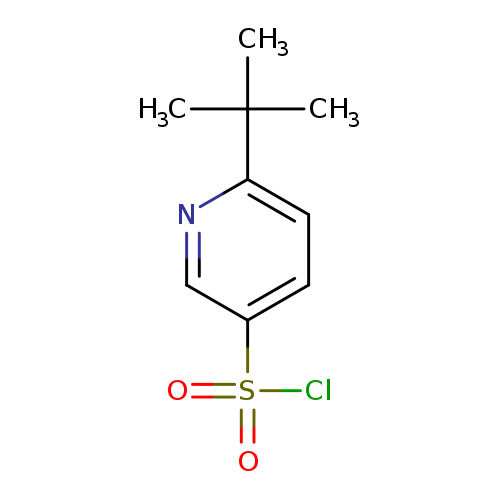
6-TERT-BUTYLPYRIDINE-3-SULFONYL CHLORIDECatalog No.:AA01DX4V CAS No.:1037241-40-9 MDL No.:MFCD19201254 MF:C9H12ClNO2S MW:233.7151 |
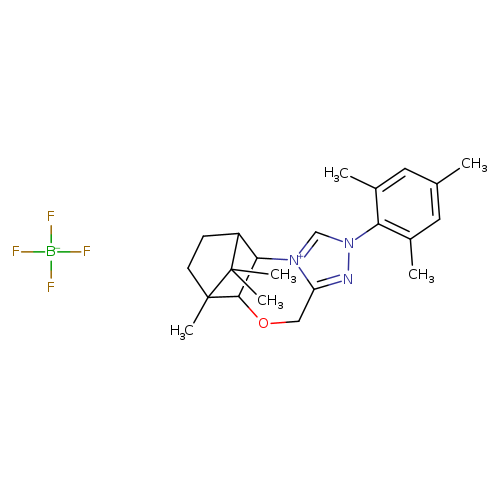
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-Hexahydro-6,11,11-triMethyl-2-(2,4,6-triMethylphenyl)-6,9-Methano-4H-[1,2,4]triazolo[3,4-c][1,4]benzoxaziniuM tetrafluoroborateCatalog No.:AA008X0V CAS No.:1037287-79-8 MDL No.:MFCD28144541 MF:C22H30BF4N3O MW:439.2977 |
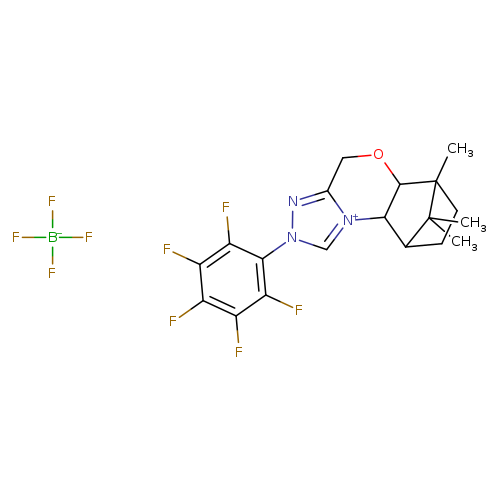
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-Hexahydro-6,11,11-triMethyl-2-(2,3,4,5,6-pentafluorophenyl)-6,9-Methano-4H-[1,2,4]triazolo[3,4-c][1,4]benzoxaziniuM tetrafluoroborateCatalog No.:AA008WE5 CAS No.:1037287-81-2 MDL No.:MFCD28144540 MF:C19H19BF9N3O MW:487.1703 |
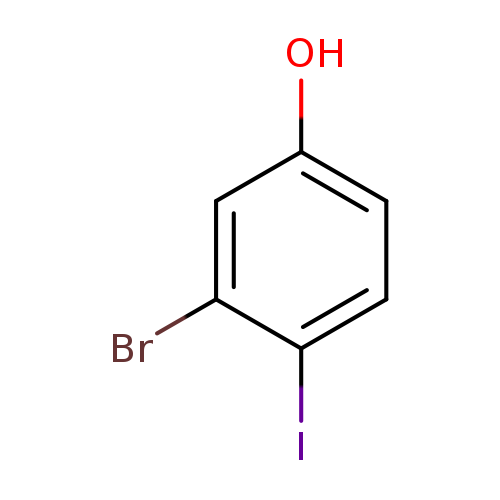
3-Bromo-4-iodophenolCatalog No.:AA003AC7 CAS No.:1037298-05-7 MDL No.:MFCD17000017 MF:C6H4BrIO MW:298.9038 |
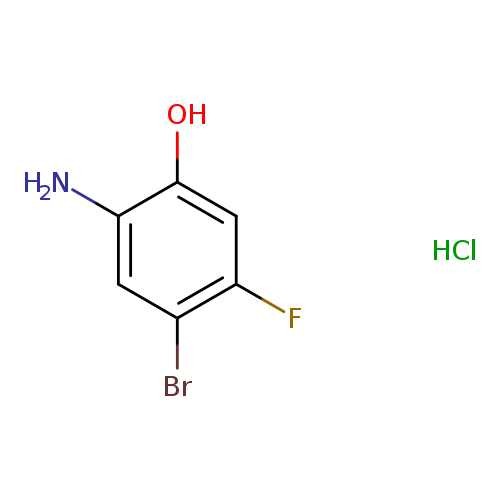
5-Bromo-4-fluoro-2-hydroxy-aniline hclCatalog No.:AA007FRL CAS No.:1037298-12-6 MDL No.:MFCD11053643 MF:C6H6BrClFNO MW:242.4733 |
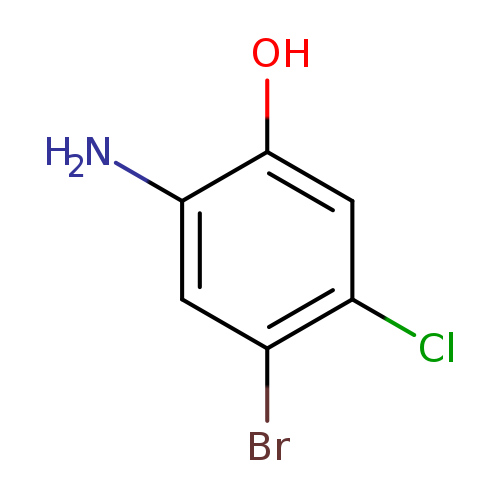
2-Amino-4-bromo-5-chlorophenolCatalog No.:AA0096XF CAS No.:1037298-14-8 MDL No.:MFCD25978069 MF:C6H5BrClNO MW:222.4670 |
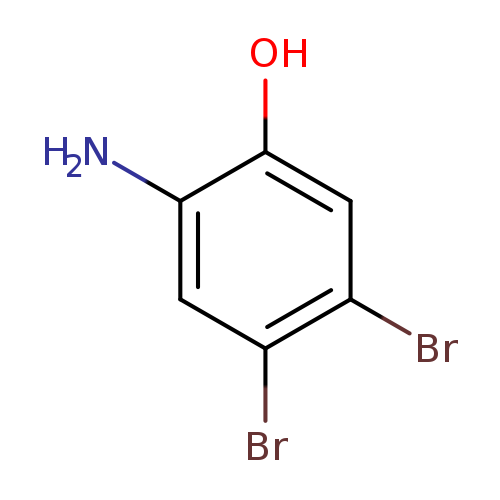
2-Amino-4,5-dibromophenolCatalog No.:AA0096KP CAS No.:1037298-16-0 MDL No.:MFCD26401820 MF:C6H5Br2NO MW:266.9180 |
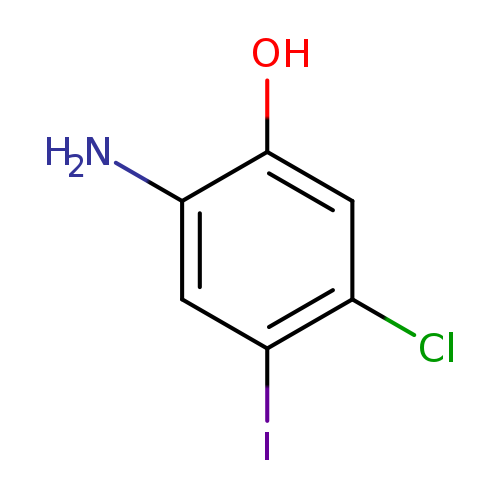
2-Amino-5-chloro-4-iodophenolCatalog No.:AA00972X CAS No.:1037298-24-0 MDL No.:MFCD27978469 MF:C6H5ClINO MW:269.4675 |
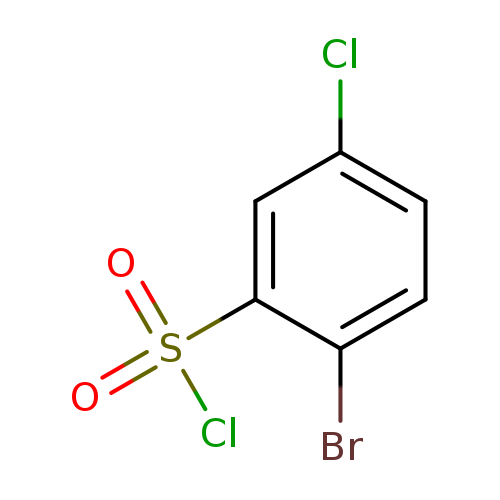
2-Bromo-5-chlorobenzene-1-sulfonyl chlorideCatalog No.:AA01A1FA CAS No.:1037299-72-1 MDL No.:MFCD18379727 MF:C6H3BrCl2O2S MW:289.9618 |
Dl-camphorquinoneCatalog No.:AA003PQW CAS No.:10373-78-1 MDL No.:MFCD00064160 MF:C10H14O2 MW:166.2170 |
H-D-Tic-otbu hclCatalog No.:AA008Y3P CAS No.:103733-29-5 MDL No.:MFCD00672372 MF:C14H20ClNO2 MW:269.7671 |
1,2,3,4-Tetrahydro-isoquinoline-1-carboxylic acid ethyl ester, HClCatalog No.:AA0085CV CAS No.:103733-33-1 MDL No.:MFCD08689587 MF:C12H16ClNO2 MW:241.7139 |
Quinapril DiketopiperazineCatalog No.:AA0085CU CAS No.:103733-49-9 MDL No.:MFCD18252326 MF:C25H28N2O4 MW:420.5008 |
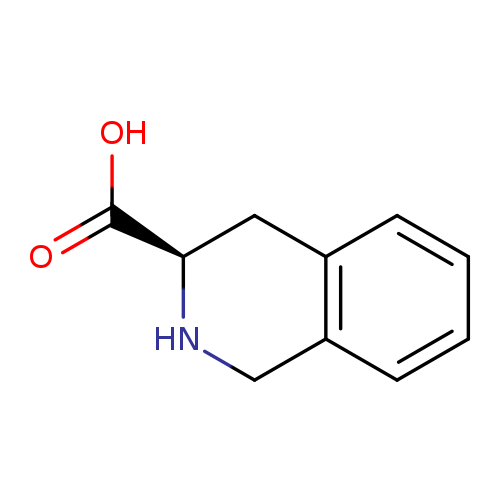
D-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acidCatalog No.:AA007FRI CAS No.:103733-65-9 MDL No.:MFCD00144038 MF:C10H11NO2 MW:177.1998 |
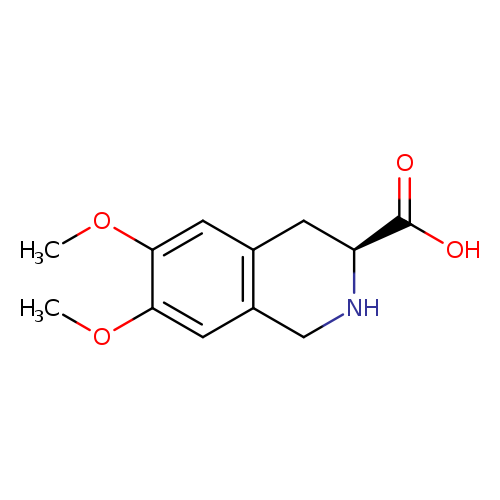
(S)-1,2,3,4-Tetrahydro-6,7-dimethoxyisoquinoline-3-carboxylic acidCatalog No.:AA0085CT CAS No.:103733-66-0 MDL No.:MFCD06796546 MF:C12H15NO4 MW:237.2518 |
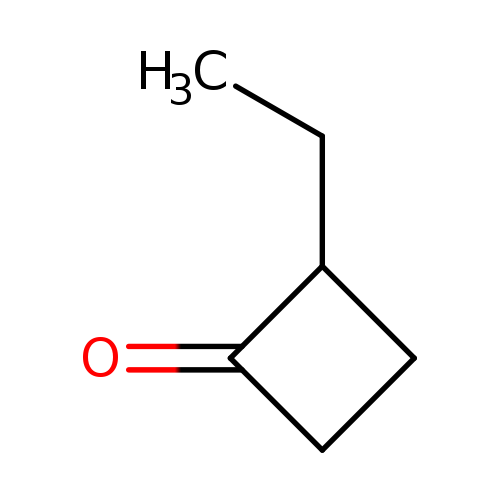
2-ETHYLCYCLOBUTANONECatalog No.:AA008T5F CAS No.:10374-14-8 MDL No.:MFCD00049150 MF:C6H10O MW:98.1430 |
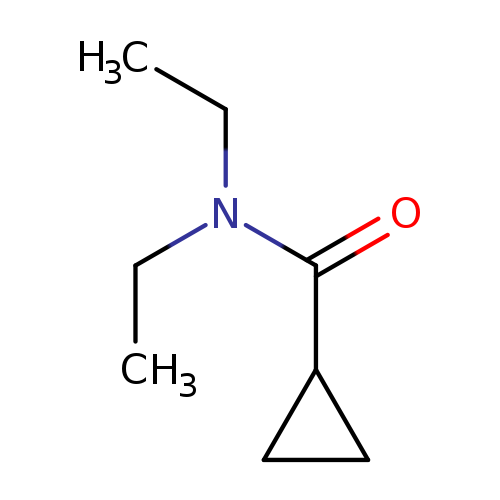
N,N-DiethylcyclopropanecarboxamideCatalog No.:AA007FRE CAS No.:10374-28-4 MDL No.:MFCD00760286 MF:C8H15NO MW:141.2108 |
4-ethoxybutanoic acidCatalog No.:AA019NBD CAS No.:10374-37-5 MDL No.:MFCD09928843 MF:C6H12O3 MW:132.1577 |
5-(Hydroxymethyl)dihydrofuran-2(3h)-oneCatalog No.:AA0035QL CAS No.:10374-51-3 MDL No.:MFCD00506240 MF:C5H8O3 MW:116.1152 |
7-TetradeceneCatalog No.:AA003NE9 CAS No.:10374-74-0 MDL No.:MFCD00009550 MF:C14H28 MW:196.3721 |
4-(3-Amino-phenyl)-thiazol-2-ylamineCatalog No.:AA007X3L CAS No.:103740-34-7 MDL No.:MFCD03015584 MF:C9H9N3S MW:191.2529 |
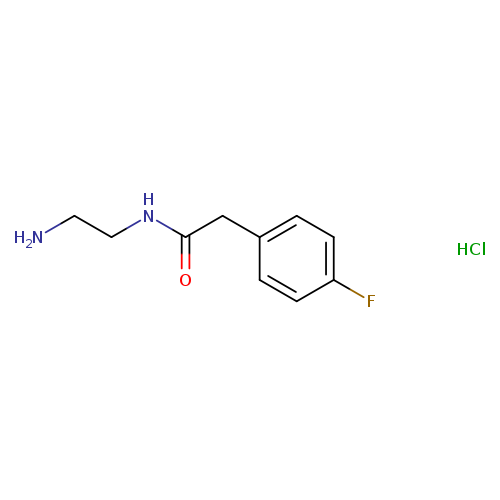
N-(2-aminoethyl)-2-(4-fluorophenyl)acetamide hydrochlorideCatalog No.:AA01EJ73 CAS No.:1037411-95-2 MDL No.:MFCD31666326 MF:C10H14ClFN2O MW:232.6824 |
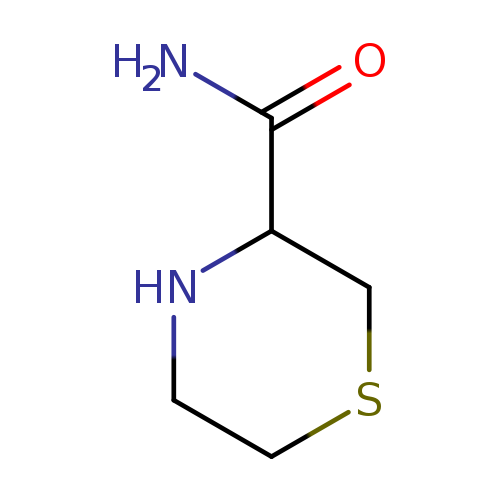
3-Thiomorpholinecarboxamide(9CI)Catalog No.:AA009SIN CAS No.:103742-31-0 MDL No.:MFCD02666349 MF:C5H10N2OS MW:146.2107 |
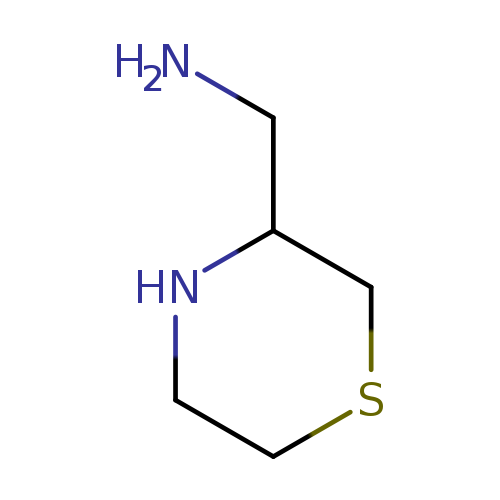
thiomorpholin-3-ylmethanamineCatalog No.:AA01A452 CAS No.:103742-33-2 MDL No.:MFCD19207011 MF:C5H12N2S MW:132.2272 |
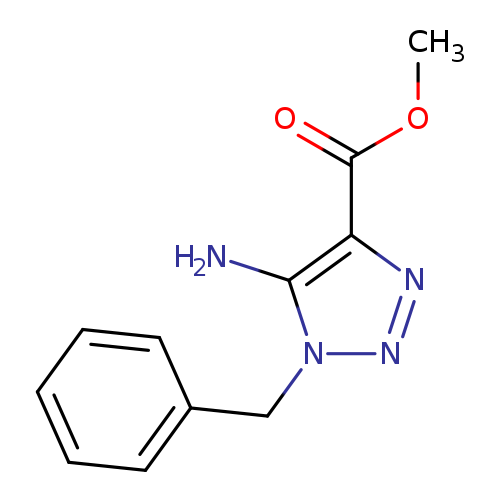
Methyl 5-amino-1-benzyl-1h-1,2,3-triazole-4-carboxylateCatalog No.:AA00J0IO CAS No.:103742-39-8 MDL No.:MFCD00100211 MF:C11H12N4O2 MW:232.2386 |
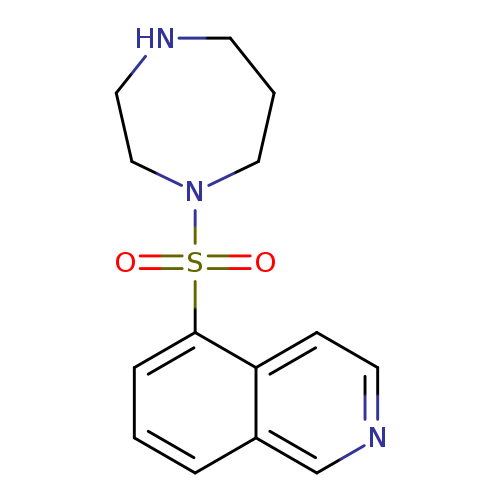
Fasudil dihydrochlorideCatalog No.:AA00HA3M CAS No.:103745-39-7 MDL No.:MFCD00153805 MF:C14H17N3O2S MW:291.3687 |
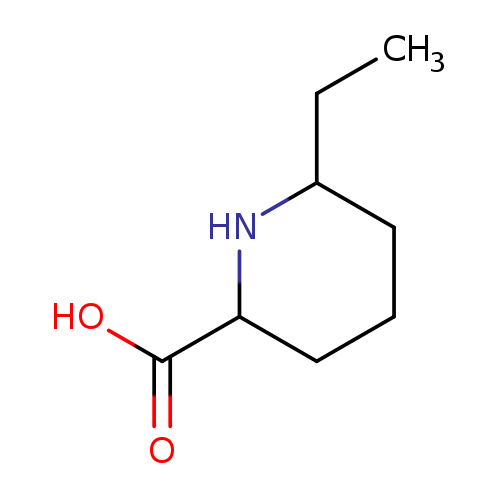
6-ETHYLPIPERIDINE-2-CARBOXYLIC ACIDCatalog No.:AA01DUUX CAS No.:1037471-93-4 MDL No.:MFCD26045085 MF:C8H15NO2 MW:157.2102 |
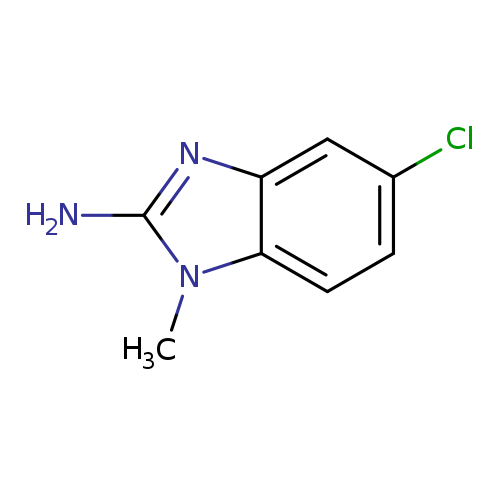
1H-Benzimidazol-2-amine,5-chloro-1-methyl-(9CI)Catalog No.:AA009P5A CAS No.:103748-25-0 MDL No.:MFCD01658290 MF:C8H8ClN3 MW:181.6222 |
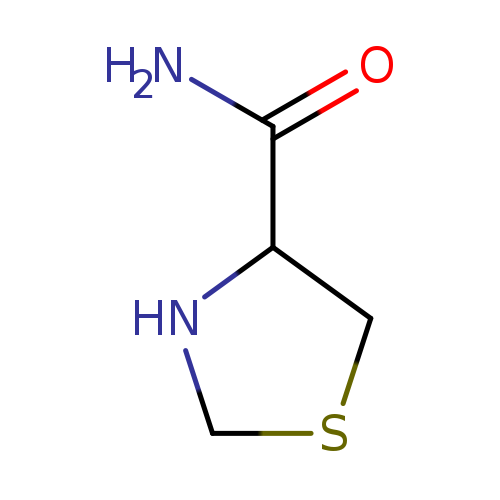
Thiazolidine-4-carboxamideCatalog No.:AA007X3K CAS No.:103749-87-7 MDL No.:MFCD07528350 MF:C4H8N2OS MW:132.1841 |
2,5-Furandicarbonyl dichlorideCatalog No.:AA007X3I CAS No.:10375-34-5 MDL No.:MFCD28044847 MF:C6H2Cl2O3 MW:192.9843 |
2,5-Dioxopyrrolidin-1-yl 5-(2,5-dioxo-2,5-dihydro-1h-pyrrol-1-yl)pentanoateCatalog No.:AA003FY0 CAS No.:103750-03-4 MDL No.:MFCD01318603 MF:C13H14N2O6 MW:294.2601 |
5-Amino-2-phenyl-2h-1,2,3-triazole-4-carboxamideCatalog No.:AA008T0B CAS No.:103752-72-3 MDL No.:MFCD02218166 MF:C9H9N5O MW:203.2007 |
3-(3,4-Dichloro-phenyl)-dihydro-furan-2-oneCatalog No.:AA007X3H CAS No.:103753-78-2 MDL No.:MFCD06796602 MF:C10H8Cl2O2 MW:231.0753 |
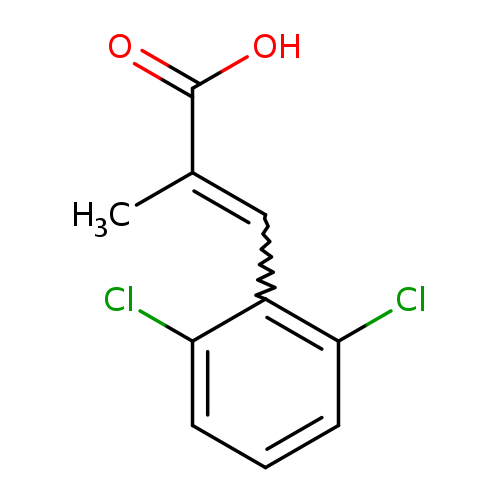
2-Propenoic acid, 3-(2,6-dichlorophenyl)-2-methyl-Catalog No.:AA01ACBL CAS No.:103753-79-3 MDL No.:MFCD00666051 MF:C10H8Cl2O2 MW:231.0753 |
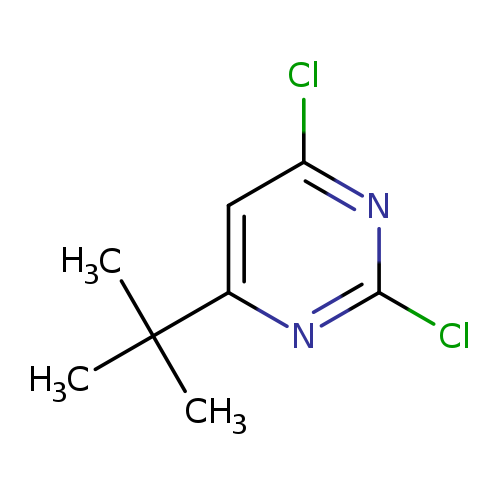
4-tert-Butyl-2,6-dichloropyrimidineCatalog No.:AA00HA3O CAS No.:1037535-38-8 MDL No.:MFCD18916413 MF:C8H10Cl2N2 MW:205.0844 |
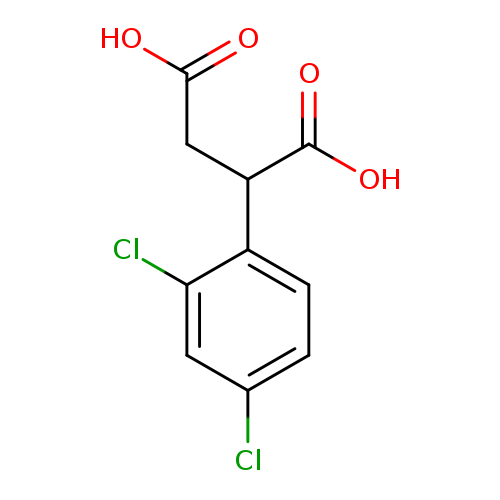
2-(2,4-Dichloro-phenyl)-succinic acidCatalog No.:AA008RM1 CAS No.:103754-45-6 MDL No.:MFCD03942288 MF:C10H8Cl2O4 MW:263.0741 |