2019-12-24 08:38:11
Jialin Xie, Zhonglin Guo, Yuanqiong Huang, Yi Qu, Hongjian Song,
Haibin Song,a Yuxiu Liu, and Qingmin Wanga, State Key Laboratory of Elemento-Organic Chemistry, Research Institute of Elemento-Organic Chemistry, College of
Chemistry, Nankai University, Tianjin 300071, People’s Republic of China
E-mail: [email protected]; [email protected] b Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, Peoples Republic of
China
Manuscript received: September 8, 2018; Revised manuscript received: November 21, 2018;
Version of record online: December 14, 2018
Supporting information for this article is available on the WWW under https://doi.org/10.1002/adsc.201801193
The majority (59%) of small-molecule drugs have nitrogen-containing heterocyclic scaffolds.[1] Indoles in particular are commonly found in biologically active molecules (Figure 1) and advanced materials and have thus attracted considerable research attention.[2] Since the classical Fischer indole synthesis was reported,[3] the number of methods for synthesizing functionalized indoles has burgeoned,[4] and the most commonmethods include metal-catalyzed cyclization reactions[5] and transition-metal-catalyzed CH bond activation reactions.[6] However, despite these advances, there are only a few methods for the construction of N-heterocyclic indoles; the most commonly used methods involve coupling reactions, such as the Buchwald-Hartwig amination and Ullman-type coupling reactions, between indoles and prefunctionalized heterocycles.[7] Except for the coupling reaction, there are few practical methods for building compounds with 2-(1H-indol-1-yl)-4,5-dihydrothiazole and 2-(1Hindol-1-yl)thiazol-5-yl aryl ketone skeletons from readily available precursors.[8]
Various nitrogen- and sulfur-containing heterocycles have been synthesized from isothiocyanate substrates.[9] In particular, o-alkynylphenyl isothiocyanates have been used to construct fused-ring compounds via cascade reactions,[10] which show higher overall efficiency than traditional stepwise syntheses because multiple CC and CX bonds can be formed in one pot.[11] For example, in 2014, Cai et al. reported the formation of 5H-benzo[d]imidazo[5,1-b][1,3]thiazines by means of copper-catalyzed [3+2] cascade cycloaddition reactions between o-alkynylphenyl isothiocyanates and isocyanides (Scheme 1a).[10c] Copper, which is abundant in nature, has a wide range of valence states and shows good affinity for molecular oxygen,[12] an environmentally friendly oxygen source for organic synthesis, although its activation remains a challenge. Therefore, copper-promoted syntheses of organic compounds under aerobic conditions have been the subject of extensive research.[13] In particular, much effort has been devoted to aerobic copperpromoted bifunctionalization of alkynes to form carbonyl compounds.[14] To our knowledge, all the reported examples of such reactions have involved aminooxygenation[14a–e] and bioxygenation[14f–h] processes (Scheme 1b), and there are no examples of alkyne bifunctionalization by means of aerobic copper-catalyzed thiooxygenation.[15] One of the limiting factors may be catalyst poisoning by sulfur species.
Inspired by the research described above, we herein report that we have achieved the first coppercatalyzed cascade bicyclization reactions between oalkynylphenyl isothiocyanates and propargylamine derivatives (Scheme 1c), despite the fact that amines are unstable to oxidants.[17] Unlike previously reported reactions of similar substrates, these reactions afforded 2-(1H-indol-1-yl)-4,5-dihydrothiazoles rather than fused-ring compounds.[10] Furthermore, the reactions could also generate 2-(1H-indol-1-yl)thiazol-5-yl aryl ketones when molecular oxygen was present.
We began by investigating the reactions of oalkynylphenyl isothiocyanates 1 and propargyl amines 2 (Scheme 2). Treatment of o-1 a (0.3 mmol) with 2 a (0.45 mmol) in the presence of CuI (15 mol%) in
CH3CN (3 mL) at 80 8C for 6 h under Ar afforded the desired product, 2-(1H-indol-1-yl)-4,5-dihydrothiazole 3 aa, in 85% yield (for detailed optimization studies, see Supporting Information [SI], Table S1).
Subsequently, we investigated the substrate scope of the reaction to afford various 2-(1H-indol-1-yl)-4,5-dihydrothiazoles 3. First, we explored the effect of substituents R1 and R2 on 1 and found that substrates 1 a–1 n, and 1 p reacted with 2 a to afford the target products in good to excellent yields. Neither the electronic properties of R1 nor its position (para or meta) markedly affected the yields of products 3 aa–3 ga.[18] The structure of 3 ca was confirmed by singlecrystal X-ray analysis (see SI, Table S3).[19] In addition, when R1 was H and R2 was an aryl group, the target products (3 ha–3 na) were obtained in satisfactory yields regardless of the electronic properties of the substituent on the aryl group. In addition, R2 could be H or cyclopropyl, but the yield of 3 oa obtained from the former substrate was relatively low (17%). The reason for the low yield of the product may be that Glaser coupling happened to the terminal alkyne substrate in the presence of amine.[20] We also investigated other aliphatic R2 groups, such as t-Bu and trimethylsilyl, but the desired products were obtained in only trace amounts.
We also evaluated the reactions of various propargylamine derivatives 2 with 1 a. To our delight, internal propargylamine derivatives (2 b–2i) containing various aryl groups were compatible with the reaction conditions, and the yields generally were70%. Even the sterically hindered substrate 3-(omethylphenyl) propargylamine (2 h) could be converted to desired product 3 ah in high yield (90%). It is noteworthy that only Z-isomers were formed, as determined by single-crystal X-ray analysis of 3 ae (see SI, Table S4);[19] this result may be due to kinetic effects, as outlined in Baldwin’s rules.[21] When the propargylamine had an a-substituent (2 j–2l), the reaction proceeded smoothly to furnish the desired products (3 aj–3 al). Surprisingly, when a nitro group was present on the phenyl ring, only aromatization product 3 am was obtained.
Intriguingly, in the presence of molecular oxygen, carbonylation product 2-(1H-indol-1-yl)thiazol-5-yl aryl ketone 4 a was obtained in 64% yield from a onepot reaction of 1 a and internal propargylamine 2 b(1.5 equiv.) in toluene at 1108C for 15 h in the presence of CuI (20 mol%) and H2O (5 equiv.) (Scheme 3; for details on the optimization of the reaction conditions, see Table S2 in the SI). Our next step was to explore the substrate scope of this aerobic reaction. First, we investigated the effect of the substituent on the aryl moiety (R3) of the internal propargylamine substrate. When the benzene ring bore a methyl group or a halogen atom or was replaced by a naphthalene ring, the desired products (4 b or 4 f, 4 c–4 e, and 4 h, respectively) were obtained in yields of 47–60%. Unfortunately, this reaction was
sensitive to steric hindrance: 4 g was not obtained from a substrate with an o-methyl group on the benzene ring. The structure of 4 c was ascertained by means of single-crystal X-ray analysis (see SI, Table S5).[19] We were pleased to find that a propargylamine with an a-aryl group furnished 4i in 40% yield.
We next examined a series of o-alkynylphenyl isothiocyanates 1, which provided products 4 j–4 u in moderate yields. Specifically, when R1 was an aryl group with an electron-donating group or an electron-withdrawing group, the desired products (4 j–4 o) were obtained in yields of 32–65%. Moreover, substrates with various aryl substituents R2 on the CC bond were also compatible with the reaction conditions, giving products 4 p–4 t regardless of the electronic properties of the substituent on the aryl group. It is noteworthy that a cyclopropane-containing substrate could also be converted to the corresponding product (4 u) in 66% yield, whereas a terminal alkyne formed only a trace of carbonylation product 4 v.
To investigate the reaction mechanism, we carried out some control experiments (Scheme 4). In the absence of a copper catalyst, reaction of 1 a with 2 a (a terminal alkyne) or 2 b (an internal alkyne) at 808C
for 4 h under argon yielded only 5; whereas when the reaction was carried out in the presence of the copper catalyst for 15 min, 5 and 2-(1H-indol-1-yl)-4,5-dihydrothiazole 3 aa or 3 ab were obtained (eq 1). Compound 6 was not detected under either of these reaction conditions. These results prove that 5 was a reaction intermediate and that it could be formed in the absence of the copper catalyst; and these tests also rule out the possibility that indole ring formation was the first step. Furthermore, we found that subjecting 5 to the optimal reaction conditions led to the formation of the final product (eq 2), confirming that 5 was indeed an intermediate.
Next, we studied the mechanism of the aerobic copper-catalyzed reaction (eqs 3–6). The reaction of 1 a and 2 b in the presence of H2 18O under an 16O2 atmosphere did not afford [18O]-4 a (eq 3), as determined by high-resolution mass spectrometry (see SI, Figure S1). This result indicates that the oxygen atom originated from molecular oxygen rather than from water. When TEMPO was added to the reaction mixture under either O2 or Ar, the radical scavenger did not markedly inhibit the reaction, and it could serve as an oxidant. Moreover, addition of the radical inhibitor BHT also did not substantially inhibit the reaction (eq 4), a result that rules out the involvement of a radical process. If the reaction was quenched after 0.5 h, we could isolate 5 b and 3 ab (eq 4), indicating that 5 b was an intermediate and that 3 ab may also have been an intermediate. To assess this possibility, we studied the reactions of 3 ab under various conditions (eq 5). Surprisingly, 4 a was obtained in 70% yield under the standard conditions (eq 5a), and only a 5% yield of 4 a was obtained in the absence of the copper salt (eq 5b). These results confirm that 3 ab was in fact an intermediate and that CuI was indispensable for the oxidation process. That 3 ab reacted in the presence of BHT confirms that a radical oxidation process was not involved (eq 5c). Moreover, neither 7 nor 3 ak underwent further oxidation under the standard conditions (eq 6), which indicates that 7 was not involved in this reaction and that the a-H of the thiazoline ring was important during oxidation.
On the basis of the results of the above-described experiments and literature reports,[11,15,22] a plausible mechanism for the formation of 3 ab and 4 a is outlined in Scheme 5. First, thiourea A is rapidly
formed by a reaction of 1 a and 2 b. Then, a coppercatalyzed intramolecular 5-exo-dig hydrothiolation reaction and a subsequent intramolecular hydroamination reaction give intermediate C. Protonation of C yields 3 ab and releases the catalyst, thus completing catalytic cycle 1. In the next step, 3 ab undergoes a copper-mediated oxidation by molecular oxygen accompanied by thiazoline-thiazole tautomerization (cycle 2)[22c] to form highly active Cu-peroxo species D, which rapidly isomerizes to organoperoxide E.
Finally, E is transformed to oxidation product 4 a and [CunOH] by removal of the benzyl proton and subsequent OO bond cleavage. [CunOH] is converted back to [Cun] by anion exchange to complete
catalytic cycle 2 and generate 1 equiv. of H2O. An alternative mechanism (cycle 3) has a low probability but has not been ruled out. This alternative mechanism involves a direct oxidative addition reaction
between a small amount of vinyl-Cu intermediate C and molecular oxygen to afford Cu-peroxo species F.
Rapid isomerization of F, OO bond cleavage, and isomerization of G generate 4 a and [CunOH]. In summary, we have developed a method for building 2-(1H-indol-1-yl)-4,5-dihydrothiazoles by means of copper-catalyzed cascade bicyclization reactions between o-alkynylphenyl isothiocyanates and propargylamine derivatives. This mild method has a wide substrate scope, shows 100% atom economy, and selectively affords Z-isomer products. When carried out in the presence of molecular oxygen, the reaction can also produce 2-(1H-indol-1-yl)thiazol-5-yl aryl ketones in moderate yields by successive formation of CC, CN, and C=O bonds. It provides an alternative method for introducing molecular oxygen, which serves both as the oxygen source and as the oxidant, into the thiazole skeleton. Studies of the use of this method for the construction of other nitrogen heterocycles are in progress in our laboratory.
Experimental Section
2-(1H-Indol-1-yl)-4,5-dihydrothiazoles: An oven-dried Schlenk tube was charged with o-alkynylphenyl isothiocyanate 1 a (0.3 mmol, 1 equiv.), propargylamine derivative 2 a(0.45 mmol, 1.5 equiv.), CuI (0.045 mmol, 15 mol%), and dry CH3CN (3.0 mL). The tube was evacuated and backfilled with Ar three times. The reaction mixture was stirred under Ar at room temperature for 15 min and then at 808C for 6 h.
After concentration of the solution in vacuo, the residue was purified via flash silica gel column chromatography with petroleum ether/ethyl acetate as the eluent to afford desired compound 3. 2-(1H-Indol-1-yl)thiazol-5-yl Aryl Ketones: A oven-dried Schlenk tube was charged with an o-alkynylphenyl isothiocyanate 1 (0.2 mmol, 1 equiv.), a propargylamine derivative 2 (0.3 mmol, 1.5 equiv.), CuI (0.040 mmol, 20 mol%), H2O (1.0 mmol, 5 equiv.), and dry toluene (3.0 mL). The tube was evacuated and backfilled with oxygen three times. The reaction mixture was stirred under oxygen at 308C for 20 min and then at 110 8C for 15 h. After concentration of the solution in vacuo, the residue was purified via flash silica gel column chromatography with petroleum ether/ethyl acetate as the eluent to afford desired compound 4.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (nos. 21602117, 21672117, 21732002) and the Tianjin Natural Science Foundation (no. 16JCZDJC32400) for generous financial support for our programs.
2-Phenyl-3-(thiophen-2-yl)acrylic acidCatalog No.:AA007VFC CAS No.:10569-35-4 MDL No.:MFCD01207139 MF:C13H10O2S MW:230.2823 |
(1S)-2-Amino-1-(4-chlorophenyl)-1-[4-(1H-pyrazol-4-yl)phenyl]ethanolCatalog No.:AA008TAT CAS No.:1056901-62-2 MDL No.:MFCD25976789 MF:C17H16ClN3O MW:313.7814 |
tert-Butyl 4-chloro-7,8-dihydropyrido[4,3-d]pyrimidine-6(5h)-carboxylateCatalog No.:AA00HAM2 CAS No.:1056934-87-2 MDL No.:MFCD08273922 MF:C12H16ClN3O2 MW:269.7273 |
Butyl 2-fluoro-6-hydroxybenzoateCatalog No.:AA01BGRZ CAS No.:1056955-09-9 MDL No.:MFCD28139412 MF:C11H13FO3 MW:212.2175 |
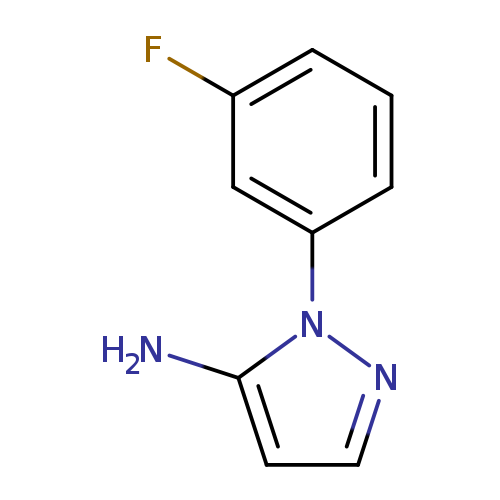
1-(3-Fluorophenyl)-1h-pyrazol-5-amineCatalog No.:AA01ACT5 CAS No.:1056999-20-2 MDL No.:MFCD14690582 MF:C9H8FN3 MW:177.1783 |
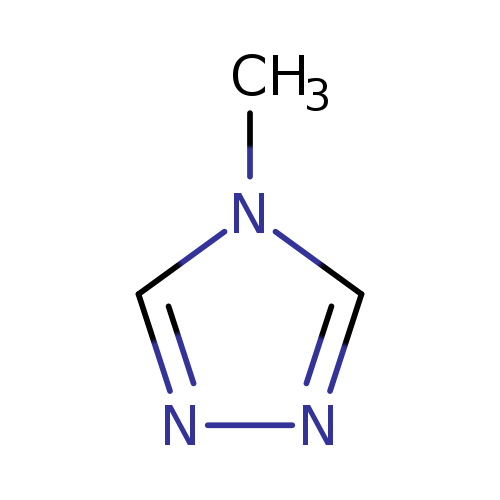
4-Methyl-4h-1,2,4-triazoleCatalog No.:AA0091WG CAS No.:10570-40-8 MDL No.:MFCD00490021 MF:C3H5N3 MW:83.0919 |
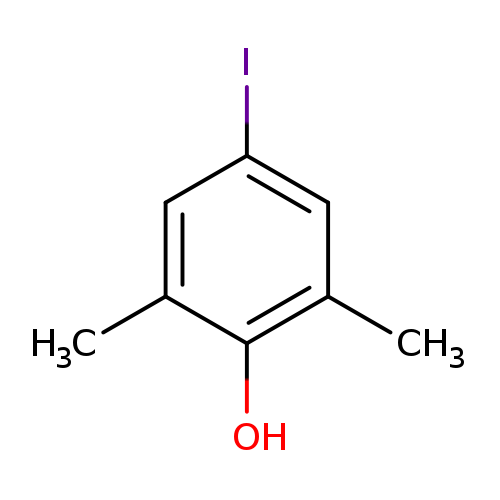
2,6-Dimethyl-4-iodophenolCatalog No.:AA008QYM CAS No.:10570-67-9 MDL No.:MFCD00094415 MF:C8H9IO MW:248.0609 |
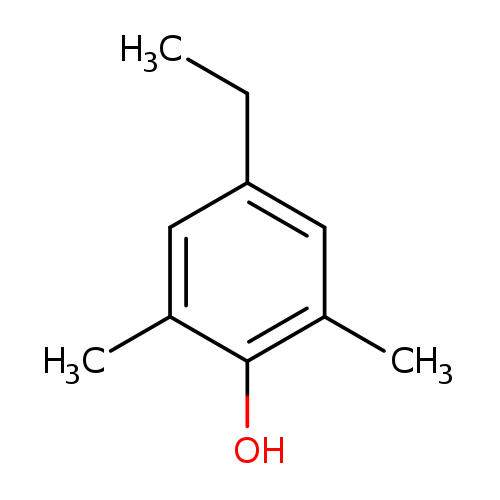
4-ethyl-2,6-dimethylphenolCatalog No.:AA01BTBI CAS No.:10570-69-1 MDL No.:MFCD24713596 MF:C10H14O MW:150.2176 |
N-Phenyl-N-(4-phenyl-2-sulfanylidene-2,3-dihydro-1,3-thiazol-3-yl)benzamideCatalog No.:AA01F48Y CAS No.:105701-07-3 MDL No.:MFCD00959668 MF:C22H16N2OS2 MW:388.5052 |
3-(Benzylamino)-2-(methylsulfanyl)-4-phenyl-1,3-thiazol-3-ium iodideCatalog No.:AA01F533 CAS No.:105701-08-4 MDL No.:MFCD00958824 MF:C17H17IN2S2 MW:440.3648 |
6-(Chloromethyl)-n-(4-fluorophenyl)-1,3,5-triazine-2,4-diamineCatalog No.:AA019OQI CAS No.:105704-32-3 MDL No.:MFCD03941309 MF:C10H9ClFN5 MW:253.6634 |
1-Cbz-2-hydroxymethyl-piperidineCatalog No.:AA0038X4 CAS No.:105706-75-0 MDL No.:MFCD04114983 MF:C14H19NO3 MW:249.3056 |
1-Cbz-piperidine-2-aldehydeCatalog No.:AA008RSX CAS No.:105706-76-1 MDL No.:MFCD02179023 MF:C14H17NO3 MW:247.2897 |
Benzyl 2-formylpyrrolidine-1-carboxylateCatalog No.:AA00859L CAS No.:105706-84-1 MDL No.:MFCD04115610 MF:C13H15NO3 MW:233.2631 |
3-(2-Methylpropyl)-1,2-oxazol-5-amineCatalog No.:AA00HAM8 CAS No.:1057064-36-4 MDL No.:MFCD10692336 MF:C7H12N2O MW:140.1830 |
1-(3-Amino-5-phenylthiophen-2-yl)ethanoneCatalog No.:AA003G84 CAS No.:105707-24-2 MDL No.:MFCD00068132 MF:C12H11NOS MW:217.2868 |
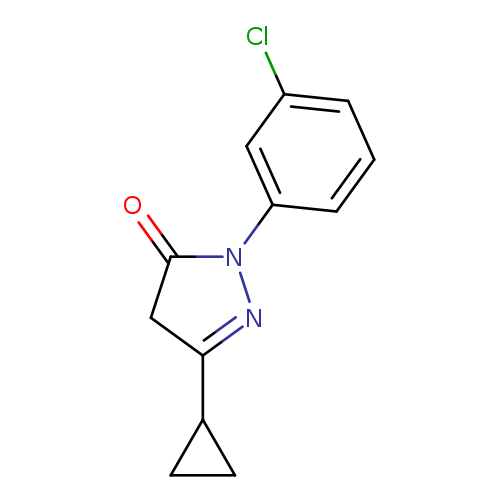
1-(3-chlorophenyl)-3-cyclopropyl-4,5-dihydro-1H-pyrazol-5-oneCatalog No.:AA01AMWH CAS No.:1057093-34-1 MDL No.:MFCD18337384 MF:C12H11ClN2O MW:234.6815 |
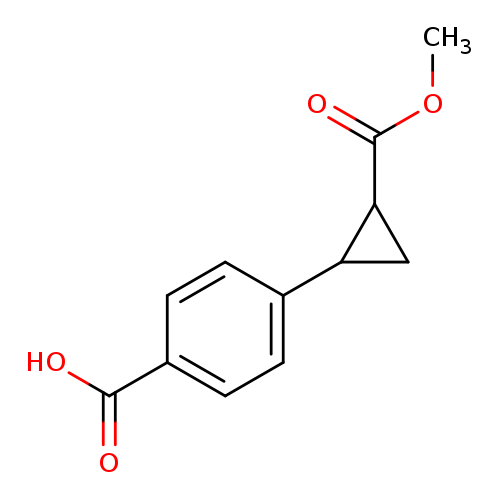
4-(2-(Methoxycarbonyl)cyclopropyl)benzoic acidCatalog No.:AA0093NV CAS No.:1057107-39-7 MDL No.:MFCD22393665 MF:C12H12O4 MW:220.2213 |
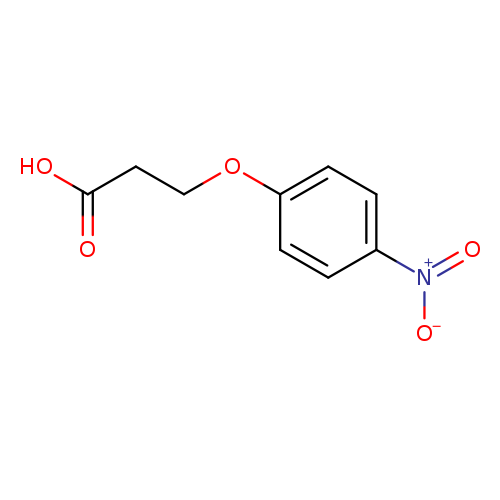
3-(4-Nitrophenoxy)propionic acidCatalog No.:AA003I2H CAS No.:10572-16-4 MDL No.:MFCD00043748 MF:C9H9NO5 MW:211.1715 |
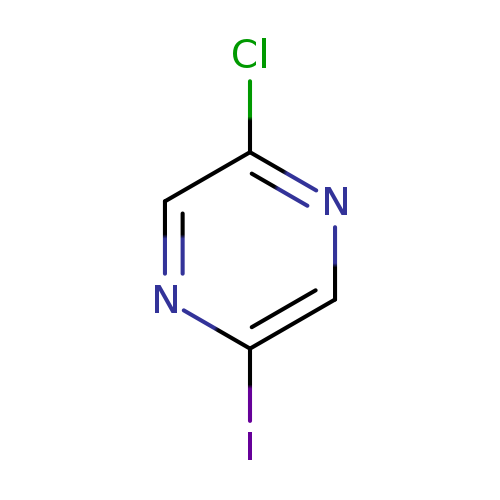
2-Chloro-5-iodopyrazineCatalog No.:AA007WYG CAS No.:1057216-55-3 MDL No.:MFCD09909701 MF:C4H2ClIN2 MW:240.4296 |
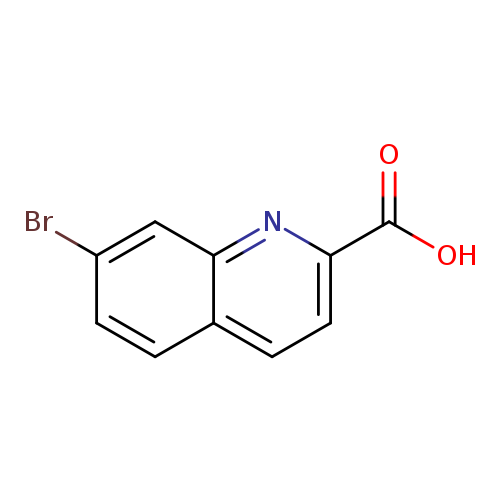
7-Bromoquinoline-2-carboxylic acidCatalog No.:AA008YYG CAS No.:1057217-63-6 MDL No.:MFCD11108693 MF:C10H6BrNO2 MW:252.0641 |
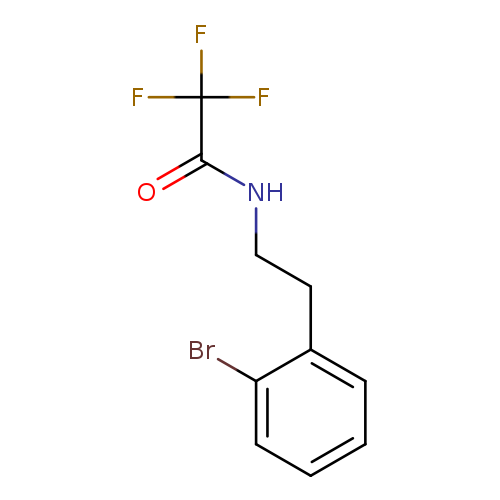
N-[2-(2-bromophenyl)ethyl]-2,2,2-trifluoroacetamideCatalog No.:AA00HAMB CAS No.:1057246-44-2 MDL No.:MFCD28096633 MF:C10H9BrF3NO MW:296.0838 |
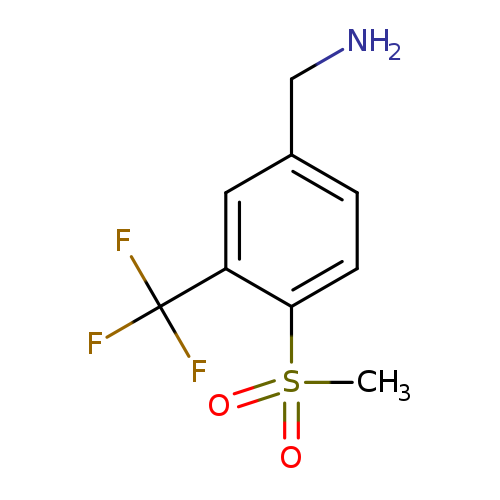
[4-methanesulfonyl-3-(trifluoromethyl)phenyl]methanamineCatalog No.:AA01BEKA CAS No.:1057260-42-0 MDL No.:MFCD26051434 MF:C9H10F3NO2S MW:253.2414 |
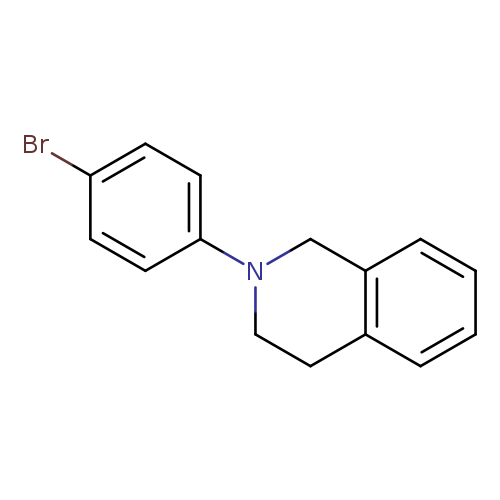
2-(4-Bromophenyl)-1,2,3,4-tetrahydroisoquinolineCatalog No.:AA0091VQ CAS No.:1057279-05-6 MDL No.:MFCD20919931 MF:C15H14BrN MW:288.1824 |
(3-FORMYL-4-NITROPHENOXY)ACETIC ACIDCatalog No.:AA008R16 CAS No.:105728-06-1 MDL No.:MFCD03840321 MF:C9H7NO6 MW:225.1550 |
2-Fluoro-5-methoxybenzaldehydeCatalog No.:AA00338L CAS No.:105728-90-3 MDL No.:MFCD00070795 MF:C8H7FO2 MW:154.1384 |
1-((3-Chloro-5-(trifluoromethyl)pyridin-2-yl)methyl)piperidin-4-oneCatalog No.:AA00IRRD CAS No.:1057282-45-7 MDL No.:MFCD20921567 MF:C12H12ClF3N2O MW:292.6847 |
1-(furan-2-yl)-2-methoxyethan-1-oneCatalog No.:AA01B8S0 CAS No.:105729-08-6 MDL No.:MFCD12062662 MF:C7H8O3 MW:140.1366 |
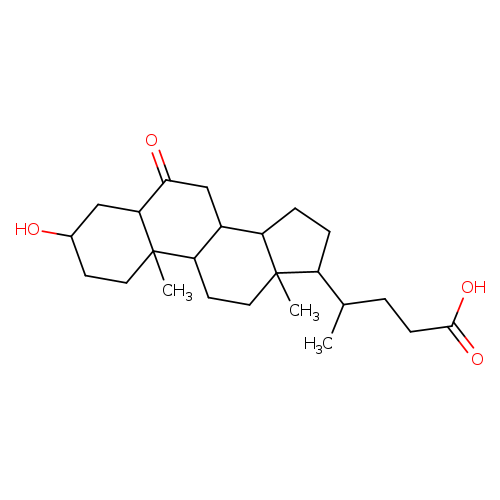
3-ALPHA-HYDROXY-6-OXO-5-ALPHA-CHOLAN-24-OIC ACIDCatalog No.:AA008XJI CAS No.:10573-17-8 MDL No.:MFCD09038290 MF:C24H38O4 MW:390.5561 |
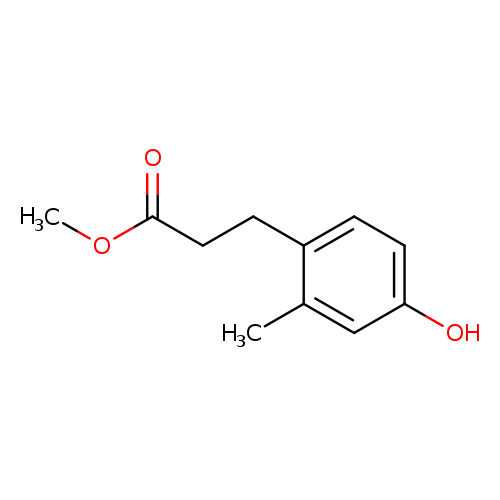
Methyl 3-(4-hydroxy-2-methylphenyl)propanoateCatalog No.:AA009K6H CAS No.:105731-18-8 MDL No.:MFCD11616255 MF:C11H14O3 MW:194.2271 |
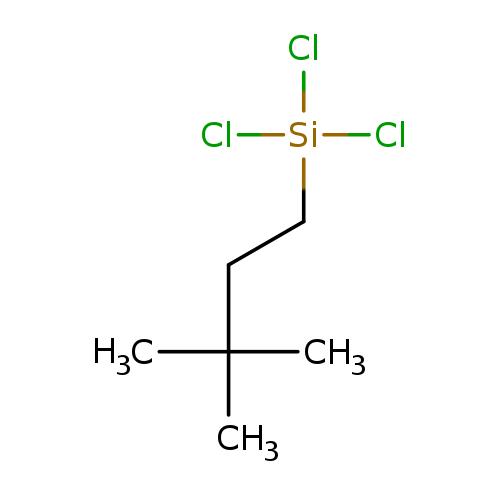
Silane,trichloro(3,3-dimethylbutyl)-Catalog No.:AA007FF1 CAS No.:105732-02-3 MDL No.:MFCD09909990 MF:C6H13Cl3Si MW:219.6119 |
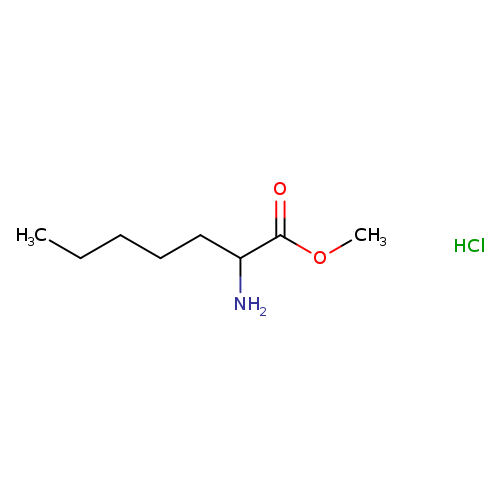
Methyl 2-aminoheptanoate hydrochlorideCatalog No.:AA019YGI CAS No.:105732-43-2 MDL No.:MFCD22196422 MF:C8H18ClNO2 MW:195.6870 |
3-Amino-5-chloro-2-iodopyridineCatalog No.:AA008XTL CAS No.:1057322-74-3 MDL No.:MFCD16556271 MF:C5H4ClIN2 MW:254.4561 |
3-amino-3-phenylprop-2-enethioamideCatalog No.:AA01AIAS CAS No.:1057326-92-7 MDL No.:MFCD18917279 MF:C9H10N2S MW:178.2541 |
tert-Butyl 4-chloro-8,9-dihydro-5h-pyrimido[5,4-d]azepine-7(6h)-carboxylateCatalog No.:AA007WY9 CAS No.:1057338-27-8 MDL No.:MFCD11045433 MF:C13H18ClN3O2 MW:283.7539 |
3-Bromo-5,6,7,8-tetrahydroimidazo[1,2-a]pyrazine dihydrochlorideCatalog No.:AA0092EC CAS No.:1057338-30-3 MDL No.:MFCD11506085 MF:C6H10BrCl2N3 MW:274.9737 |
4-Methyl-2-oxo-2h-chromen-7-yl chloroacetateCatalog No.:AA007FEG CAS No.:105738-24-7 MDL No.:MFCD01034923 MF:C12H9ClO4 MW:252.6505 |
(2E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-oneCatalog No.:AA00IRGJ CAS No.:1057383-66-0 MDL No.:MFCD02187201 MF:C13H17NO3 MW:235.2790 |
1-(cyclopropanesulfonyl)piperazine hydrochlorideCatalog No.:AA01AQG6 CAS No.:1057385-13-3 MDL No.:MFCD16653430 MF:C7H15ClN2O2S MW:226.7242 |
1-(4-Iodopyridin-2-yl)hydrazineCatalog No.:AA0093K1 CAS No.:1057393-44-8 MDL No.:MFCD10000014 MF:C5H6IN3 MW:235.0257 |
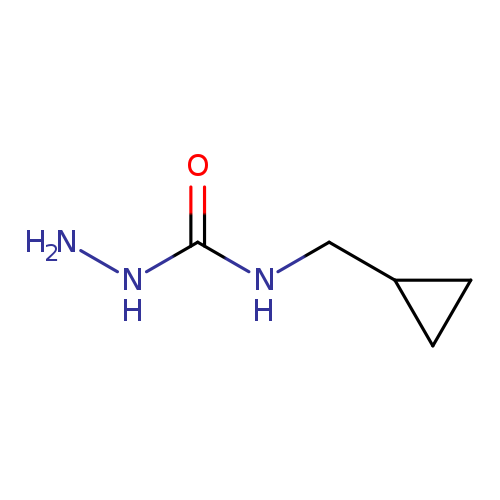
3-Amino-1-(cyclopropylmethyl)ureaCatalog No.:AA019XRB CAS No.:1057393-54-0 MDL No.:MFCD11212750 MF:C5H11N3O MW:129.1603 |
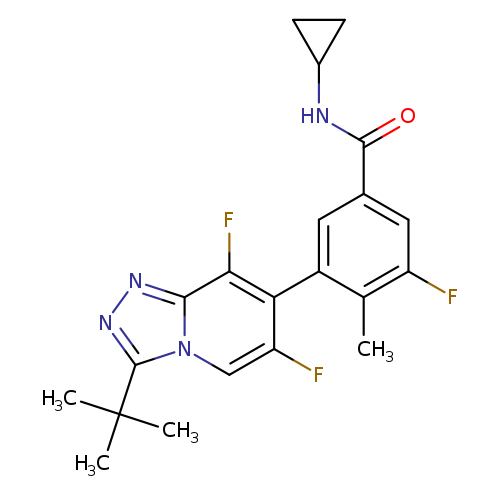
AL8697Catalog No.:AA01DZEE CAS No.:1057394-06-5 MDL No.:MFCD28133371 MF:C21H21F3N4O MW:402.4128 |
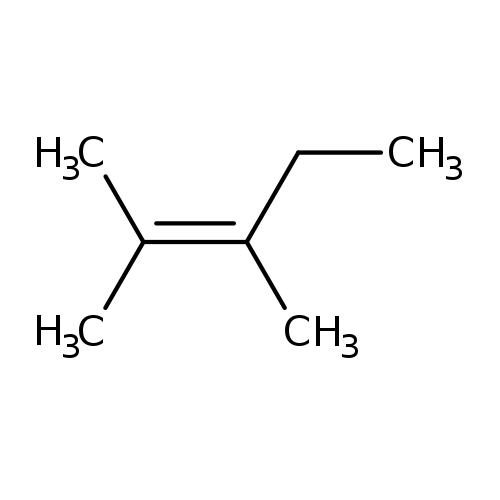
2,3-DIMETHYL-2-PENTENECatalog No.:AA008T5O CAS No.:10574-37-5 MDL No.:MFCD00027048 MF:C7H14 MW:98.1861 |
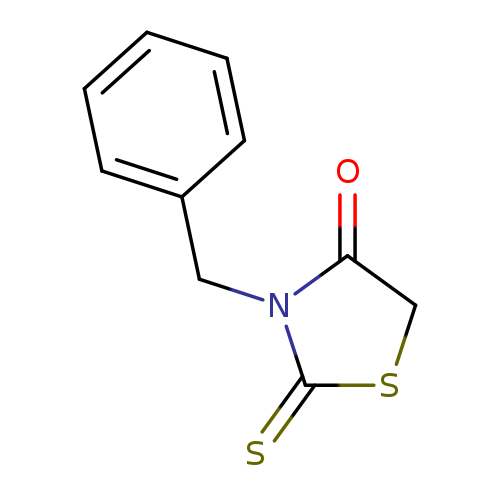
3-BenzylrhodanineCatalog No.:AA003IW8 CAS No.:10574-69-3 MDL No.:MFCD00086924 MF:C10H9NOS2 MW:223.3146 |
3-chloro-5-methyl-1H-indole-2-carboxylic acidCatalog No.:AA019MPW CAS No.:1057404-44-0 MDL No.:MFCD11052580 MF:C10H8ClNO2 MW:209.6290 |
Ganoderic acid C6Catalog No.:AA008Z6N CAS No.:105742-76-5 MDL No.:MFCD21333706 MF:C30H42O8 MW:530.6497 |
H-Alpha-me-leu-ohCatalog No.:AA008R0K CAS No.:105743-53-1 MDL No.:MFCD01716279 MF:C7H15NO2 MW:145.1995 |
BrassininCatalog No.:AA00851W CAS No.:105748-59-2 MDL No.:MFCD01632490 MF:C11H12N2S2 MW:236.3564 |
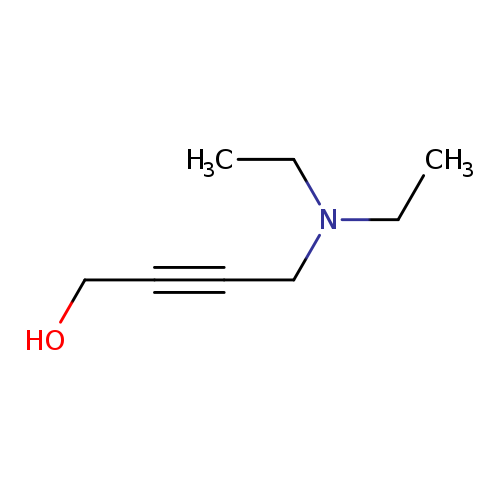
4-(Diethylamino)but-2-yn-1-olCatalog No.:AA003L7H CAS No.:10575-25-4 MDL No.:MFCD00671356 MF:C8H15NO MW:141.2108 |
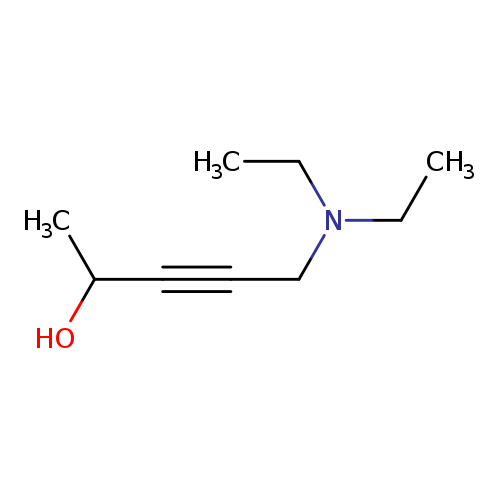
3-Pentyn-2-ol,5-(diethylamino)-Catalog No.:AA00851T CAS No.:10575-26-5 MDL No.:MFCD01763223 MF:C9H17NO MW:155.2374 |
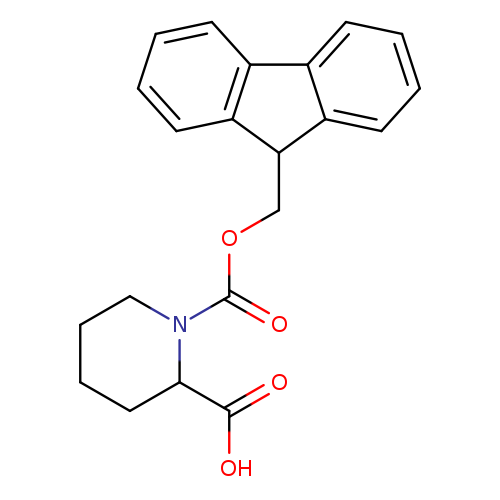
Fmoc-dl-pipecolic acidCatalog No.:AA008RTY CAS No.:105751-19-7 MDL No.:MFCD01823265 MF:C21H21NO4 MW:351.3957 |
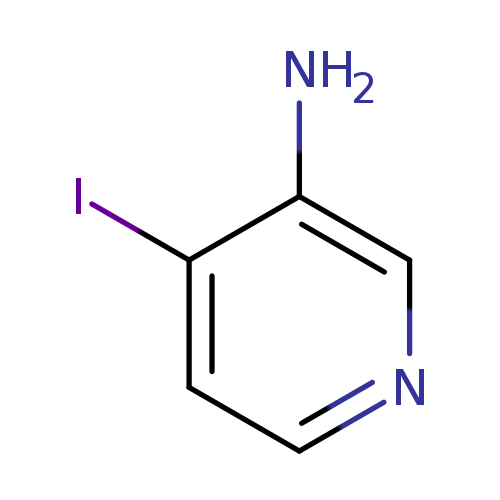
3-Amino-4-iodopyridineCatalog No.:AA0037YA CAS No.:105752-11-2 MDL No.:MFCD04971334 MF:C5H5IN2 MW:220.0111 |
Ethyl acetohydroximateCatalog No.:AA008R3L CAS No.:10576-12-2 MDL No.:MFCD00002114 MF:C4H9NO2 MW:103.1198 |
(R)-2-Aminobut-3-enoic acid hydrochlorideCatalog No.:AA0097DT CAS No.:105763-41-5 MDL No.:MFCD01862356 MF:C4H8ClNO2 MW:137.5648 |
2,4-Dichloro-6-methoxyquinazolineCatalog No.:AA003FNZ CAS No.:105763-77-7 MDL No.:MFCD09954880 MF:C9H6Cl2N2O MW:229.0627 |
5-(Hydroxymethyl)-2-methoxybenzene-1-sulfonamideCatalog No.:AA01BIBZ CAS No.:105764-06-5 MDL No.:MFCD21298877 MF:C8H11NO4S MW:217.2422 |
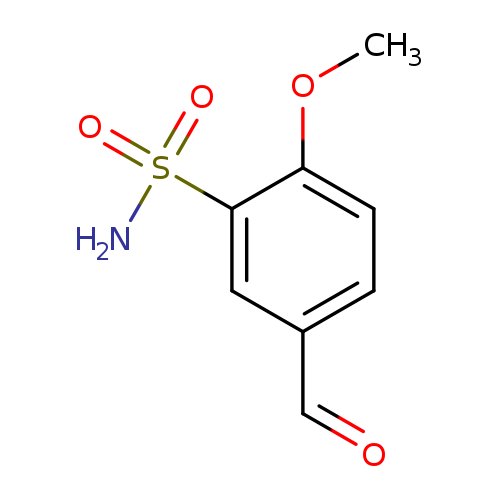
5-Formyl-2-methoxybenzenesulfonamideCatalog No.:AA008WIR CAS No.:105764-07-6 MDL No.:MFCD25955005 MF:C8H9NO4S MW:215.2264 |
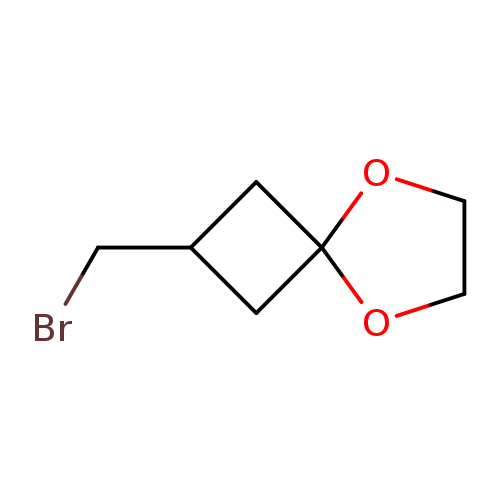
2-Bromomethyl-5,8-dioxaspiro[3.4]octaneCatalog No.:AA00HAMQ CAS No.:1057641-71-0 MDL No.:MFCD23106219 MF:C7H11BrO2 MW:207.0650 |
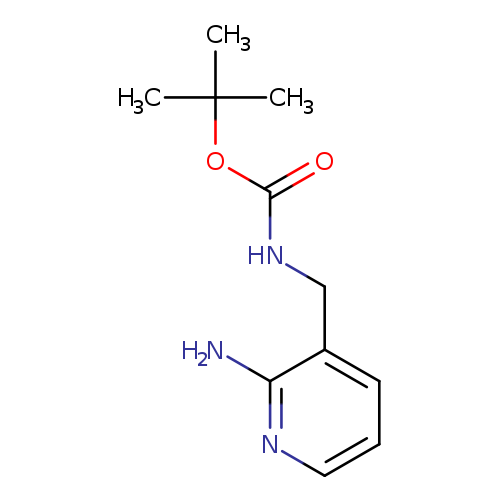
tert-Butyl (2-amino-3-pyridinyl)methylcarbamateCatalog No.:AA01C19K CAS No.:1057650-80-2 MDL No.:MFCD16293635 MF:C11H17N3O2 MW:223.2716 |
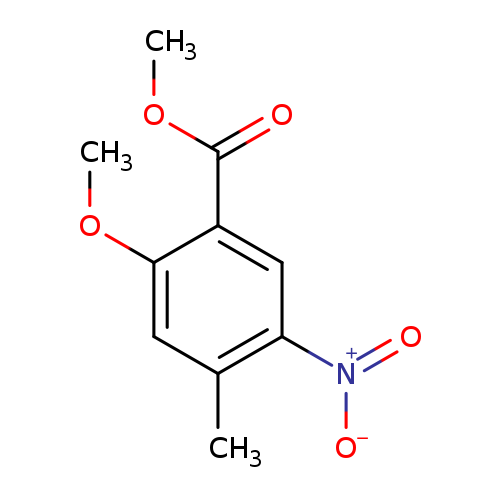
Methyl 2-methoxy-4-methyl-5-nitrobenzoateCatalog No.:AA008TF1 CAS No.:1057652-79-5 MDL No.:MFCD19443222 MF:C10H11NO5 MW:225.1980 |
4-Bromo-3-cyclopropyl-5-(trifluoromethyl)-1H-pyrazoleCatalog No.:AA00HAMR CAS No.:1057659-72-9 MDL No.:MFCD28991862 MF:C7H6BrF3N2 MW:255.0351 |
2-Bromo-1-ethynyl-4-fluorobenzeneCatalog No.:AA0093RM CAS No.:1057670-01-5 MDL No.:MFCD08703532 MF:C8H4BrF MW:199.0198 |
2-Chloro-4-ethynyl-1-fluorobenzeneCatalog No.:AA0093S6 CAS No.:1057670-04-8 MDL No.:MFCD08703540 MF:C8H4ClF MW:154.5688 |
5-Ethynyl-2,3-dimethylthiopheneCatalog No.:AA01BGXK CAS No.:1057670-09-3 MDL No.:MFCD08703552 MF:C8H8S MW:136.2141 |
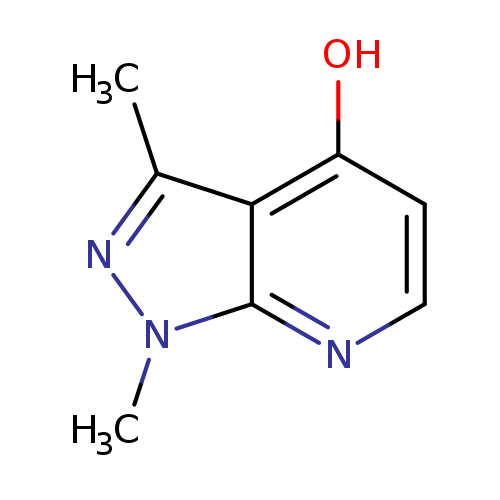
1,3-dimethyl-1H-pyrazolo[3,4-b]pyridin-4-olCatalog No.:AA01BFGZ CAS No.:1057670-30-0 MDL No.:MFCD00109112 MF:C8H9N3O MW:163.1766 |
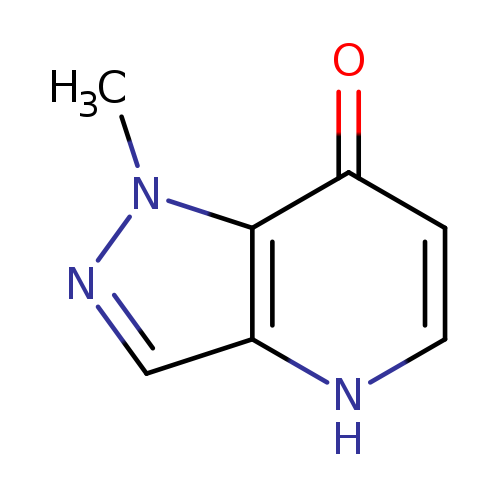
1-Methyl-1H-pyrazolo[4,3-b]pyridin-7-olCatalog No.:AA008XE8 CAS No.:1057670-31-1 MDL No.:MFCD08704235 MF:C7H7N3O MW:149.1500 |
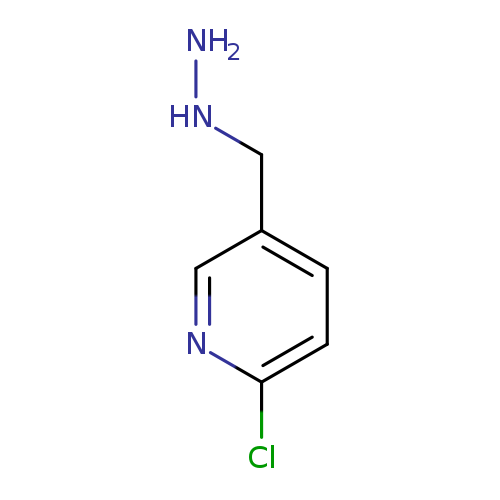
2-Chloro-5-(hydrazinylmethyl)pyridineCatalog No.:AA008UA7 CAS No.:1057670-48-0 MDL No.:MFCD08704288 MF:C6H8ClN3 MW:157.6008 |
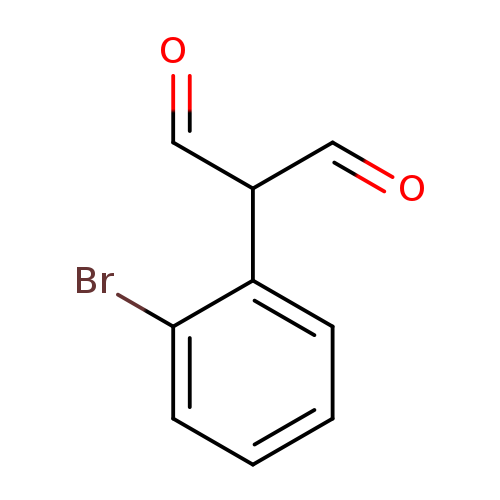
2-(2-bromophenyl)propanedialCatalog No.:AA01DX6M CAS No.:1057670-77-5 MDL No.:MFCD02261893 MF:C9H7BrO2 MW:227.0547 |
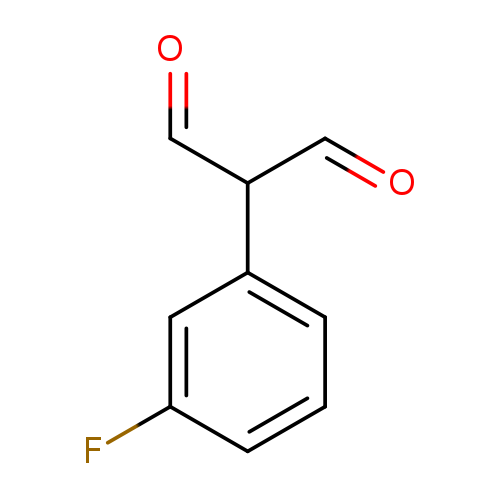
2-(3-fluorophenyl)propanedialCatalog No.:AA01DX6N CAS No.:1057670-78-6 MDL No.:MFCD02261921 MF:C9H7FO2 MW:166.1491 |
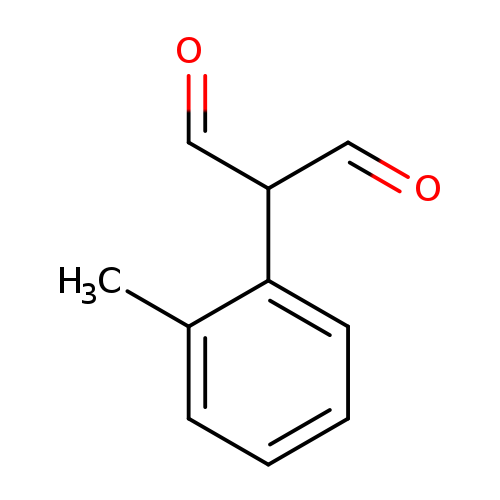
2-(2-methylphenyl)propanedialCatalog No.:AA01DX6O CAS No.:1057670-80-0 MDL No.:MFCD12545735 MF:C10H10O2 MW:162.1852 |
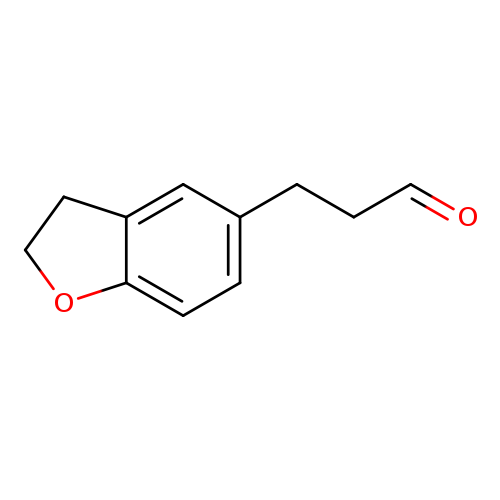
3-(2,3-dihydro-1-benzofuran-5-yl)propanalCatalog No.:AA01AD4Y CAS No.:1057670-88-8 MDL No.:MFCD09028563 MF:C11H12O2 MW:176.2118 |
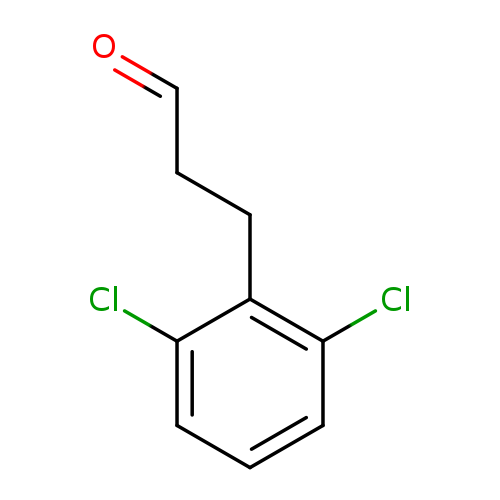
Benzenepropanal, 2,6-dichloro-Catalog No.:AA00989Z CAS No.:1057670-91-3 MDL No.:MFCD09028571 MF:C9H8Cl2O MW:203.0652 |
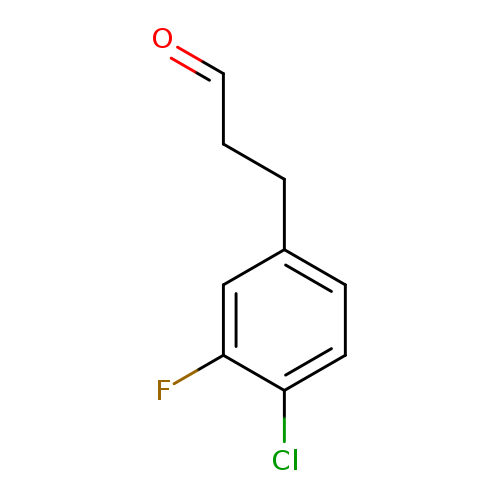
3-(4-chloro-3-fluorophenyl)propanalCatalog No.:AA01FPW1 CAS No.:1057671-10-9 MDL No.:MFCD09028617 MF:C9H8ClFO MW:186.6106 |
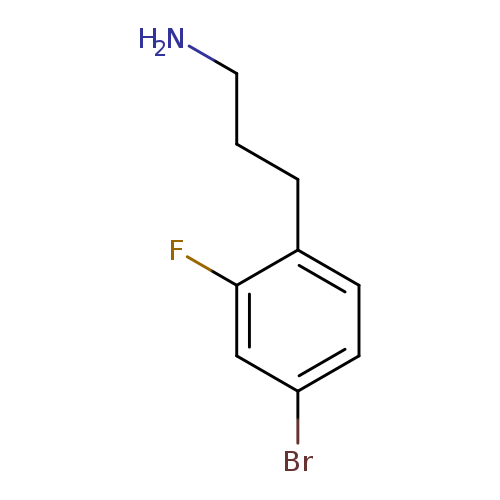
3-(4-bromo-2-fluorophenyl)propan-1-amineCatalog No.:AA01AIRK CAS No.:1057671-72-3 MDL No.:MFCD09744460 MF:C9H11BrFN MW:232.0927 |
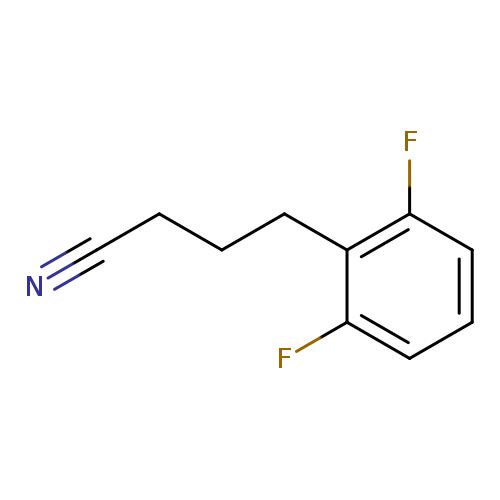
4-(2,6-difluorophenyl)butanenitrileCatalog No.:AA01C5G8 CAS No.:1057672-27-1 MDL No.:MFCD09744527 MF:C10H9F2N MW:181.1820 |
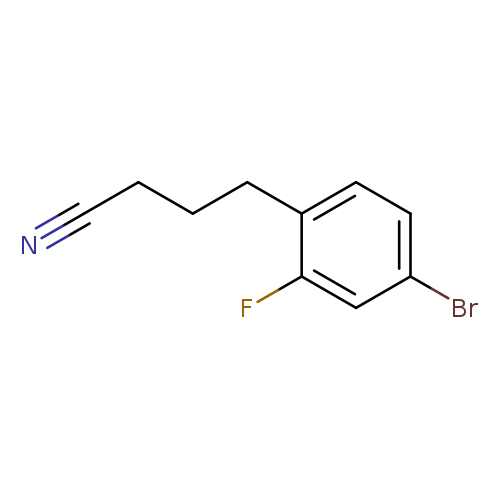
4-(4-Bromo-2-fluorophenyl)butanenitrileCatalog No.:AA0093Z5 CAS No.:1057672-39-5 MDL No.:MFCD09744540 MF:C10H9BrFN MW:242.0876 |
3,6-Dichloro-4-(trifluoromethyl)pyridazineCatalog No.:AA008XEB CAS No.:1057672-68-0 MDL No.:MFCD00052597 MF:C5HCl2F3N2 MW:216.9760 |
4-chloro-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridineCatalog No.:AA01AHE7 CAS No.:1057672-77-1 MDL No.:MFCD09744140 MF:C8H8ClN3 MW:181.6222 |
2-(3-methylcyclohexyl)ethan-1-amineCatalog No.:AA01AIR4 CAS No.:1057674-38-0 MDL No.:MFCD07772983 MF:C9H19N MW:141.2539 |
ethyl 3-[5-bromo-2-(difluoromethoxy)phenyl]propanoateCatalog No.:AA00VS0D CAS No.:1057674-78-8 MDL No.:MFCD09028856 MF:C12H13BrF2O3 MW:323.1306 |
(4-(Difluoromethoxy)phenyl)propanenitrileCatalog No.:AA019ZU9 CAS No.:1057676-27-3 MDL No.:MFCD09744618 MF:C10H9F2NO MW:197.1814 |
3-(2-chloro-6-fluorophenyl)propanenitrileCatalog No.:AA01BIBR CAS No.:1057676-61-5 MDL No.:MFCD09744658 MF:C9H7ClFN MW:183.6100 |
1-(3-bromopropyl)-3-(trifluoromethoxy)benzeneCatalog No.:AA01ELS1 CAS No.:1057678-65-5 MDL No.:MFCD09744370 MF:C10H10BrF3O MW:283.0850 |
(R)-6-(3-Methylpiperazin-1-yl)nicotinonitrileCatalog No.:AA007FDO CAS No.:1057682-05-9 MDL No.:MFCD17488802 MF:C11H14N4 MW:202.2557 |