2020-01-16 08:51:18
Lukasz Skalniak1 , Aleksandra Twarda-Clapa1, Constantinos G. Neochoritis2, Ewa Surmiak1 , Monika Machula1, Aneta Wisniewska1, Beata Labuzek1, Ameena M. Ali2, Sylwia Krzanik3, Grzegorz Dubin3,4, Matthew Groves2, Alexander Do€mling2 and Tad A. Holak1
Faculty of Chemistry, Jagiellonian University, Krakow, Poland
Department of Drug Design, University of Groningen, The Netherlands
Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland 4 Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland
Introduction
The protein p53 is a well-known tumor suppressor, activated in a response to several different stimuli, including DNA damage. Due to its tumor-suppressive activity, p53 is dysregulated in almost every single cancer [1]. It is estimated that 50% of cancers inactivate p53 by gaining loss-of-function mutations or deletions in the TP53 gene [2]. The remaining 50% of cancers utilize natural mechanisms of negative regulation of p53, enhanced to the extent that disallows its activation. In this regard, the increase of the inhibitory potential of MDM2 protein is the most common way to keep p53 inactive. MDM2 has the ability to inhibit p53 in the two different ways: by masking the transactivation domain of p53, and by directly ubiquitinating p53, directing the protein to proteasomal degradation [3]. The gene encoding MDM2 protein is very frequently amplified in human cancers, leading to MDM2 overexpression [3]. Overexpressed MDM2 binds to p53, leading to the permanent inactivation of this protein. Accordingly, forced disruption of p53/ MDM2 complexes became a favored strategy of the restoration of p53 functioning in p53wt cancers [4].
Over the last years, numerous small molecule scaffolds have been proposed that are able to disrupt the MDM2/p53 complexes. Among these compounds, several presented truly promising preclinical results and are currently undergoing clinical trials [5]. This includes small molecule derivatives of nutlin-3, RG7112, and RG7388 from Roche [6,7], the successor of AM-8553 compound, AMG 232 from Amgen [8], CGM097 and HDM201 from Novartis [9], and spiro- oxindole compounds, SAR405838 (MI-77301) from Sanofi [10], DS-3032b from Daiichi Sankyo [11], and APG-115 from Ascentage Pharma Group [12]. More- over, several stapled peptides have been described with high affinity toward MDM2 and MDMX, with repre- sentative molecule, ALRN-6924 (Aileron Therapeu- tics), currently undergoing phase I and II clinical trials in patients suffering from solid tumors, lymphoma, and myeloid leukemias [5].
In recent years, we have successfully designed and synthesized MDM2 antagonists based on the well- recognized three-point binding model using a pharma- cophore-based screening approach and multicompo- nent reaction chemistry [13–21]. Among these reports, we have reported the design, synthesis, and activity of a library of MDM2 antagonists, substituted with fluo- rine at various positions and synthesized by a classical Ugi four-component reaction (U-4CR) [22]. We showed that the introduction of fluorine substituents to the benzyl group can considerably improve the in vitro MDM2 binding activity of the antagonists due to the electrostatic interaction between small molecules and the His96 residue of the protein. We have then identified the most active compound, which was able to disrupt the p53/MDM2 interaction with Ki values of 0.13 lM and 0.4 lM for the corresponding acid and ester form, respectively [22]. In the current report, we demonstrate the biological activity of our most potent trifluoro-substituted compound in comparison to known MDM2 antagonists nutlin-3a and Idasanutlin (RG7388). In addition, we report the structural basis of the interaction of our compound (de-esterified) with human MDM2 protein for further structure-based optimization of its properties.
Results
The synthesis of the compounds
Based on our previous work [22], we resynthesized and scaled-up the most promising trifluoro-substituted compound (Fig. 1) for further evaluation of its activity employing biochemical, biophysical, and cellular assays. The synthesis involves a convergent Ugi 4- component reaction and the MDM2 antagonists can be assembled in just two steps from commercially available starting materials [22]. The equimolar mixing of the 3,4,5-trifluorobenzylamine (1), indole-3-carbox-aldehyde (2), tert-butyl isocyanide (3), and formic acid(4) led to the desired p53–MDM2 antagonist (5) (for the detailed description of synthetic procedures and analytical data see Supporting Information). The resulting racemic mixture was subjected to preparative chiral separation yielding the two enantiomers, (R)-5a and (S)-5b (see the section 1.5 of Supporting Information, Fig. S1 and Table S1 for the details on the sepa- ration of enantiomers). The two latter compounds were hydrolyzed into the corresponding acids (R)-6a
and (S)-6b (Fig. 1).
The compounds 5 and 6 bind to MDM2
Three complementary assays based on independent physicochemical principles, fluorescence polarization (FP), 1H-15N HSQC NMR, and microscale ther- mophoresis (MST) were used to test the in vitro activ- ity of the compounds. The FP assay was performed to compare the MDM2–p53 inhibitory activity of new batches of the compounds with the data reported in our previous study [22]. The assay was performed for the enantiomers R of the compounds (5) and (6), as these enantiomers presented higher activity in our previous study [22]. Both compounds presented Ki values much lower than 1 lM, which corresponds well with the values reported previously (see Tables 1 and S2).
1H-15N HSQC NMR titration was used to verify the results of the FP assay. This method is based on moni- toring the chemical shift changes in protein amide backbone resonances upon protein interaction with a small molecule [23–25]. The binding to MDM2 protein was confirmed for all the final compounds, i.e., (R)-5a, (S)-5b, (R)-6a and (S)-6b. The pattern of chemical shift changes observed for the compound (R)-6a was similar to the pattern reported in our previous work and also correlates well with the FP data [22]. Tight binding, characterized by the slow chemical exchange and observed as doubling of NMR signals [26] was noted for the compounds (R)-5a, (R)-6a, and (S)-6b (Figs 2, S2 and S3). Such a behavior is characteristic for the compounds with dissociation constants KD values below 1 lM. NMR results showed that acids (R)-6a and (S)-6b were more potent compared to their esters (R)-5a and (S)-5b (Table 1). This was most evident for the compounds (S)-5b/6b, for which only acid (S)-6b, but not the ester (S)-5b, was able to evoke peaks dou- bling (Fig. S4). For (S)-5b, though, the spectrum is characteristic for intermediate chemical exchange range which is indicated by broadening and disappearing of peaks like Gly58 or Gly12. For the compounds that undergo intermediate chemical exchange, it is feasible to determine the KD value [27,28]. The calculated KD for (S)-5b was 10.7 T 6.5 lM (Fig. S4). The compar- ison of enantiomers shows that from the pair of the ester compounds (R/S)-5a/b the R enantiomer is far more potent than the S enantiomer. In the case of acids (R/S)-6a/b, both enantiomers exhibit similar potency in the tested system.
Finally, a third assay, microscale thermophoresis (MST) was employed to cross-validate the binding affinity of tested compounds. The technique relies on the thermophoretic motion of the molecule in a tem- perature gradient induced by a high precision IR-laser beam. Changes in the molecular diffusion resulting from binding events provide information on affinity [29,30]. In the experimental setup, the concentration of a flu- orescently labeled MDM2 was fixed at 100 nM, whereas the concentration of an unlabeled binding compound was varied. All the tested compounds showed an affinity toward MDM2 in the low lM range (Table 1, raw data available in the Supporting Information). The KD values were 7.0 lM and 1.3 lM for the (R)-6a and (S)-6b enantiomers, respectively. Likewise, the binding of ester enantiomers, (R)-5a and (S)-5b to the fluorescently labeled MDM2 led to KD values of 3.0 lM and 7.7 lM, respectively.
The compounds 5 and 6 disrupt the MDM2–p53 complex
As described above, the compounds 5 and 6 bind to MDM2 and are able to displace p53-derived peptide complexed with MDM2 protein. To verify if the com- pounds are also able to dissociate the p53–MDM2 complex, the compounds were tested by the Antago- nist Induced Dissociation Assay (AIDA-NMR) [31].
The method is based on the NMR spectroscopy of the complex formed by the human p53 (residues 1–321) and human MDM2 (residues 1–125), and its subsequent dissociation upon the titration with the inhibitor. Usually the methodology is performed using 15N-labeled protein. However, if the distinguished, well-separated peaks are observed in 1H spectra, it is feasible to perform the AIDA-NMR with unlabeled proteins. The analyzed p53–MDM2 complex have the required characteristic signals (around 10 ppm region), originating from the N-terminal p53 domain NHe
indole residues of Trp23, Trp53, and Trp91. Therefore, we decided to perform a proton AIDA-NMR experi- ments for all the compounds with FP Ki values below 1 lM, and defined as ‘tight binders’ by the NMR spectroscopy, i.e., the compounds (R)-5a, (R)-6a, and (S)-6b), along with a known MDM2 antagonist, nutlin-3a as an reference.
On the 1D proton spectrum of the reference p53– MDM2 complex shows only two peaks, corresponding to Trp91 and Trp53 of p53 (Fig. S5). The peak corre- sponding to the Trp23 is not visible, because this resi- due is buried inside the protein complex, as it participates in the complex formation. The residues Trp53 and Trp91 are accessible for NMR spectroscopy even when p53 is bound to MDM2 and their corre- sponding signals are not expected to vary between MDM2-bound and unbound p53. Upon the titration of the complex with MDM2 antagonists, the formation of the peak originating from Trp23 is observed, as exemplified for nutlin-3a (Fig. S5A). This indicates the release of p53 from its complex with MDM2. The p53–MDM2 complex titrated with each of the tested compounds resulted in similar observa- tions, suggesting that the compounds indeed disrupt p53–MDM2 complex in solution (Fig. S5B–D).
(R)-6a binds to MDM2 with a classical three- finger binding mode
To characterize the structural basis of the interactions of the evaluated compounds at the hydrophobic pocket of the p53-binding domain of MDM2, we determined the crystal structure of (R)-6a in complex with MDM2(18–125) (fragment of MDM2 comprising residues 18–125). The compound (R)-6a was chosen as the most potent one in the FP assay. The obtained crystals diffracted to 2.0 A˚ resolution and contained one protein-inhibitor complex in the asymmetric unit, with (R)-6a well defined by its electron density (Fig. S6) and with no additional inhibitor molecules found within the structure (data collection and refine- ment statistics shown in the Table S3 in the Support- ing Information).
(R)-6a binds to MDM2 with a classical three-finger binding mode (Fig. 3A,B), occupying all three p53- binding subpockets defined by following residues of p53: Phe19, Trp23, and Leu26 (Fig. 3C). Concerning the similarities to the native interaction, the binding of (R)-6a into the cleft of MDM2 is primarily stabilized by hydrophobic interactions with Leu54, Leu57, Gly58, Ile61, Met62, Tyr67, Val75, Phe86, Phe91, Val93, Ile99, Tyr100, and Ile103 (residues labeled at Fig. 4A). Another resemblance is noticed for the 6- chloroindole substituent of (R)-6a that inserts into a Trp23 subpocket and assumes an orientation almost identical to the Trp side chain of p53 in the native p53–MDM2 complex. The chlorine atom introduced in (R)-6a stabilizes the interaction at the bottom of the hydrophobic cleft. Such an arrangement of 6-chloroin-dole moiety repeating the p53’s Trp23 indole interac- tion follows a canonical binding observed in many different classes of MDM2 inhibitors [32]. Further,
comparable to p53 interaction, the NH group within 6-chloroindole participates in a hydrogen bond with the carbonyl oxygen of Leu54 (length of 2.6 Fig. 4A).
Concerning the remaining MDM2 subpockets which
are naturally occupied by p53’s Phe19 and Leu26, the differences can be easily spotted as the character (alkyl/aryl) of substituents of (R)-6a differs from p53’s side chains. The Phe19 subpocket accommodates the tert-butyl substituent of (R)-6a directed at Tyr67. The 3,4,5-trifluorobenzyl substituent of (R)-6a fills the Leu26 subpocket pointing toward Tyr100 and is involved in p-stacking with His-96 side chain (Fig. 4A) [22]. Moreover, this stacking results also in the change of the overall shape of Leu26 subpocket when compared to the native interaction, as in case of (R)-6a it gets ‘closed’ and less exposed to the solvent.
Additionally, the inhibitor’s scaffold and carboxylic group of (R)-6a further contribute to the formation of several hydrogen bonds with water molecules interact- ing with MDM2 (Fig. 4B). Taken together, hydropho- bic interactions, hydrogen bonds and p-stacking define the high affinity of (R)-6a to p53-binding pocket of MDM2.
The MDM2 antagonist (R)-5a activates p53 and induces the expression of p53-dependent genes
The disruption of the p53/MDM2 interaction in p53wt cells leads to the release of transcriptionally active p53, which then regulates the expression of a panel of target genes. The protein p21 is a product of such a well-known p53-regulated gene and changes in its expres- sion are often examined to track the activation of p53. To test the bioactivity of the compounds, human osteosarcoma U-2 OS cells were treated with increasing concentrations of the compounds (R)-5a and (R)-6a for 24 h, followed by western blot analysis of the expression of p21 protein. As a control, two MDM2 antagonists were used: Nutlin-3 and RG7388 as one of the best-studied compounds and one of the most potent antagonists, respectively. Both compounds increased the expression of p21 and p53 in U-2 OS cells (Fig. 5A). The compound (R)-5a induced a strong expression of both p53 and p21 protein, proving the efficient release of p53 from the negative influence of MDM2 (Fig. 5A). This effect was observed at the con- centrations of 5 and 10 lM of the compound. The car- boxylic acid version of (R)-5a, compound (R)-6a, did not alter the expression of the examined proteins (Fig. 5A).
To check the stereoselectivity of the compound 5 in living cells, both enantiomers of the compound 5, (R)-5a and (S)-5b, were tested in a western blot experiment. The analysis confirmed a strong activity of (R)-5a, while the enantiomer (S)-5b was found inactive (Fig. 5B). This corresponded well with the FP, NMR, and MST results, where the enantiomer S was found less active than the R enantiomer (Table 1).
While the increase of p53 protein levels in response to MDM2 antagonists is due to increased stability of the p53 protein, the increase of expression of p53 targets, p21 and MDM2, is related to transcriptional induction of CDKN1A and MDM2 genes, respectively. To verify if the increased protein levels are associated with increase abundance of corresponding transcript, a real-time PCR was performed with the detection of MDM2, CDKN1A, and TP53 transcripts. The results clearly show that the treatment of U-2 OS cells with MDM2 antagonists, RG7388 or (R)-5a, leads to a sig- nificant increase of MDM2 and CDKN1A transcripts (Fig. 5C). This suggests that the (R)-5a compound indeed indices transcriptional activity of p53 in living cells.
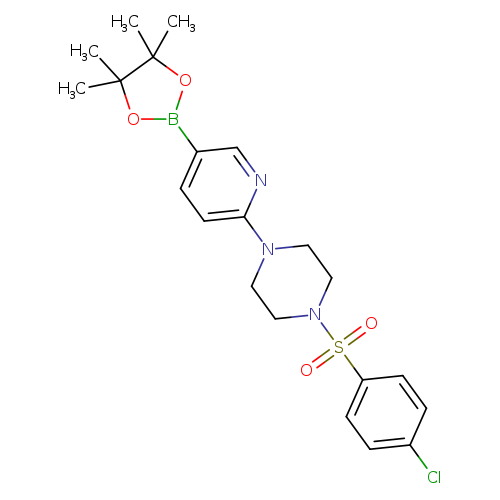
1-((4-Chlorophenyl)sulfonyl)-4-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)piperazineCatalog No.:AA007EKQ CAS No.:1073354-20-7 MDL No.:MFCD06797988 MF:C21H27BClN3O4S MW:463.7858 |
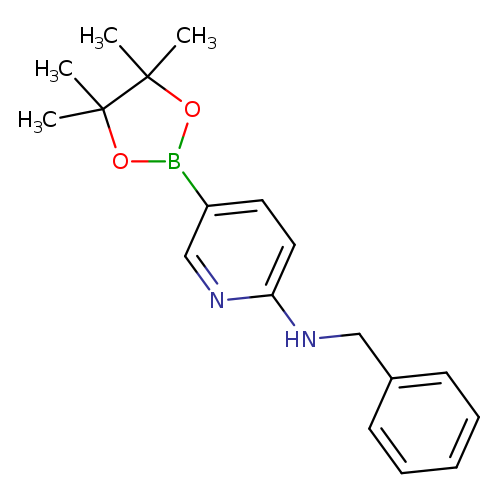
6-(Benzylamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SOP CAS No.:1073354-27-4 MDL No.:MFCD06798270 MF:C18H23BN2O2 MW:310.1984 |
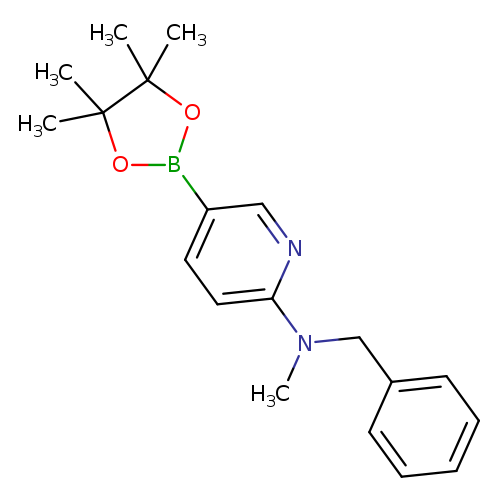
N-Benzyl-N-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amineCatalog No.:AA003SPF CAS No.:1073354-30-9 MDL No.:MFCD06798271 MF:C19H25BN2O2 MW:324.2250 |
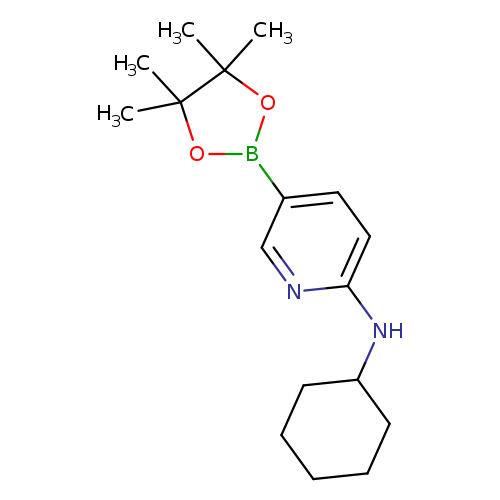
6-(Cyclohexylamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SUA CAS No.:1073354-34-3 MDL No.:MFCD06798277 MF:C17H27BN2O2 MW:302.2195 |
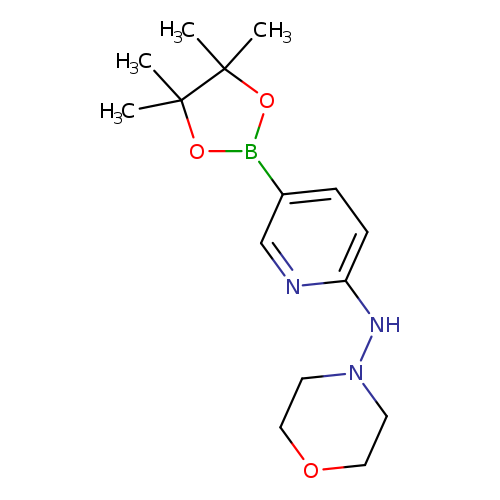
6-(4-Morpholineamino)pyridine-3-boronic acid pinacol esterCatalog No.:AA003SAO CAS No.:1073354-38-7 MDL No.:MFCD06798279 MF:C15H24BN3O3 MW:305.1804 |
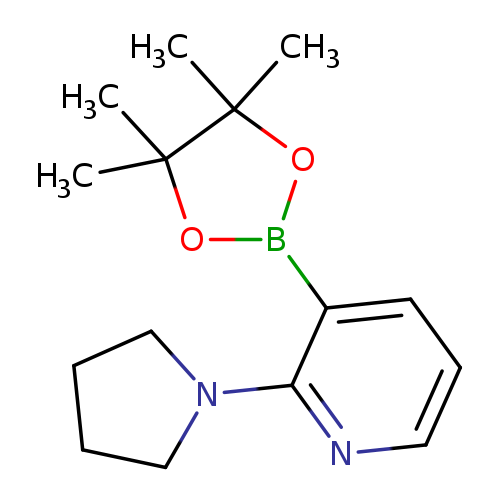
2-Pyrrolidinopyridine-3-boronic acid, pinacol esterCatalog No.:AA003F1R CAS No.:1073354-41-2 MDL No.:MFCD06798280 MF:C15H23BN2O2 MW:274.1663 |
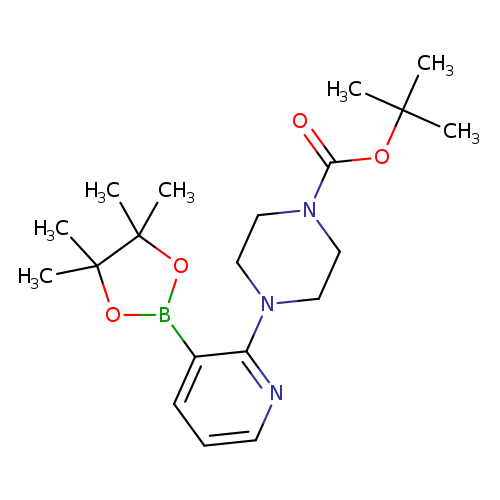
2-(4-BOC-Piperazino)pyridine-3-boronic acid, pinacol esterCatalog No.:AA003UH4 CAS No.:1073354-42-3 MDL No.:MFCD07781131 MF:C20H32BN3O4 MW:389.2968 |
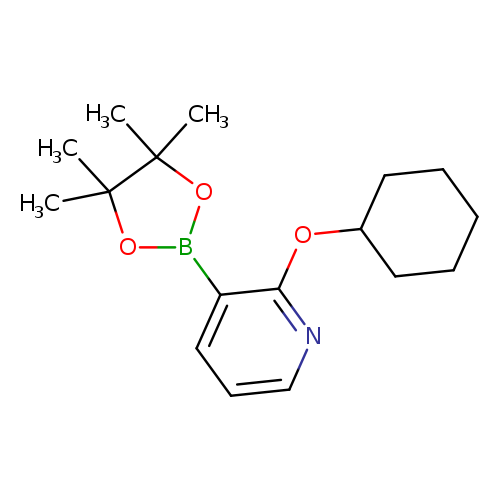
2-Cyclohexyloxypyridine-3-boronic acid, pinacol esterCatalog No.:AA003EY1 CAS No.:1073354-43-4 MDL No.:MFCD07781171 MF:C17H26BNO3 MW:303.2042 |
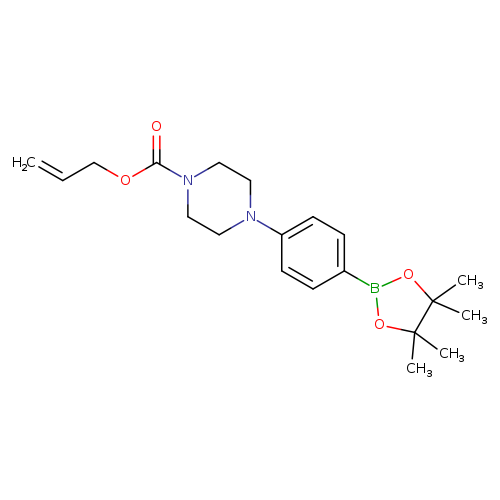
4-(4'-Allyloxycarbonylpiperizino)phenylboronic acid, pinacol esterCatalog No.:AA003JZB CAS No.:1073354-49-0 MDL No.:MFCD07781206 MF:C20H29BN2O4 MW:372.2663 |
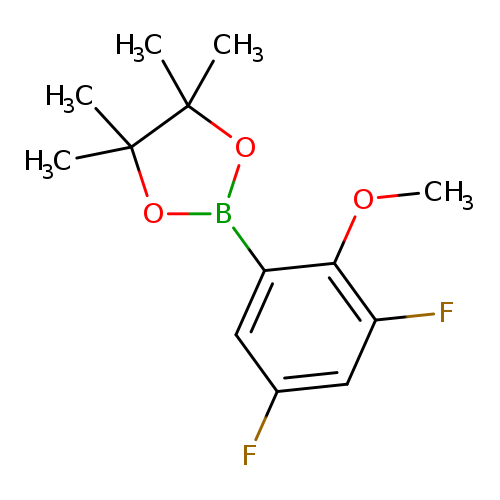
3,5-Difluoro-2-methoxyphenylboronic acid, pinacol esterCatalog No.:AA003IJN CAS No.:1073354-50-3 MDL No.:MFCD07781207 MF:C13H17BF2O3 MW:270.0801 |
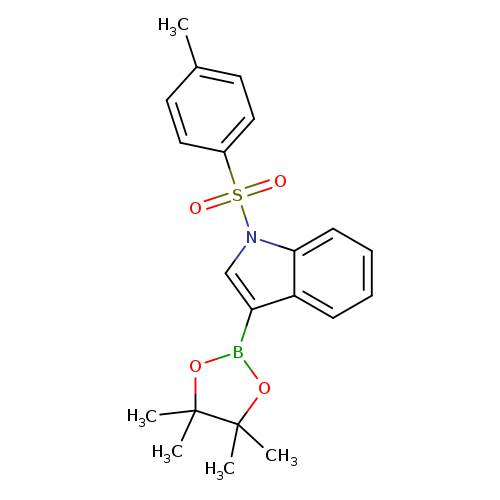
1-(Toluene-4-sulfonyl)-1H-indole-3-boronic acid pinacol esterCatalog No.:AA003I00 CAS No.:1073354-51-4 MDL No.:MFCD08063118 MF:C21H24BNO4S MW:397.2956 |
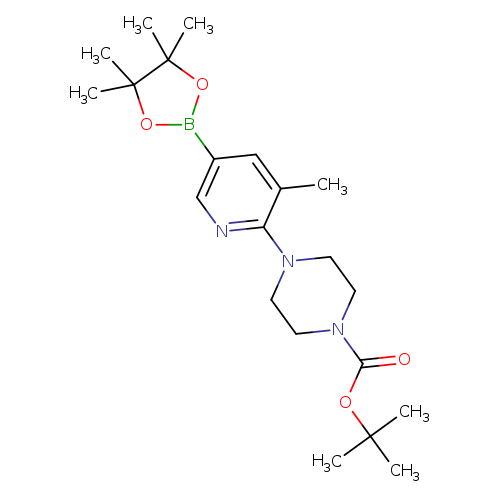
2-(4-Boc-piperazin-1-yl)-3-methylpyridine-5-boronic acid pinacol esterCatalog No.:AA003UH7 CAS No.:1073354-54-7 MDL No.:MFCD08064052 MF:C21H34BN3O4 MW:403.3234 |
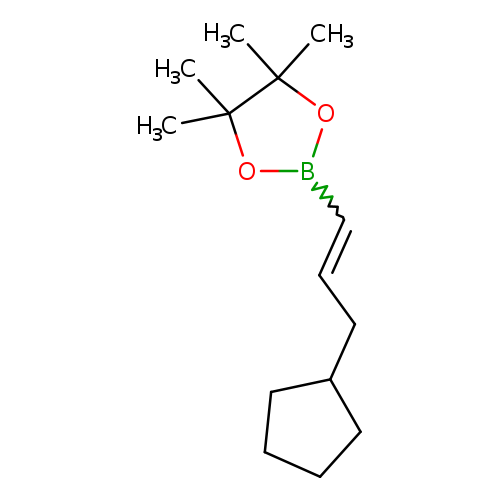
trans-3-Cyclopentylpropen-1-ylboronic acid, pinacol esterCatalog No.:AA003EQZ CAS No.:1073354-57-0 MDL No.:MFCD08276877 MF:C14H25BO2 MW:236.1581 |
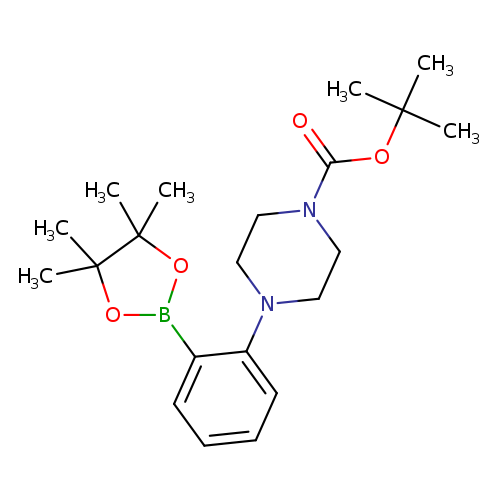
2-[4-(N-Boc)piperazin-1-yl]phenylboronic acid pinacol esterCatalog No.:AA007EKP CAS No.:1073354-59-2 MDL No.:MFCD08669542 MF:C21H33BN2O4 MW:388.3087 |
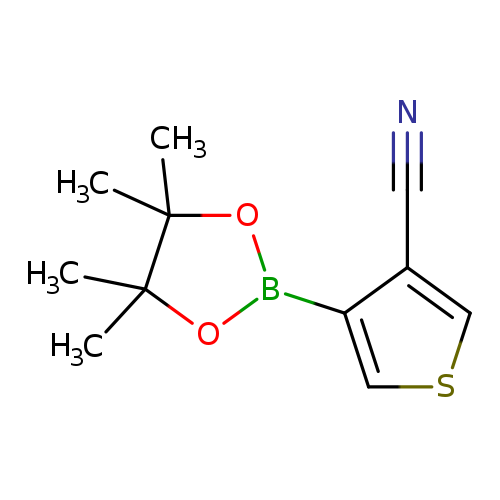
3-Cyanothiophene-4-boronic acid pinacol esterCatalog No.:AA003J9T CAS No.:1073354-61-6 MDL No.:MFCD08669563 MF:C11H14BNO2S MW:235.1104 |
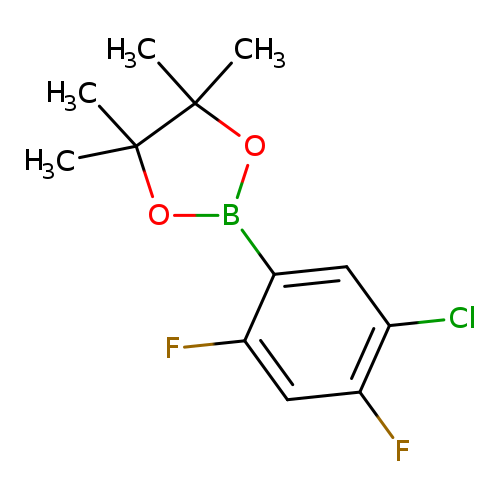
2-(5-Chloro-2,4-difluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolaneCatalog No.:AA007EKO CAS No.:1073354-65-0 MDL No.:MFCD08669564 MF:C12H14BClF2O2 MW:274.4992 |
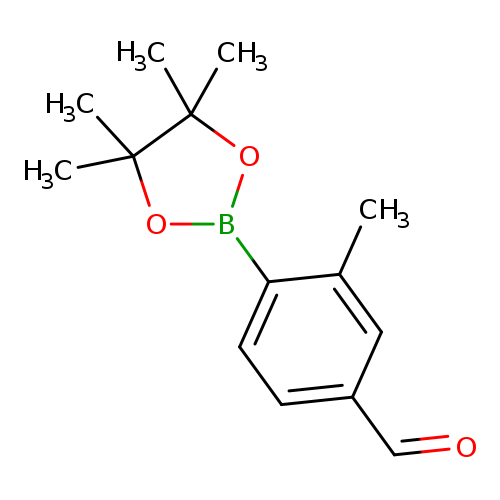
4-Formyl-2-methylphenylboronic acid pinacol esterCatalog No.:AA003JMY CAS No.:1073354-66-1 MDL No.:MFCD08669583 MF:C14H19BO3 MW:246.1099 |
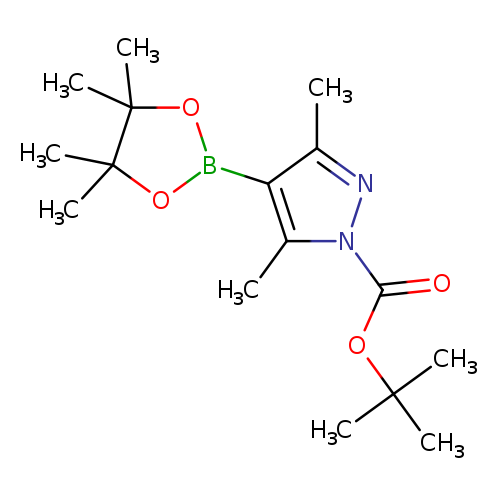
1-Boc-3,5-dimethylpyrazole-4-boronic acid pinacol esterCatalog No.:AA003UGS CAS No.:1073354-70-7 MDL No.:MFCD09027070 MF:C16H27BN2O4 MW:322.2076 |
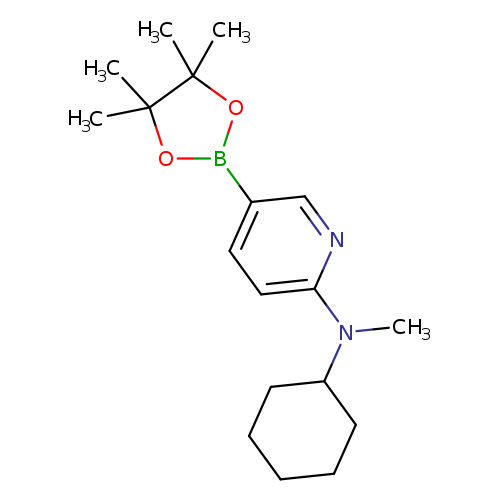
6-[Cyclohexyl(methyl)amino]pyridine-3-boronic acid pinacol esterCatalog No.:AA003SUG CAS No.:1073354-73-0 MDL No.:MFCD09027071 MF:C18H29BN2O2 MW:316.2461 |
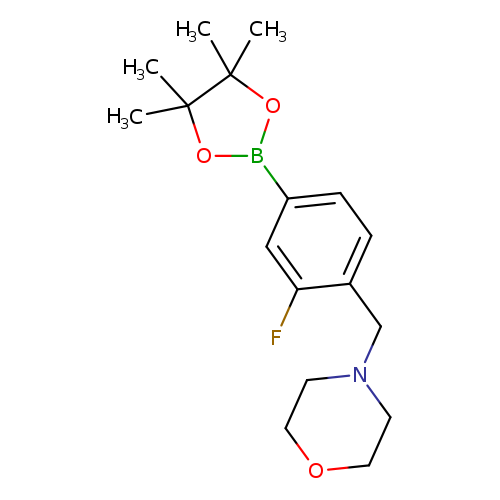
3-Fluoro-4-(N-morpholinomethyl)phenylboronic acid, pinacol esterCatalog No.:AA003JWX CAS No.:1073354-74-1 MDL No.:MFCD09027076 MF:C17H25BFNO3 MW:321.1947 |
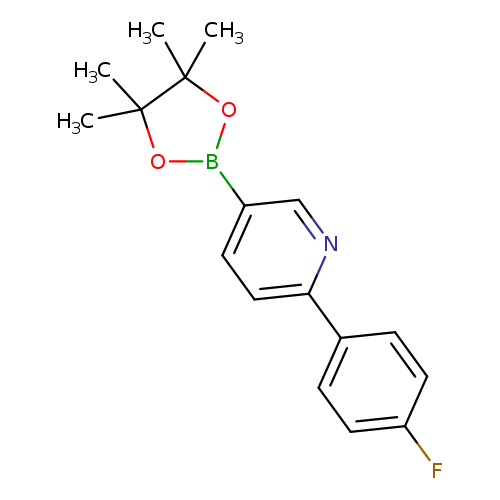
6-(4-Fluorophenyl)pyridine-3-boronic acid pinacol esterCatalog No.:AA003ETI CAS No.:1073354-81-0 MDL No.:MFCD09037494 MF:C17H19BFNO2 MW:299.1477 |
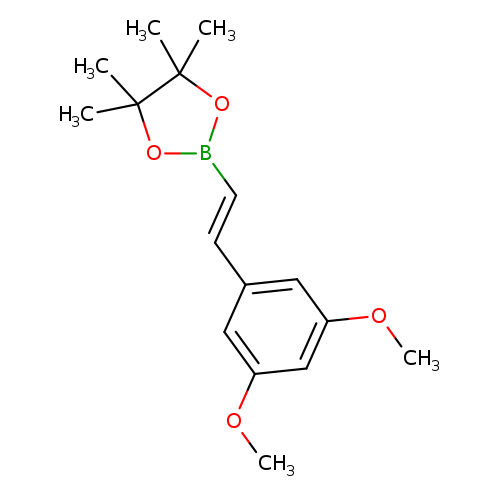
E-2-(3,5-Dimethoxyphenyl)vinylboronic acid pinacol esterCatalog No.:AA003BXG CAS No.:1073354-86-5 MDL No.:MFCD09038437 MF:C16H23BO4 MW:290.1624 |
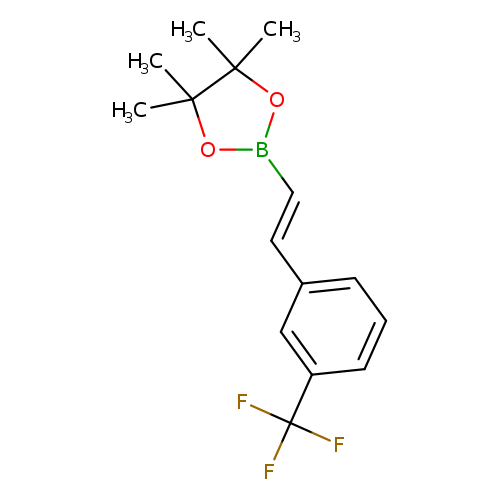
E-2-(3-Trifluoromethylphenyl)vinylboronic acid pinacol esterCatalog No.:AA003PU3 CAS No.:1073354-88-7 MDL No.:MFCD09038444 MF:C15H18BF3O2 MW:298.1084 |
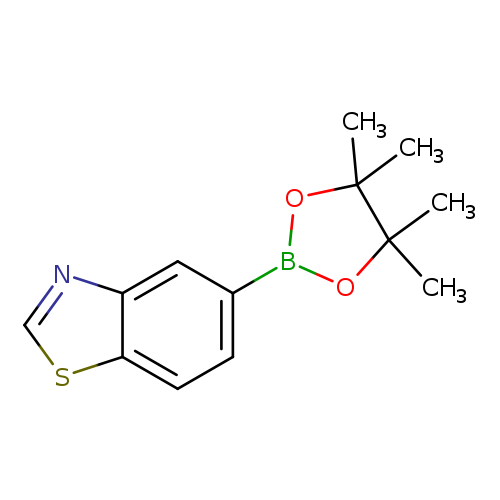
Benzothiazole-5-boronic acid pinacol esterCatalog No.:AA003M26 CAS No.:1073354-91-2 MDL No.:MFCD09260439 MF:C13H16BNO2S MW:261.1476 |
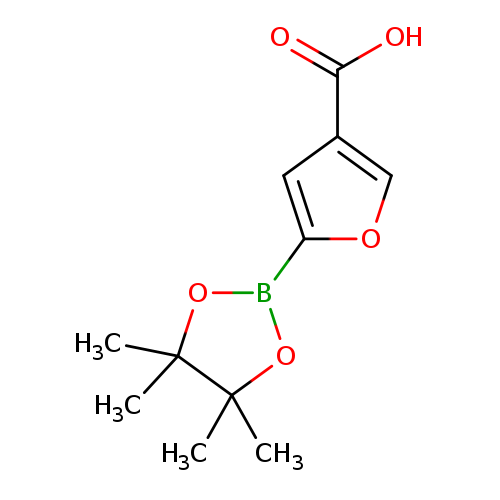
4-Carboxyfuran-2-boronic acid pinacol esterCatalog No.:AA003M28 CAS No.:1073354-94-5 MDL No.:MFCD09260445 MF:C11H15BO5 MW:238.0448 |
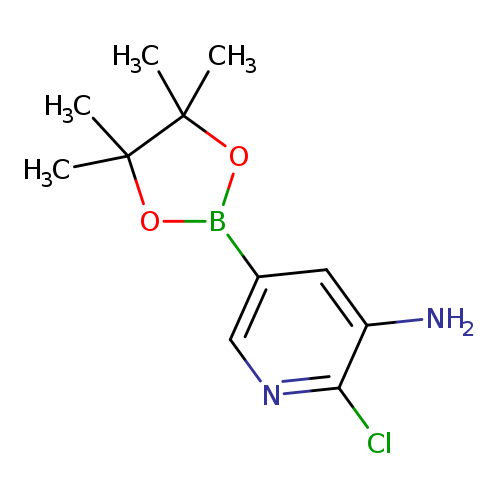
3-Amino-2-chloropyridine-5-boronic acid, pinacol esterCatalog No.:AA003GV8 CAS No.:1073354-96-7 MDL No.:MFCD09260487 MF:C11H16BClN2O2 MW:254.5209 |
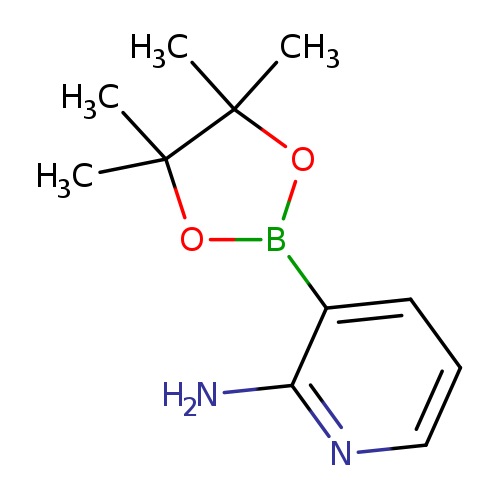
2-Aminopyridine-3-boronic acid, pinacol esterCatalog No.:AA008SDL CAS No.:1073354-97-8 MDL No.:MFCD09260488 MF:C11H17BN2O2 MW:220.0759 |
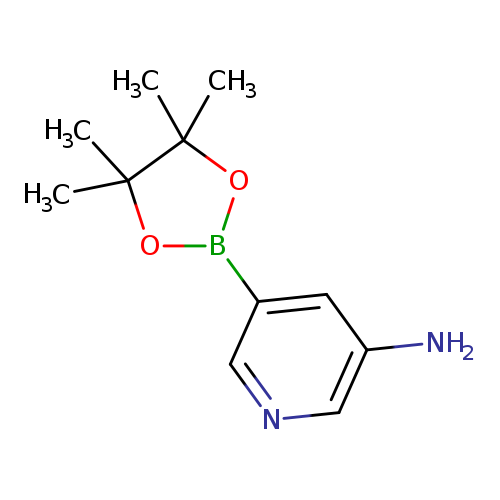
3-Aminopyridine-5-boronic acid, pinacol esterCatalog No.:AA003IVB CAS No.:1073354-99-0 MDL No.:MFCD09260489 MF:C11H17BN2O2 MW:220.0759 |
2-((tert-Butyldimethylsilanyl)ethynyl) boronic acid pinacol esterCatalog No.:AA003UIU CAS No.:1073355-02-8 MDL No.:MFCD09265092 MF:C14H27BO2Si MW:266.2595 |
3-[3-(Ethoxycarbonyl)piperidine-1-carbonyl]phenylboronic acid pinacol esterCatalog No.:AA007WEU CAS No.:1073355-04-0 MDL No.:MFCD09266178 MF:C21H30BNO5 MW:387.2776 |
3-Benzylphenylboronic acid pinacol esterCatalog No.:AA007W8Q CAS No.:1073355-05-1 MDL No.:MFCD09266179 MF:C19H23BO2 MW:294.1957 |
3-(Tetrahydrofurfurylaminocarbonyl)phenylboronic acid pinacol esterCatalog No.:AA0090C0 CAS No.:1073355-06-2 MDL No.:MFCD09266189 MF:C18H26BNO4 MW:331.2143 |
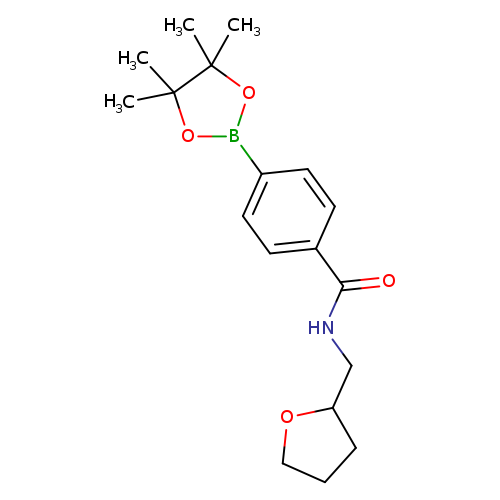
4-(Tetrahydrofurfurylaminocarbonyl)phenylboronic acid pinacol esterCatalog No.:AA0090C4 CAS No.:1073355-09-5 MDL No.:MFCD09266200 MF:C18H26BNO4 MW:331.2143 |
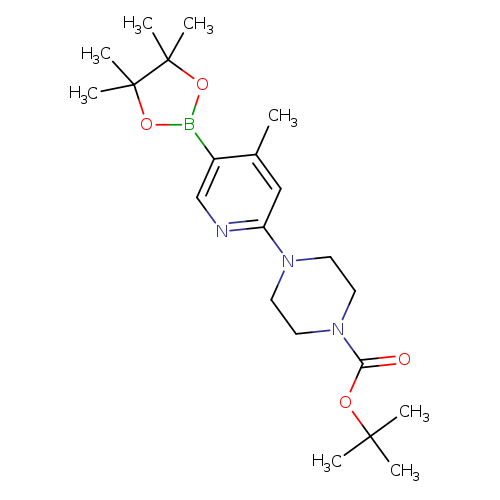
2-(4-Boc-piperazin-1-yl)-4-methylpyridine-5-boronic acid pinacol esterCatalog No.:AA003UH9 CAS No.:1073355-13-1 MDL No.:MFCD09271789 MF:C21H34BN3O4 MW:403.3234 |
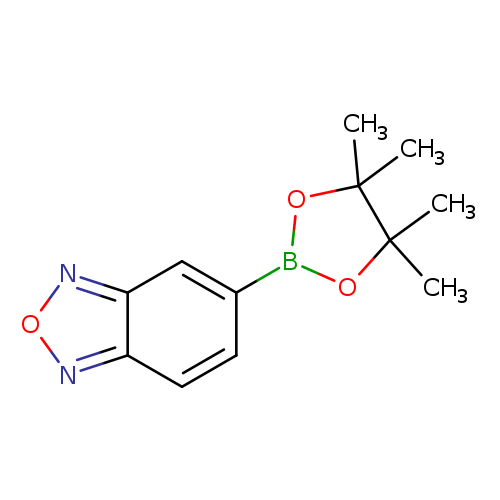
Benzofurazan-5-boronic acid, pinacol esterCatalog No.:AA007W8O CAS No.:1073355-14-2 MDL No.:MFCD09842717 MF:C12H15BN2O3 MW:246.0701 |
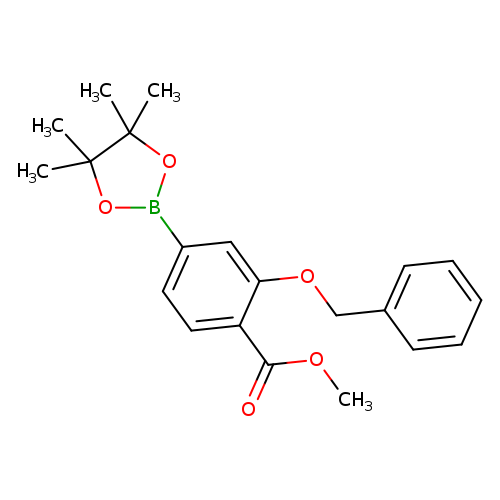
3-Benzyloxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA0084SZ CAS No.:1073355-16-4 MDL No.:MFCD09864186 MF:C21H25BO5 MW:368.2312 |
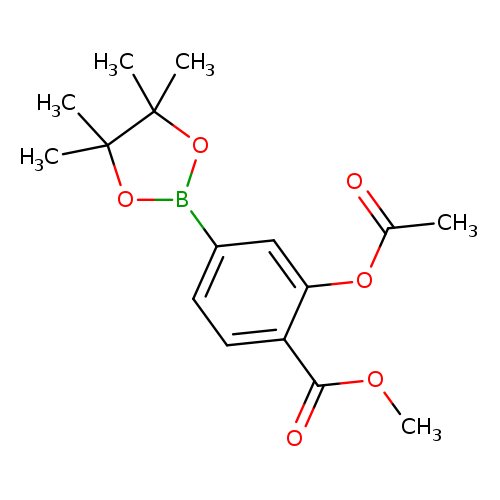
3-Acetoxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA003RJG CAS No.:1073355-18-6 MDL No.:MFCD09864187 MF:C16H21BO6 MW:320.1453 |
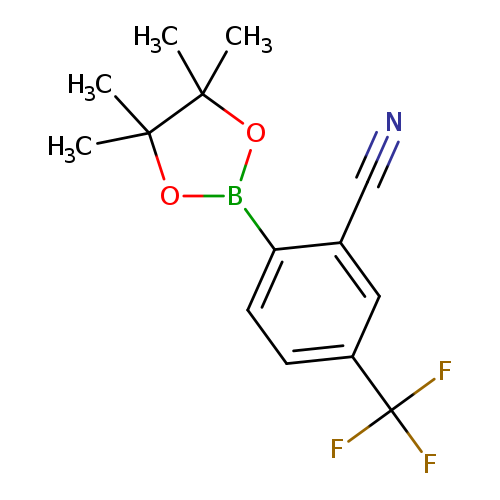
2-Cyano-4-(trifluoromethyl)phenylboronic acid pinacol esterCatalog No.:AA007EKN CAS No.:1073355-21-1 MDL No.:MFCD09878539 MF:C14H15BF3NO2 MW:297.0806 |
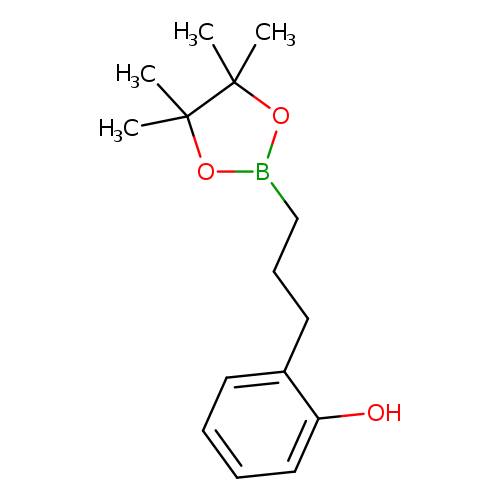
3-(2-Hydroxyphenyl)propylboronic acid, pinacol esterCatalog No.:AA003EQ0 CAS No.:1073355-25-5 MDL No.:MFCD09953481 MF:C15H23BO3 MW:262.1523 |
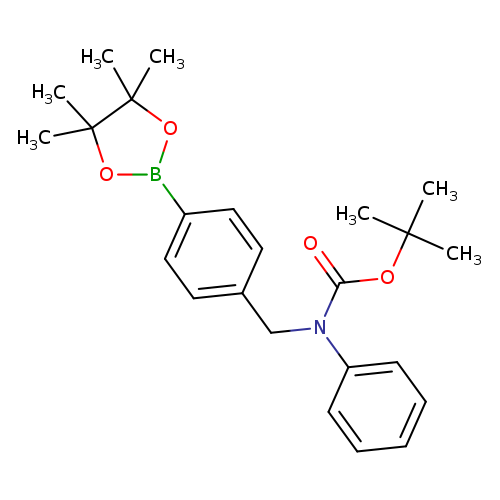
4-(N-Boc-phenylaminomethyl)phenylboronic acid pinacol esterCatalog No.:AA0090XV CAS No.:1073371-71-7 MDL No.:MFCD09953500 MF:C24H32BNO4 MW:409.3262 |
4-(Hydroxy(thiazol-2-yl)methyl)phenylboronic acid, pinacol esterCatalog No.:AA00906S CAS No.:1073371-73-9 MDL No.:MFCD09953504 MF:C16H20BNO3S MW:317.2109 |
2-(t-Butyldimethylsilyl)thiophene-5-boronic acid pinacol esterCatalog No.:AA003UIV CAS No.:1073371-74-0 MDL No.:MFCD10567054 MF:C16H29BO2SSi MW:324.3618 |
4-(3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)morpholine hydrochlorideCatalog No.:AA008UMU CAS No.:1073371-76-2 MDL No.:MFCD06657893 MF:C17H27BClNO3 MW:339.6652 |
2-Amino-5-chlorophenylboronic acid, pinacol esterCatalog No.:AA007W8M CAS No.:1073371-77-3 MDL No.:MFCD06795672 MF:C12H17BClNO2 MW:253.5329 |
4-((N,N-Dimethylamino)methyl)phenylboronic acid pinacol ester HClCatalog No.:AA0090D7 CAS No.:1073371-85-3 MDL No.:MFCD07771993 MF:C15H25BClNO2 MW:297.6285 |
5-(Morpholine-4-carbonyl)pyridine-3-boronic acid, pinacol esterCatalog No.:AA003S3H CAS No.:1073371-92-2 MDL No.:MFCD07781248 MF:C16H23BN2O4 MW:318.1758 |
2-Nitropyridine-5-boronic acid, pinacol esterCatalog No.:AA007W8L CAS No.:1073371-93-3 MDL No.:MFCD08063079 MF:C11H15BN2O4 MW:250.0588 |
2-Fluoro-5-methylpyridine-3-boronic acid pinacol esterCatalog No.:AA0084SW CAS No.:1073371-96-6 MDL No.:MFCD08063092 MF:C12H17BFNO2 MW:237.0783 |
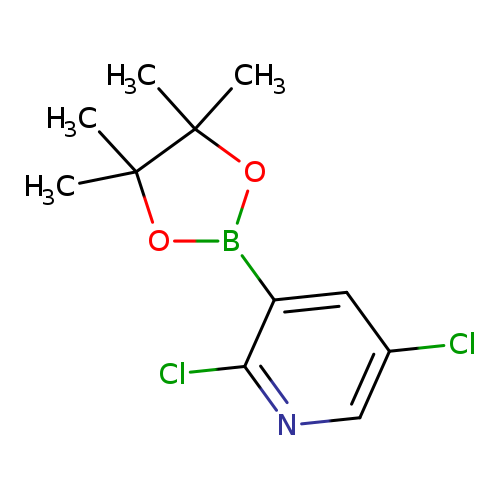
2,5-Dichloropyridine-3-boronic acid pinacol esterCatalog No.:AA003FU7 CAS No.:1073371-98-8 MDL No.:MFCD08063094 MF:C11H14BCl2NO2 MW:273.9514 |
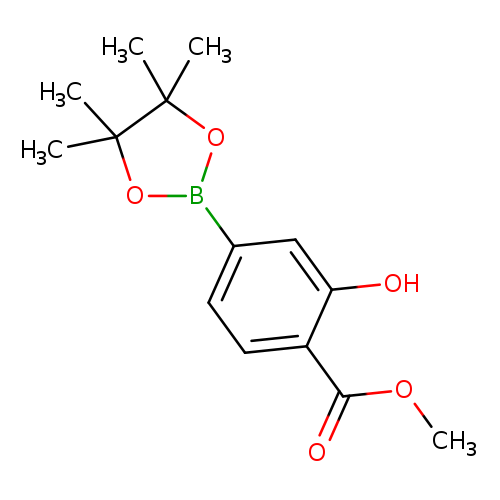
3-Hydroxy-4-methoxycarbonylphenylboronic acid, pinacol esterCatalog No.:AA007W8K CAS No.:1073371-99-9 MDL No.:MFCD08458200 MF:C14H19BO5 MW:278.1087 |
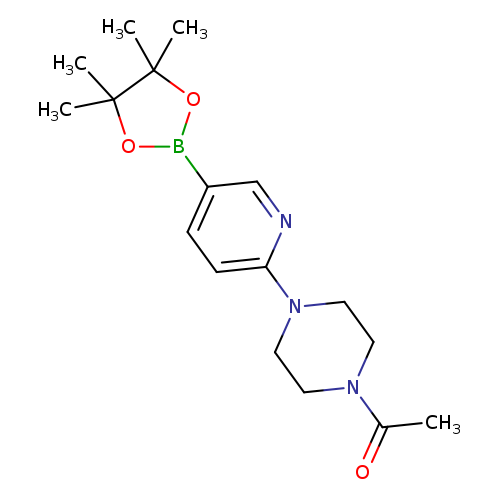
2-(4-Acetylpiperazin-1-yl)pyridine-5-boronic acid, pinacol esterCatalog No.:AA007W8J CAS No.:1073372-01-6 MDL No.:MFCD09027063 MF:C17H26BN3O3 MW:331.2176 |
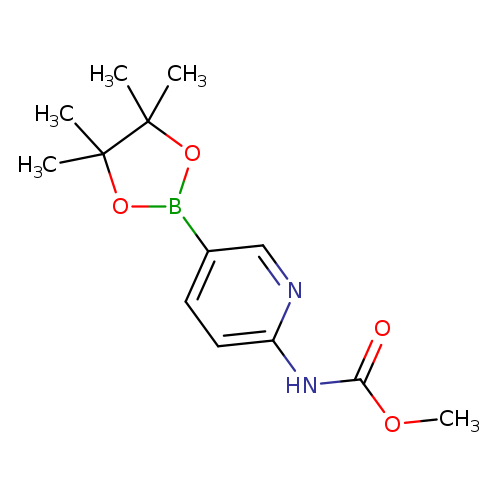
2-Methoxycarbonylaminopyridine-5-boronic acid, pinacol esterCatalog No.:AA008TRP CAS No.:1073372-02-7 MDL No.:MFCD09027078 MF:C13H19BN2O4 MW:278.1120 |
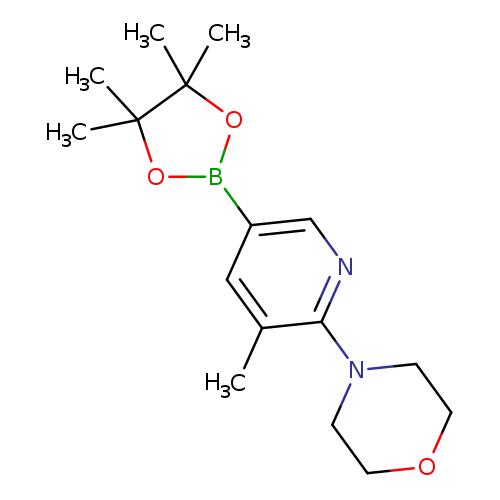
5-Methyl-6-morpholinylpyridine-3-boronic acid pinacol esterCatalog No.:AA003JYI CAS No.:1073372-03-8 MDL No.:MFCD09037483 MF:C16H25BN2O3 MW:304.1923 |
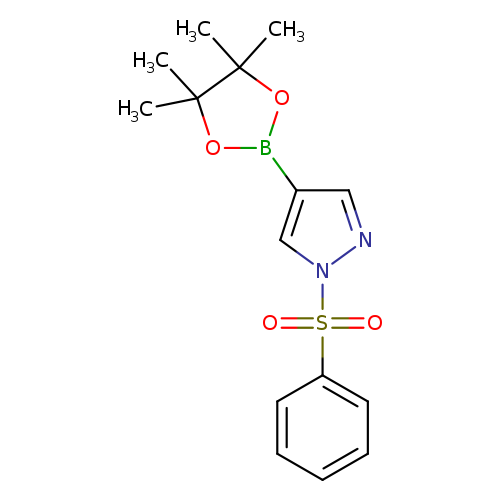
1-(Phenylsulfonyl)pyrazole-4-boronic acid, pinacol esterCatalog No.:AA0032GF CAS No.:1073372-04-9 MDL No.:MFCD09801236 MF:C15H19BN2O4S MW:334.1984 |
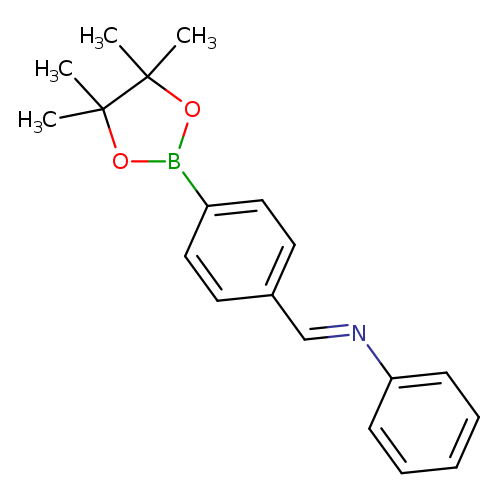
4-((Phenylimino)methyl)phenylboronic acid pinacol esterCatalog No.:AA0090XY CAS No.:1073372-06-1 MDL No.:MFCD09266199 MF:C19H22BNO2 MW:307.1945 |
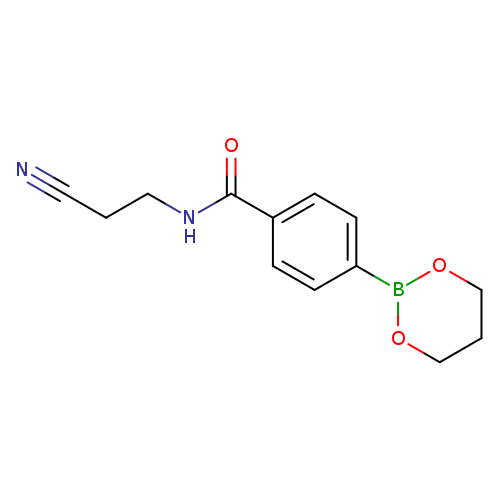
N-(2-Cyanoethyl)-4-(1,3,2-dioxaborinan-2-yl)benzamideCatalog No.:AA003S6E CAS No.:1073372-08-3 MDL No.:MFCD11053850 MF:C13H15BN2O3 MW:258.0808 |
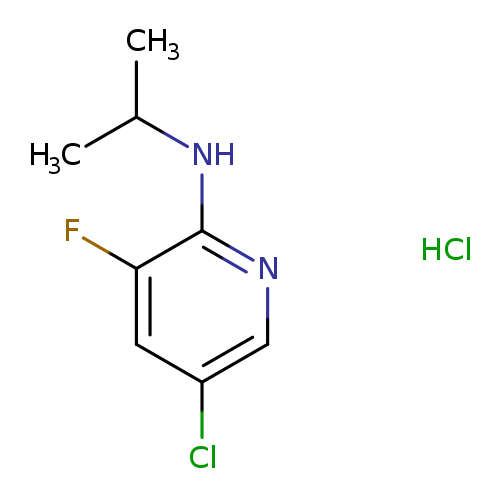
5-Chloro-3-fluoro-2-(N-isopropylamino)pyridine, HClCatalog No.:AA003ML5 CAS No.:1073372-10-7 MDL No.:MFCD09972198 MF:C8H11Cl2FN2 MW:225.0907 |
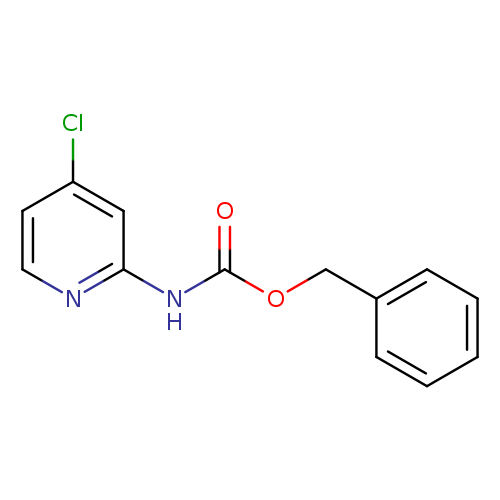
Benzyl 4-chloropyridin-2-ylcarbamateCatalog No.:AA003O0I CAS No.:1073372-14-1 MDL No.:MFCD11504979 MF:C13H11ClN2O2 MW:262.6916 |
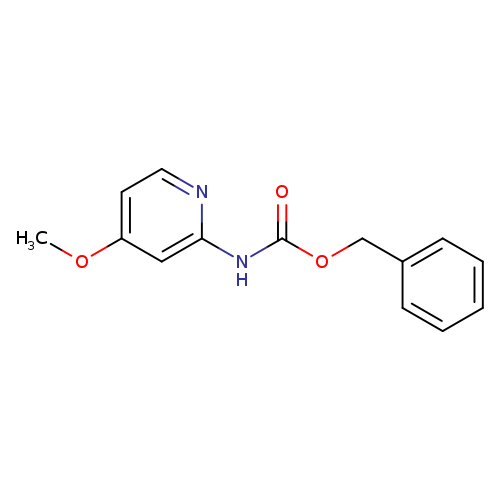
Benzyl 4-methoxypyridin-2-ylcarbamateCatalog No.:AA003O0J CAS No.:1073372-17-4 MDL No.:MFCD11504982 MF:C14H14N2O3 MW:258.2726 |
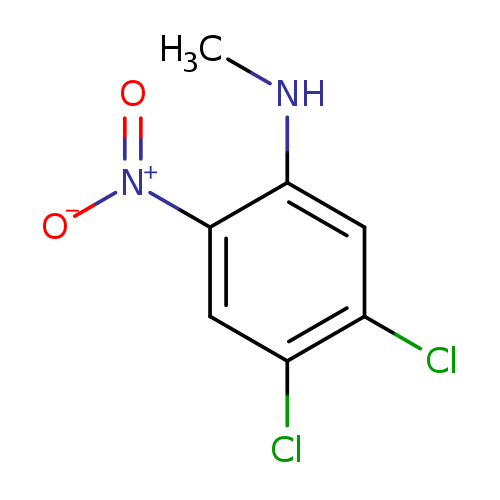
4,5-Dichloro-n-methyl-2-nitroanilineCatalog No.:AA007W8H CAS No.:107342-18-7 MDL No.:MFCD00186241 MF:C7H6Cl2N2O2 MW:221.0407 |
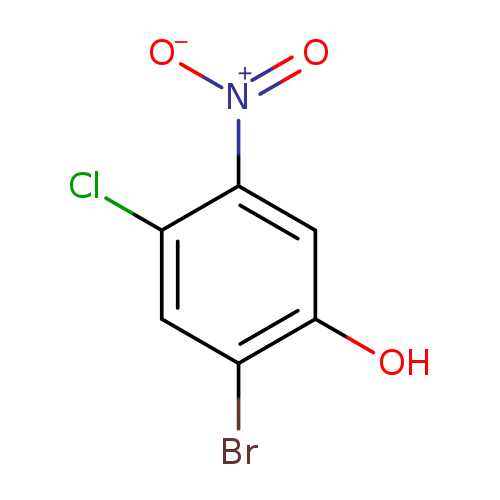
2-BROMO-4-CHLORO-5-NITROPHENOLCatalog No.:AA019EKP CAS No.:1073437-11-2 MDL No.:MFCD30534472 MF:C6H3BrClNO3 MW:252.4499 |
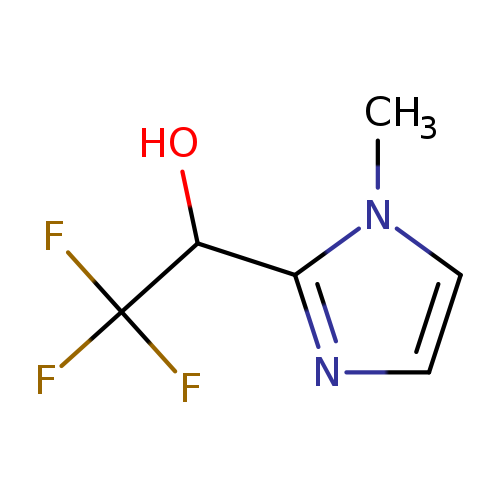
2,2,2-trifluoro-1-(1-methyl-1H-imidazol-2-yl)ethan-1-olCatalog No.:AA019K1L CAS No.:107344-63-8 MDL No.:MFCD08264986 MF:C6H7F3N2O MW:180.1278 |
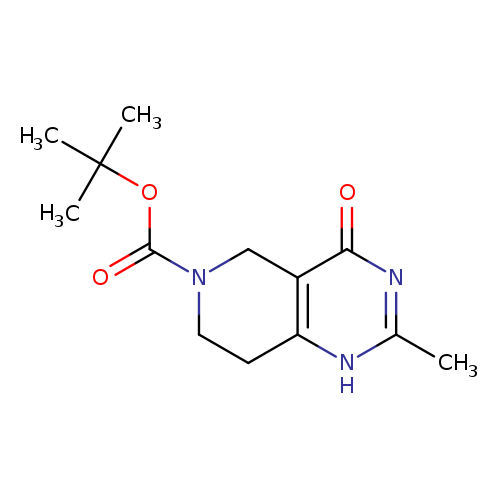
tert-Butyl 4-hydroxy-2-methyl-5H,7H,8H-pyrido[4,3-d]pyrimidine-6-carboxylateCatalog No.:AA0093HG CAS No.:1073440-84-2 MDL No.:MFCD13189665 MF:C13H19N3O3 MW:265.3083 |
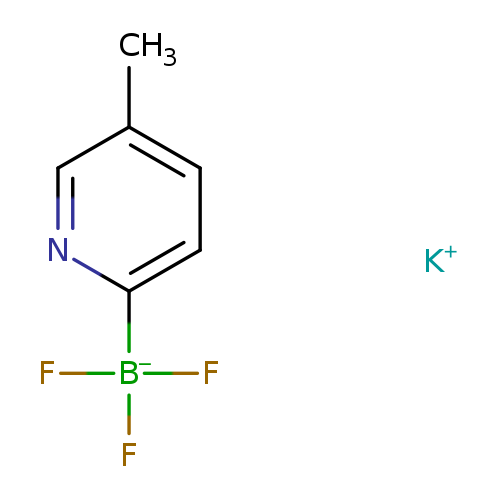
Potassium 5-methylpyridine-2-trifluoroborateCatalog No.:AA003TSW CAS No.:1073468-31-1 MDL No.:MFCD09992972 MF:C6H6BF3KN MW:199.0230 |
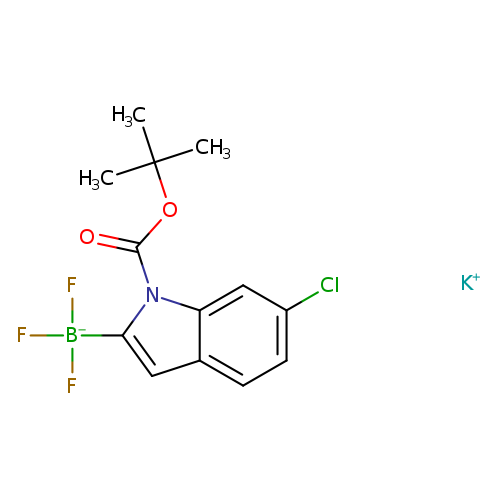
Potassium 1-Boc-6-chloroindole-2-trifluoroborateCatalog No.:AA008SRK CAS No.:1073468-33-3 MDL No.:MFCD11054034 MF:C13H13BClF3KNO2 MW:357.6053 |
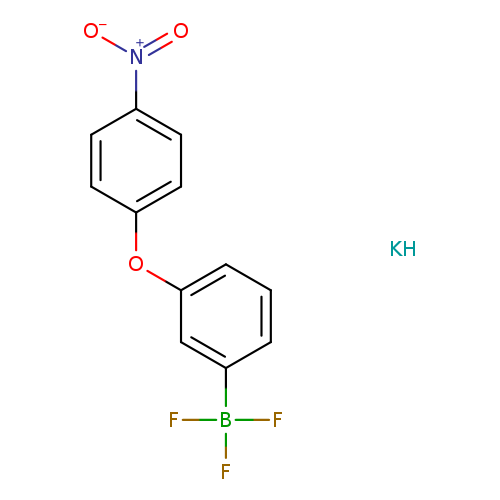
Potassium 3-(4-nitrophenoxy)phenyltrifluoroborateCatalog No.:AA008SR4 CAS No.:1073468-34-4 MDL No.:MFCD09972205 MF:C12H9BF3KNO3 MW:322.1093 |
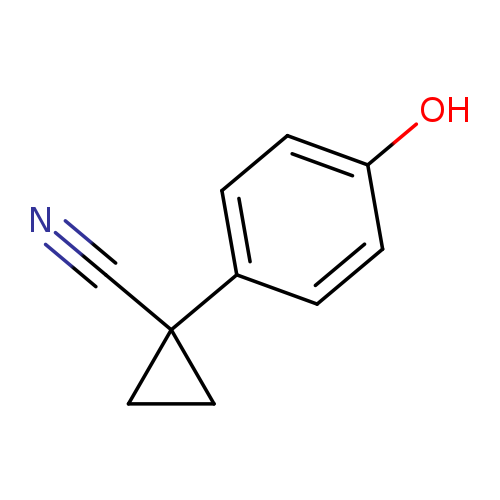
1-(4-Hydroxyphenyl)cyclopropane-1-carbonitrileCatalog No.:AA0093K8 CAS No.:1073477-06-1 MDL No.:MFCD19692083 MF:C10H9NO MW:159.1846 |
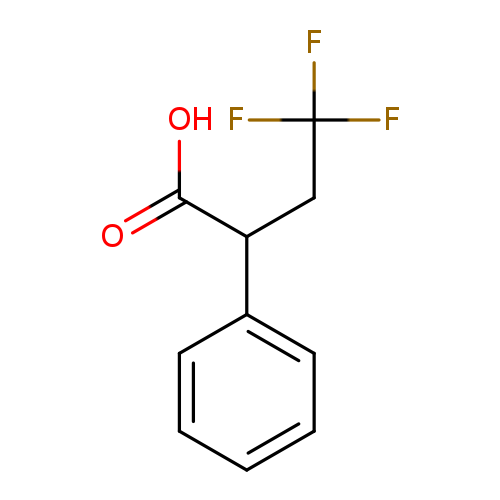
4,4,4-trifluoro-2-phenylbutanoic acidCatalog No.:AA01DX76 CAS No.:1073477-18-5 MDL No.:MFCD21922772 MF:C10H9F3O2 MW:218.1725 |
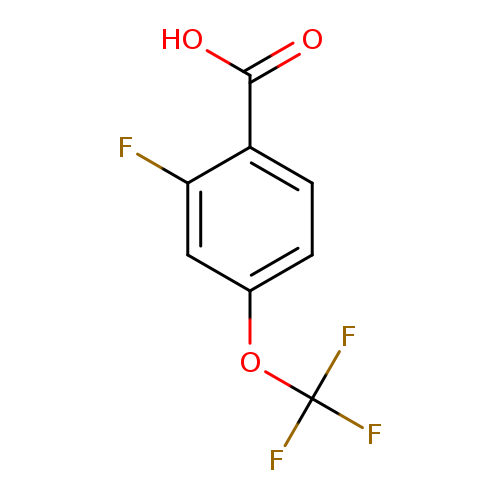
2-Fluoro-4-(trifluoromethoxy)benzoic acidCatalog No.:AA00971U CAS No.:1073477-22-1 MDL No.:MFCD16652439 MF:C8H4F4O3 MW:224.1092 |
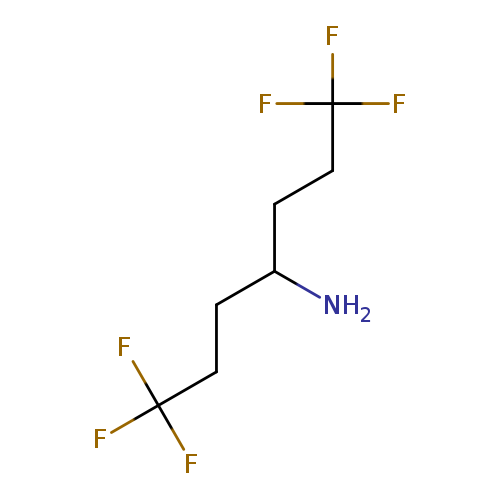
1,1,1,7,7,7-hexafluoroheptan-4-amineCatalog No.:AA01B625 CAS No.:1073477-67-4 MDL No.:MFCD23801863 MF:C7H11F6N MW:223.1594 |
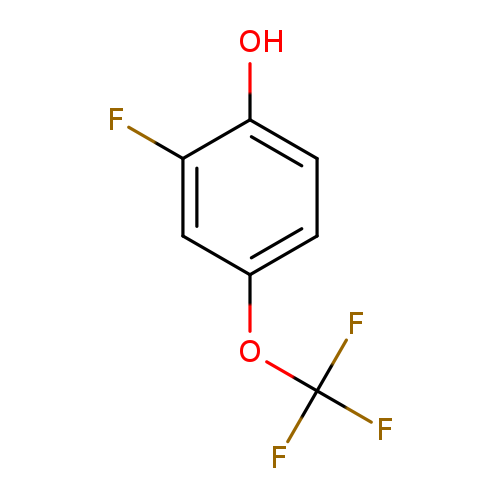
2-Fluoro-4-(trifluoromethoxy)phenolCatalog No.:AA0095B0 CAS No.:1073477-74-3 MDL No.:MFCD23111095 MF:C7H4F4O2 MW:196.0991 |
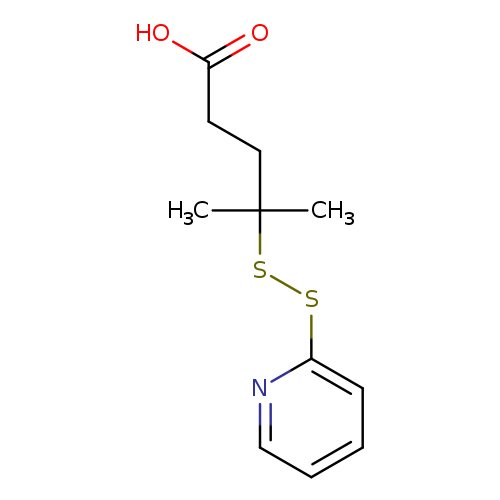
4-methyl-4-(pyridin-2-yldisulfanyl)pentanoic acidCatalog No.:AA01DHU3 CAS No.:107348-49-2 MDL No.:MFCD27935396 MF:C11H15NO2S2 MW:257.3723 |
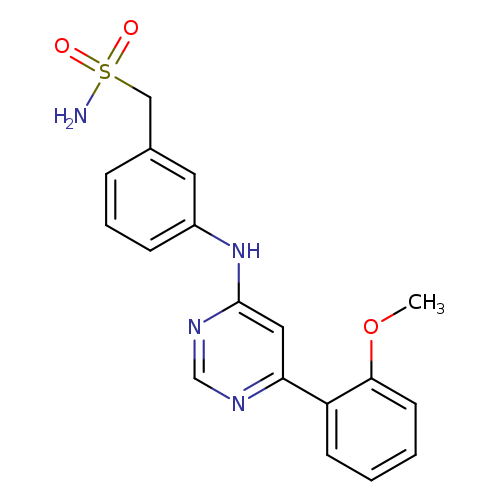
Ldc000067Catalog No.:AA008TEH CAS No.:1073485-20-7 MDL No.:MFCD28137788 MF:C18H18N4O3S MW:370.4255 |
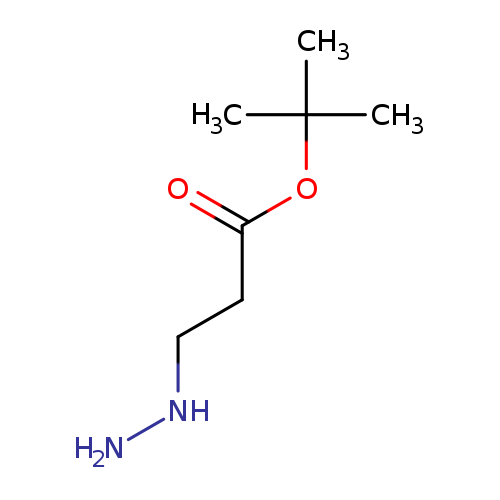
Propanoic acid, 3-hydrazinyl-, 1,1-diMethylethyl esterCatalog No.:AA0092WQ CAS No.:107349-78-0 MDL No.:MFCD14707427 MF:C7H16N2O2 MW:160.2141 |
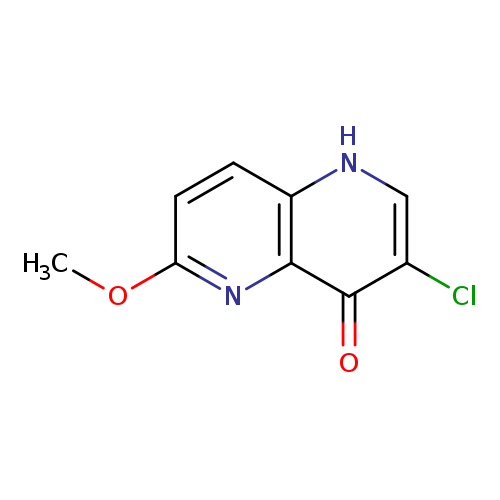
3-Chloro-6-methoxy-1,5-naphthyridin-4(1h)-oneCatalog No.:AA0096V8 CAS No.:1073495-87-0 MDL No.:MFCD24396067 MF:C9H7ClN2O2 MW:210.6171 |
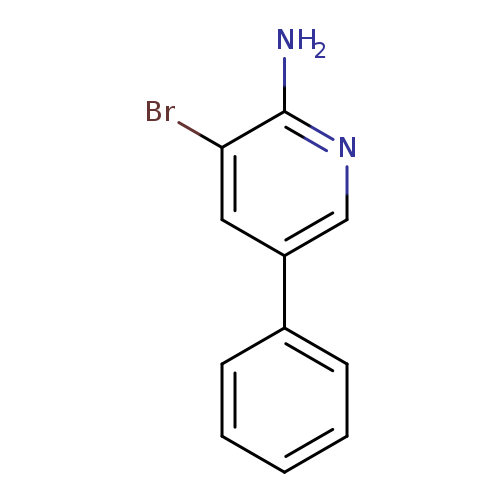
3-Bromo-5-phenylpyridin-2-amineCatalog No.:AA007W8C CAS No.:107351-80-4 MDL No.:MFCD02183569 MF:C11H9BrN2 MW:249.1066 |
2-AMINO-3-METHYLAMINO-5-PHENYLPYRIDINECatalog No.:AA007W8B CAS No.:107351-81-5 MDL No.:MFCD03701121 MF:C12H13N3 MW:199.2517 |
2-Bromo-5-phenylpyridineCatalog No.:AA0084N5 CAS No.:107351-82-6 MDL No.:MFCD00869668 MF:C11H8BrN MW:234.0919 |
4-OXO-3,3-DIPHENYL-[1,2]DIAZETIDINE-1,2-DICARBOXYLIC ACID DIMETHYL ESTERCatalog No.:AA01DU0Q CAS No.:1073529-41-5 MDL No.:MFCD28053514 MF:C17H20N2O5 MW:332.3511 |
3-Cyclopropyl-3-(4-hydroxyphenyl)propanoic acidCatalog No.:AA00HAX1 CAS No.:1073545-88-6 MDL No.:MFCD22574044 MF:C12H14O3 MW:206.2378 |
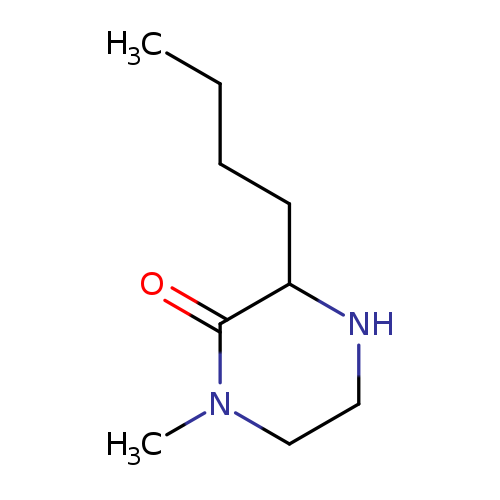
3-butyl-1-methylpiperazin-2-oneCatalog No.:AA008VHC CAS No.:1073556-04-3 MDL No.:MFCD08060031 MF:C9H18N2O MW:170.2520 |
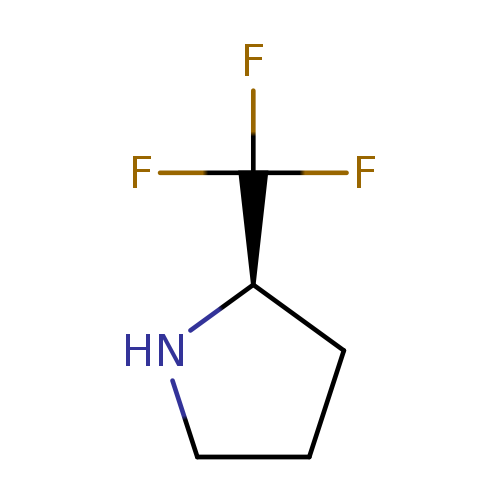
(R)-2-(Trifluoromethyl)pyrrolidineCatalog No.:AA008SCZ CAS No.:1073556-31-6 MDL No.:MFCD07784180 MF:C5H8F3N MW:139.1189 |
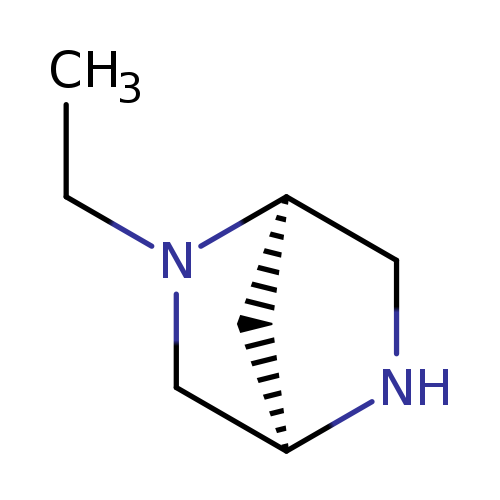
(1R,4R)-2-Ethyl-2,5-diaza-bicyclo[2.2.1]heptaneCatalog No.:AA00HAX2 CAS No.:1073556-32-7 MDL No.:MFCD19237471 MF:C7H14N2 MW:126.1995 |
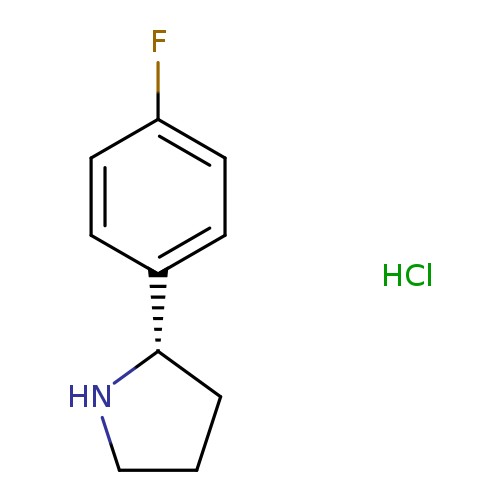
(S)-2-(4-Fluorophenyl)pyrrolidine hydrochlorideCatalog No.:AA00HAX3 CAS No.:1073556-40-7 MDL No.:MFCD08751463 MF:C10H13ClFN MW:201.6683 |
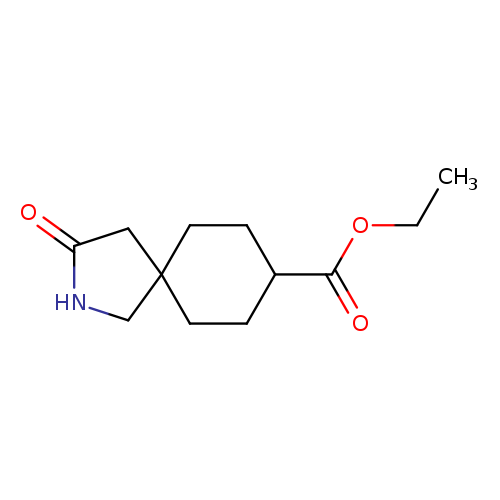
Ethyl 3-oxo-2-azaspiro[4.5]decane-8-carboxylateCatalog No.:AA009982 CAS No.:1073559-59-7 MDL No.:MFCD20488669 MF:C12H19NO3 MW:225.2842 |
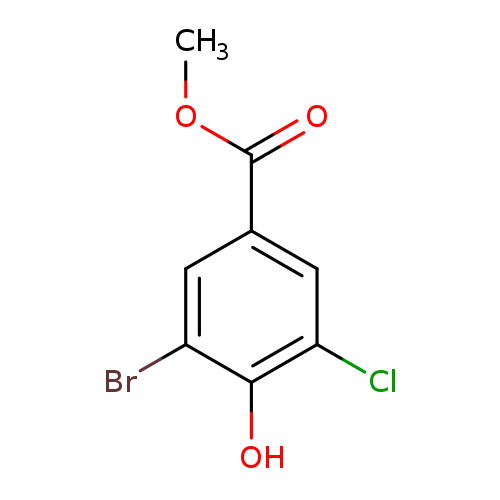
methyl 3-bromo-5-chloro-4-hydroxybenzoateCatalog No.:AA01BQZ4 CAS No.:107356-01-4 MDL No.:MFCD25955178 MF:C8H6BrClO3 MW:265.4884 |
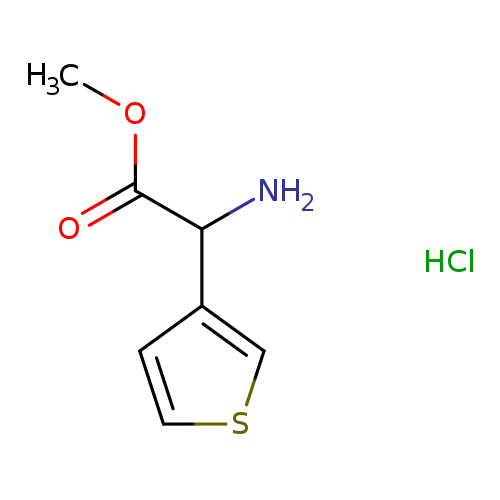
methyl 2-amino-2-(thiophen-3-yl)acetate hydrochlorideCatalog No.:AA01ACN3 CAS No.:107357-02-8 MDL No.:MFCD26936076 MF:C7H10ClNO2S MW:207.6778 |
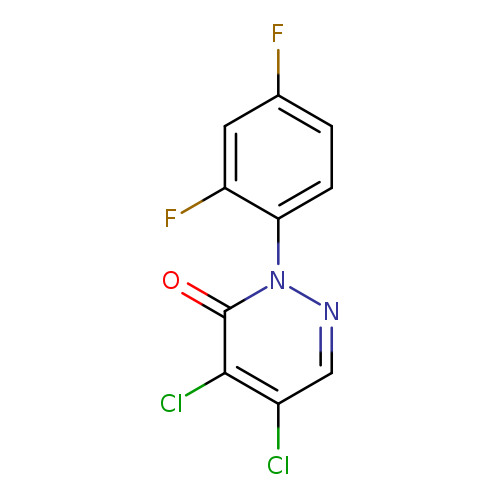
4,5-dichloro-2-(2,4-difluorophenyl)-2,3-dihydropyridazin-3-oneCatalog No.:AA019U8C CAS No.:107360-91-8 MDL No.:MFCD09051393 MF:C10H4Cl2F2N2O MW:277.0544 |
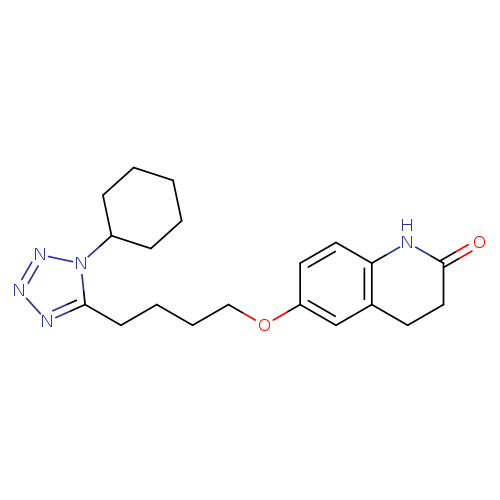
Cilostazol-d11Catalog No.:AA008VYE CAS No.:1073608-02-2 MDL No.:MFCD28138296 MF:C20H27N5O2 MW:369.4607 |
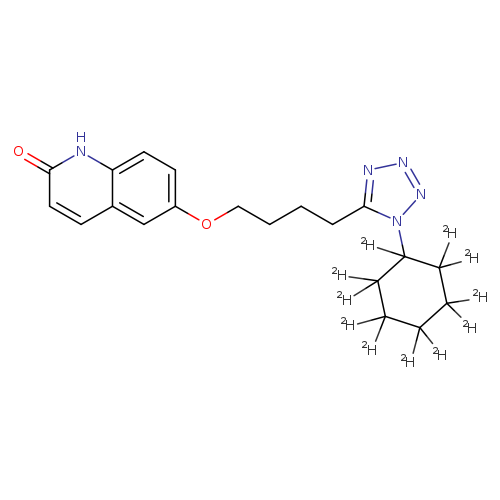
3,4-DehydroCilostazol-d11Catalog No.:AA01DZFU CAS No.:1073608-13-5 MDL No.:MFCD09840314 MF:C20H14D11N5O2 MW:378.5126 |
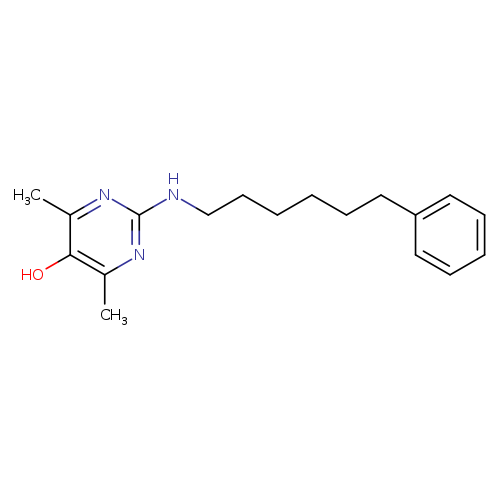
EnazadremCatalog No.:AA008SEV CAS No.:107361-33-1 MDL No.:MFCD00866625 MF:C18H25N3O MW:299.4106 |
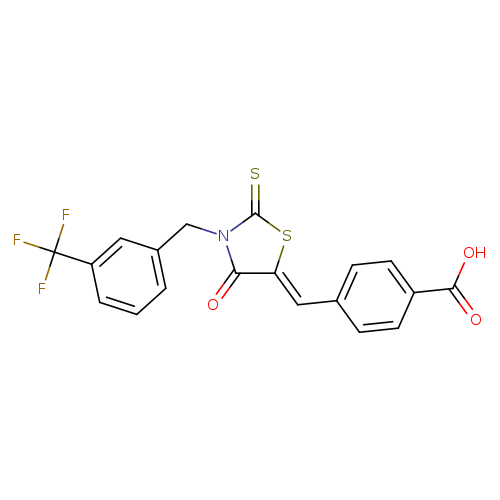
Cy-09Catalog No.:AA01DEX8 CAS No.:1073612-91-5 MDL No.:MFCD31619349 MF:C19H12F3NO3S2 MW:423.4287 |
3-(1H-Pyrrol-1-yl)benzo[b]thiophene-2-carbohydrazideCatalog No.:AA0084N1 CAS No.:107363-01-9 MDL No.:MFCD06200944 MF:C13H11N3OS MW:257.3109 |
(3-Bromo-4-oxo-cyclohexyl)-carbamic acid tert-butyl esterCatalog No.:AA0093HS CAS No.:1073632-93-5 MDL No.:MFCD23115355 MF:C11H18BrNO3 MW:292.1695 |
(2-Amino-5,6,7,8-tetrahydro-quinazolin-6-yl)-carbamic acid tert-butyl esterCatalog No.:AA0093I0 CAS No.:1073633-86-9 MDL No.:MFCD24843107 MF:C13H20N4O2 MW:264.3235 |
9-methyl-9H-carbazole-3-sulfonamideCatalog No.:AA01C3UK CAS No.:1073653-45-8 MDL No.:MFCD29762920 MF:C13H12N2O2S MW:260.3116 |
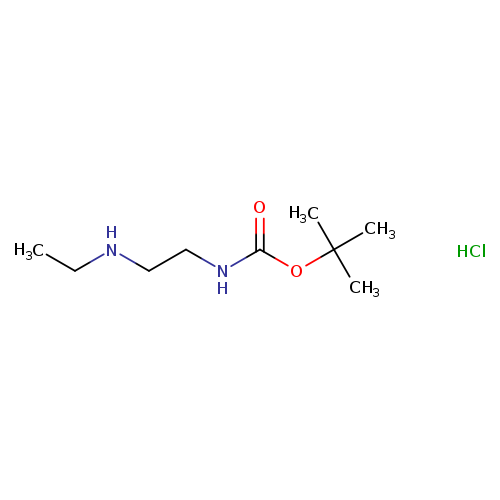
Boc-eda-et hclCatalog No.:AA008UY3 CAS No.:1073659-87-6 MDL No.:MFCD06796899 MF:C9H21ClN2O2 MW:224.7282 |
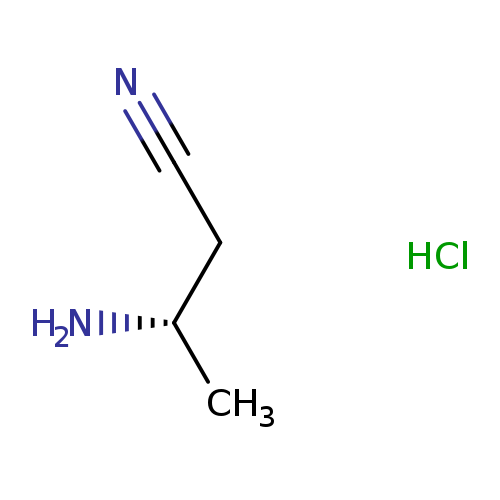
(S)-3-Aminobutanenitrile hydrochlorideCatalog No.:AA003A91 CAS No.:1073666-54-2 MDL No.:MFCD18651598 MF:C4H9ClN2 MW:120.5807 |
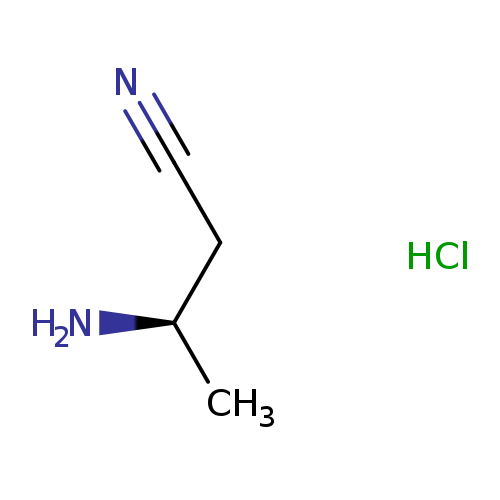
(R)-3-Aminobutanenitrile hclCatalog No.:AA009695 CAS No.:1073666-55-3 MDL No.:MFCD18651597 MF:C4H9ClN2 MW:120.5807 |
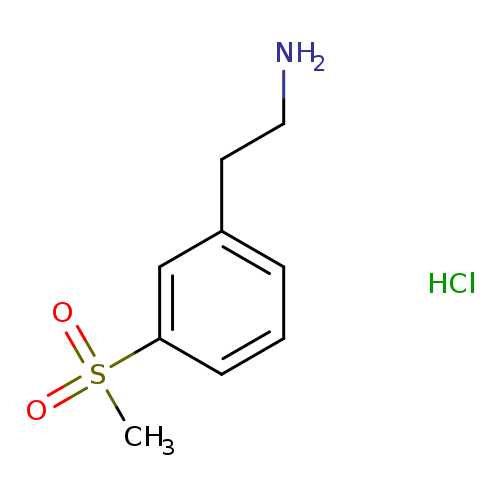
2-[3-(Methylsulfonyl)phenyl]ethylamine HydrochlorideCatalog No.:AA00HAX6 CAS No.:1073666-57-5 MDL No.:MFCD27986815 MF:C9H14ClNO2S MW:235.7310 |
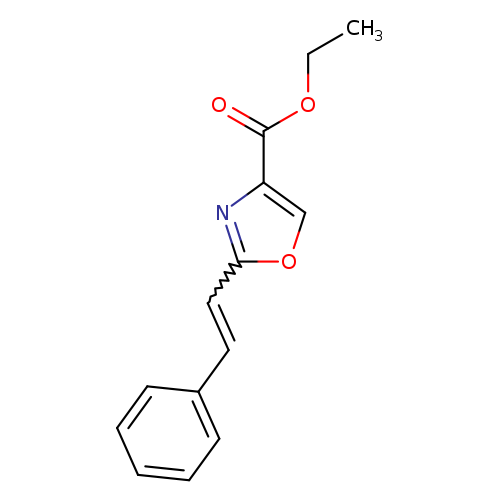
ethyl 2-(2-phenylethenyl)-1,3-oxazole-4-carboxylateCatalog No.:AA01ACPX CAS No.:107367-69-1 MDL No.:MFCD00793709 MF:C14H13NO3 MW:243.2579 |
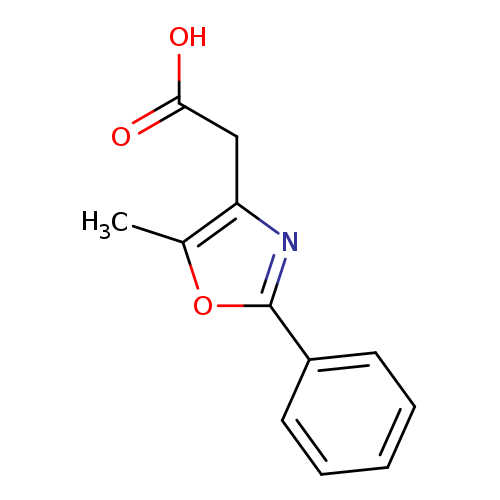
2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)acetic acidCatalog No.:AA007EKE CAS No.:107367-98-6 MDL No.:MFCD00100005 MF:C12H11NO3 MW:217.2206 |
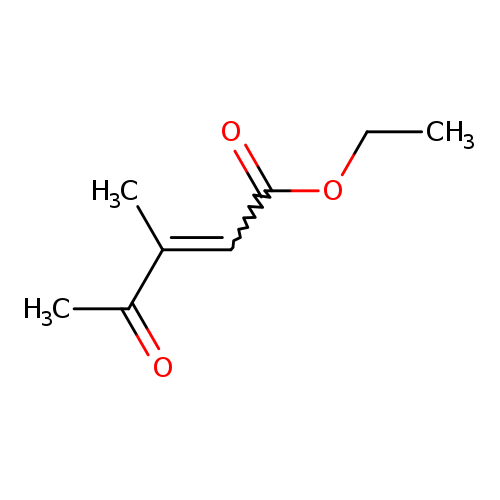
2-Pentenoic acid, 3-methyl-4-oxo-, ethyl ester, (Z)-Catalog No.:AA01B9LI CAS No.:107368-27-4 MDL No.:MFCD28383942 MF:C8H12O3 MW:156.1791 |
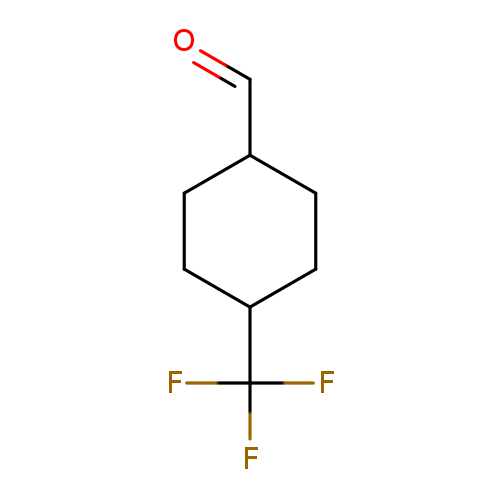
4-(trifluoromethyl)cyclohexane-1-carbaldehyde, Mixture of diastereomersCatalog No.:AA01ACIO CAS No.:1073722-40-3 MDL No.:MFCD21321762 MF:C8H11F3O MW:180.1675 |
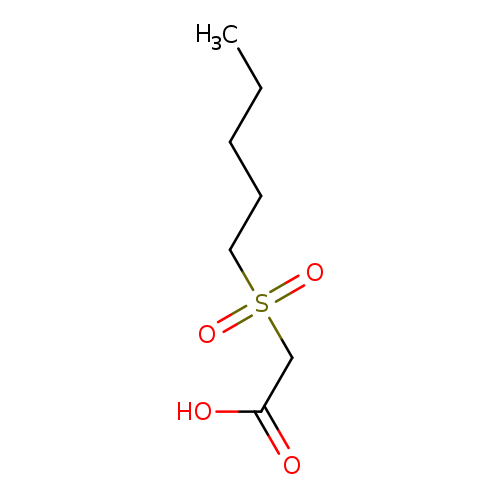
2-(pentane-1-sulfonyl)acetic acidCatalog No.:AA01AA6W CAS No.:107375-91-7 MDL No.:MFCD09940130 MF:C7H14O4S MW:194.2487 |
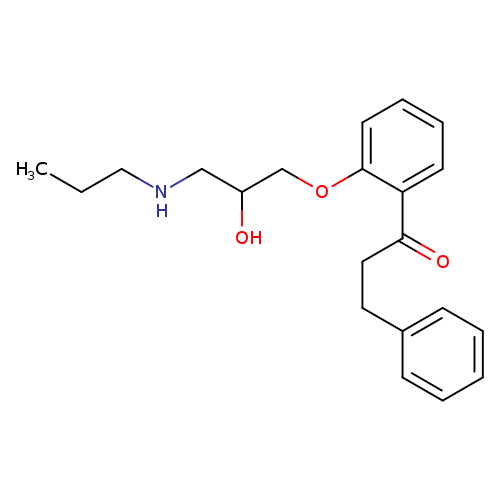
(R)-PropafenoneCatalog No.:AA008W4Q CAS No.:107381-31-7 MDL No.:MFCD00869669 MF:C21H27NO3 MW:341.4440 |
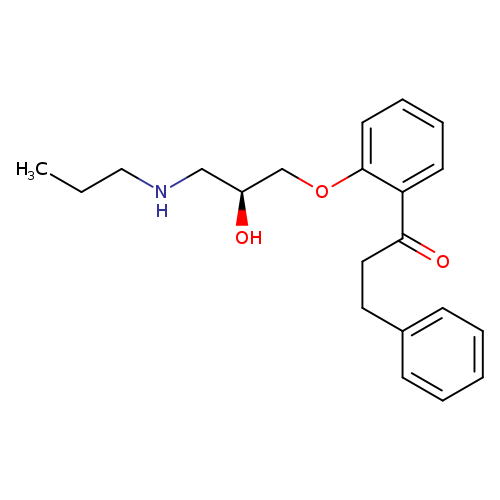
1-Propanone,1-[2-[(2S)-2-hydroxy-3-(propylamino)propoxy]phenyl]-3-phenyl-Catalog No.:AA0084MV CAS No.:107381-32-8 MDL No.:MFCD00869668 MF:C21H27NO3 MW:341.4440 |
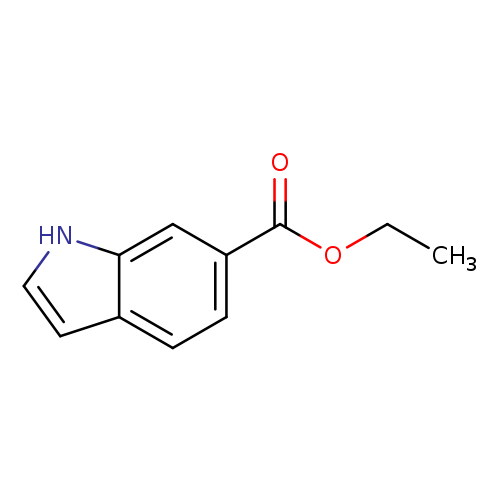
1H-Pyrrolo[3,2-c]pyridine-6-carboxylic acid, ethyl esterCatalog No.:AA0095VV CAS No.:107384-68-9 MDL No.:MFCD20527767 MF:C11H11NO2 MW:189.2105 |
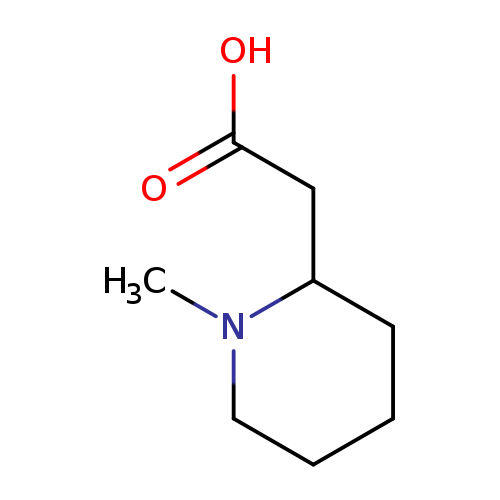
2-(1-Methylpiperidin-2-yl)acetic acidCatalog No.:AA0084MU CAS No.:107388-72-7 MDL No.:MFCD05860069 MF:C8H15NO2 MW:157.2102 |
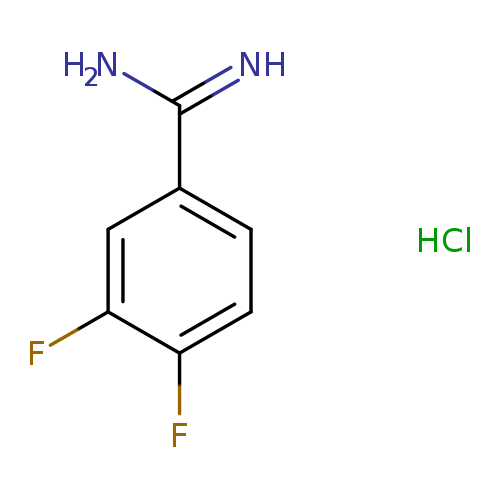
3,4-Difluoro-benzamidine, HClCatalog No.:AA007EET CAS No.:107392-33-6 MDL No.:MFCD04114434 MF:C7H7ClF2N2 MW:192.5937 |
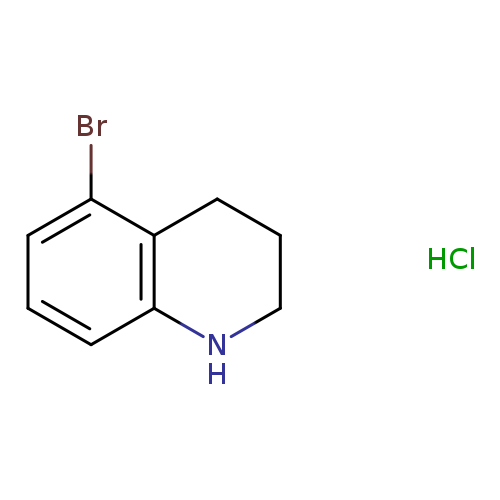
5-Bromo-1,2,3,4-tetrahydroquinoline, HClCatalog No.:AA007W7X CAS No.:1073968-64-5 MDL No.:MFCD09026771 MF:C9H11BrClN MW:248.5473 |
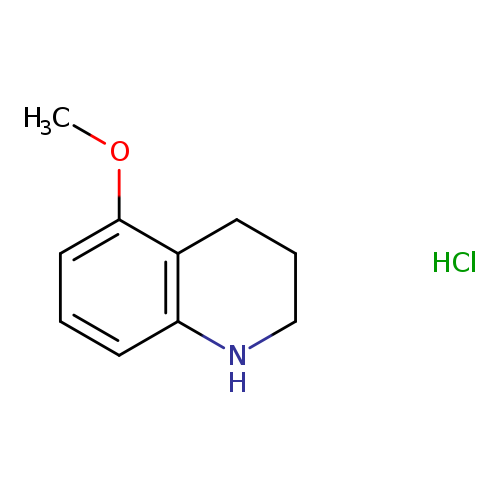
5-Methoxy-1,2,3,4-tetrahydroquinoline hydrochlorideCatalog No.:AA008YZE CAS No.:1073968-65-6 MDL No.:MFCD09026772 MF:C10H14ClNO MW:199.6773 |
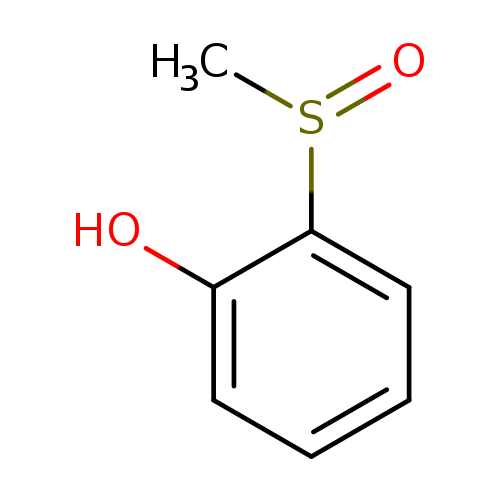
2-(Methylsulfinyl)phenolCatalog No.:AA0084MP CAS No.:1074-02-8 MDL No.:MFCD00127350 MF:C7H8O2S MW:156.2022 |

PhenylglyoxalCatalog No.:AA0035EE CAS No.:1074-12-0 MDL No.:MFCD00006959 MF:C8H6O2 MW:134.1320 |
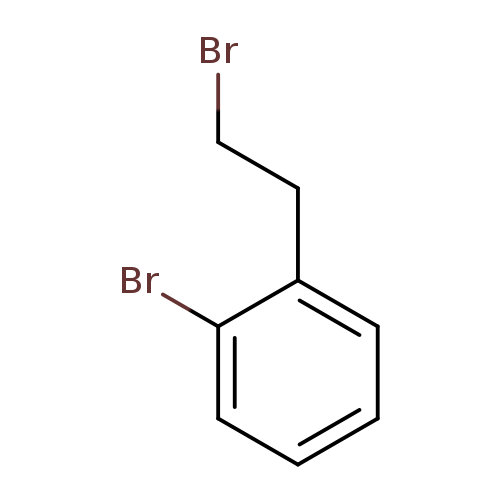
1-bromo-2-(2-bromoethyl)benzeneCatalog No.:AA008RMA CAS No.:1074-15-3 MDL No.:MFCD00027929 MF:C8H8Br2 MW:263.9571 |
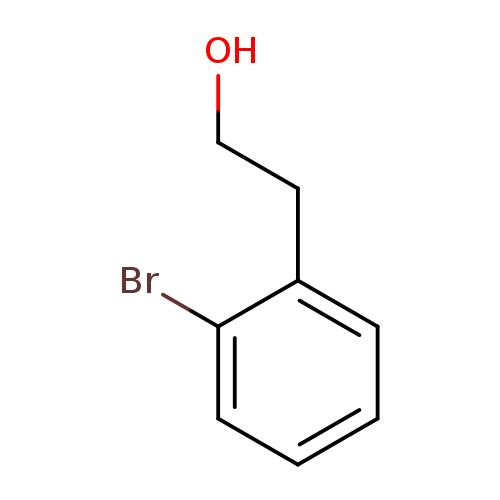
2-(2-Bromophenyl)ethanolCatalog No.:AA0032RR CAS No.:1074-16-4 MDL No.:MFCD00093566 MF:C8H9BrO MW:201.0605 |
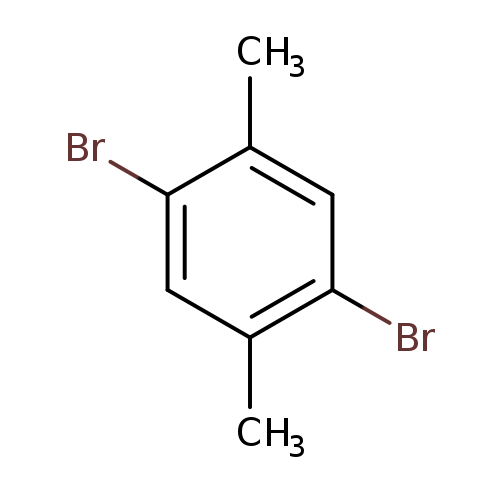
2,5-Dibromo-p-xyleneCatalog No.:AA0032JV CAS No.:1074-24-4 MDL No.:MFCD00000091 MF:C8H8Br2 MW:263.9571 |
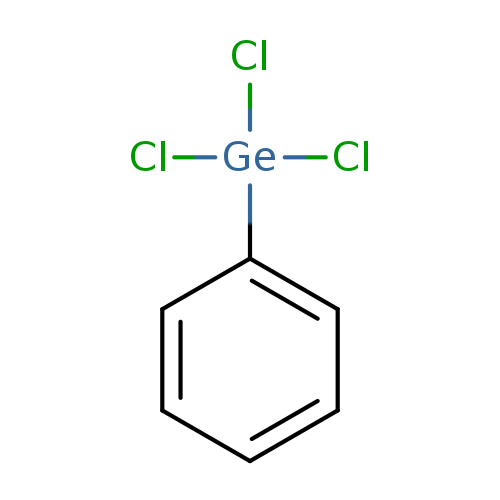
PhenyltrichlorogermaneCatalog No.:AA003TNS CAS No.:1074-29-9 MDL No.:MFCD00000460 MF:C6H5Cl3Ge MW:256.1029 |
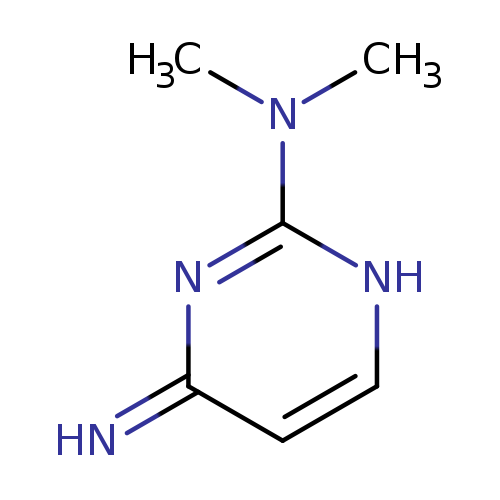
N2,N2-Dimethylpyrimidine-2,4-diamineCatalog No.:AA009QTJ CAS No.:1074-34-6 MDL No.:MFCD16103668 MF:C6H10N4 MW:138.1704 |
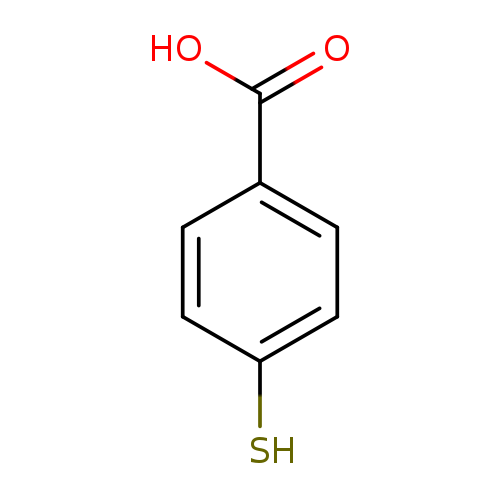
4-Mercaptobenzoic acidCatalog No.:AA00340D CAS No.:1074-36-8 MDL No.:MFCD00016617 MF:C7H6O2S MW:154.1863 |
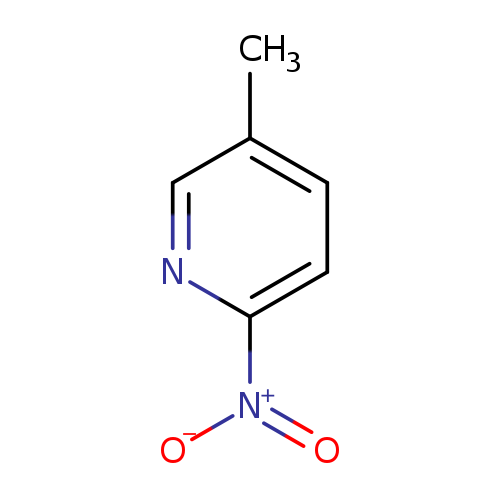
2-Nitro-5-methylpyridineCatalog No.:AA007EEF CAS No.:1074-38-0 MDL No.:MFCD00234182 MF:C6H6N2O2 MW:138.1240 |
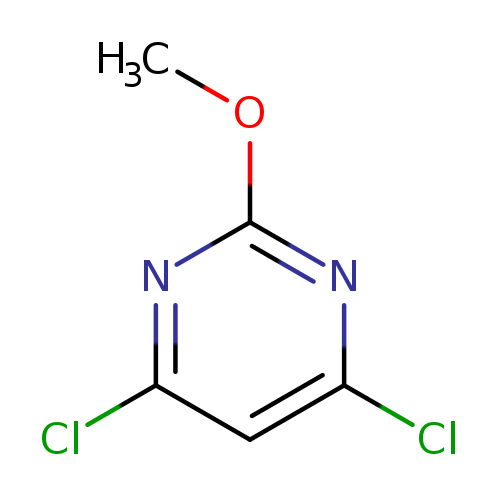
4,6-Dichloro-2-methoxypyrimidineCatalog No.:AA003KGE CAS No.:1074-40-4 MDL No.:MFCD03428360 MF:C5H4Cl2N2O MW:179.0041 |
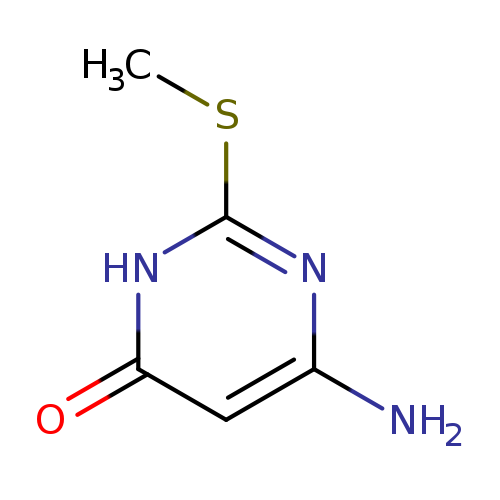
4-Amino-2-(methylthio)-6-pyrimidinolCatalog No.:AA003KL4 CAS No.:1074-41-5 MDL No.:MFCD00055198 MF:C5H7N3OS MW:157.1936 |
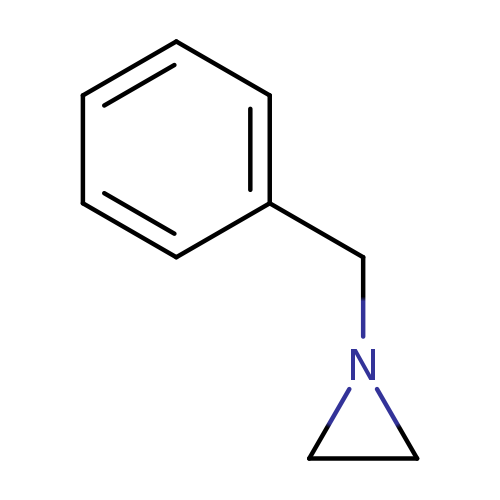
Aziridine,1-(phenylmethyl)-Catalog No.:AA0084ME CAS No.:1074-42-6 MDL No.:MFCD00871539 MF:C9H11N MW:133.1903 |
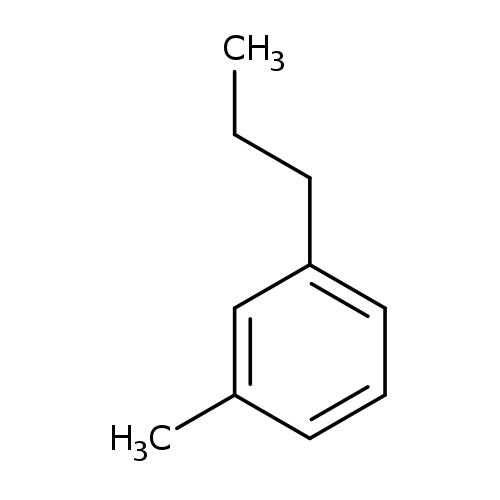
1-Methyl-3-propylbenzeneCatalog No.:AA003EF4 CAS No.:1074-43-7 MDL No.:MFCD00059247 MF:C10H14 MW:134.2182 |
1-(Piperazin-1-yl)propan-2-olCatalog No.:AA0084MC CAS No.:1074-54-0 MDL No.:MFCD00130195 MF:C7H16N2O MW:144.2147 |
3-(Imidazol-4-yl)propionic acidCatalog No.:AA007EE9 CAS No.:1074-59-5 MDL No.:MFCD00237415 MF:C6H8N2O2 MW:140.1399 |
(4-Vinylphenyl)methanolCatalog No.:AA008R08 CAS No.:1074-61-9 MDL No.:MFCD00016872 MF:C9H10O MW:134.1751 |
3-(Cyclopentylamino)propionitrileCatalog No.:AA008RUY CAS No.:1074-63-1 MDL No.:MFCD00060286 MF:C8H14N2 MW:138.2102 |
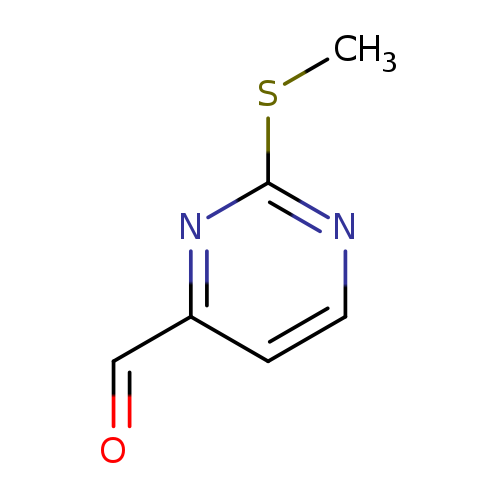
2-(Methylthio)pyrimidine-4-carbaldehydeCatalog No.:AA008R7C CAS No.:1074-68-6 MDL No.:MFCD02683211 MF:C6H6N2OS MW:154.1896 |
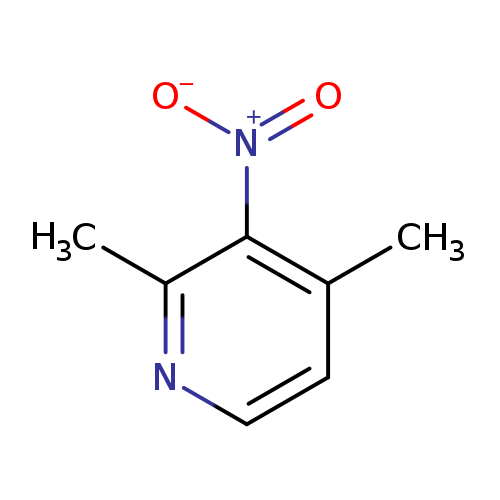
2,4-Dimethyl-3-nitropyridineCatalog No.:AA0084M6 CAS No.:1074-76-6 MDL No.:MFCD08235269 MF:C7H8N2O2 MW:152.1506 |
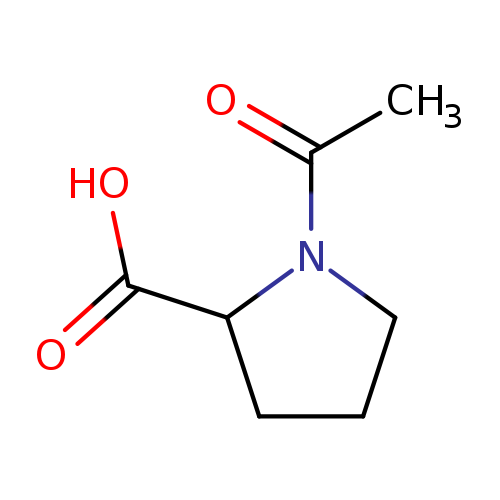
Ac-DL-Pro-OHCatalog No.:AA0084M3 CAS No.:1074-79-9 MDL No.:MFCD02678612 MF:C7H11NO3 MW:157.1671 |
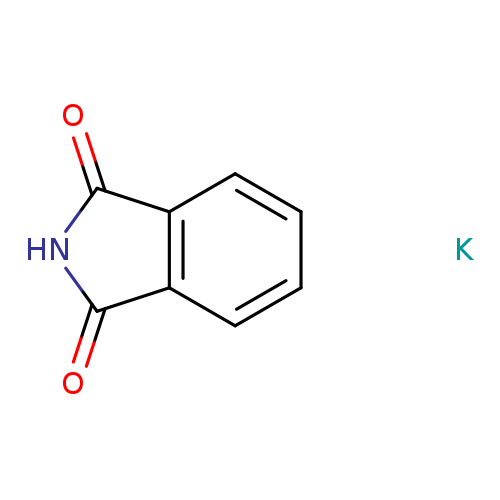
Potassium phthalimideCatalog No.:AA0035FX CAS No.:1074-82-4 MDL No.:MFCD00005887 MF:C8H5KNO2 MW:186.2291 |
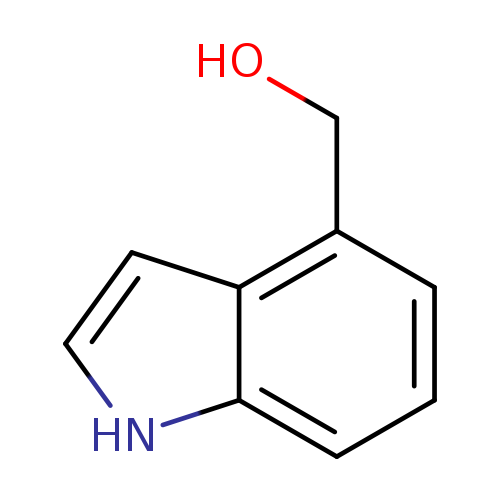
Indole-4-methanolCatalog No.:AA0038WW CAS No.:1074-85-7 MDL No.:MFCD01632220 MF:C9H9NO MW:147.1739 |
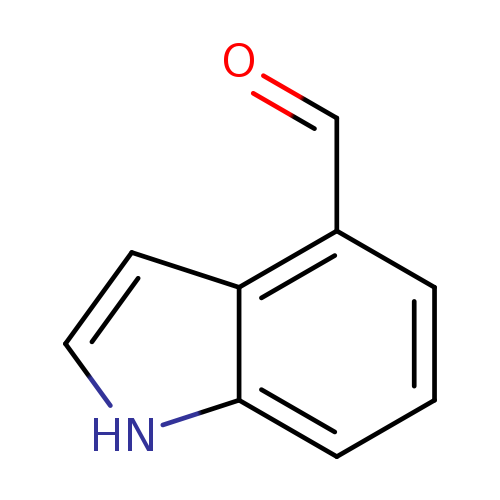
4-FormylindoleCatalog No.:AA0037XQ CAS No.:1074-86-8 MDL No.:MFCD01632221 MF:C9H7NO MW:145.1580 |
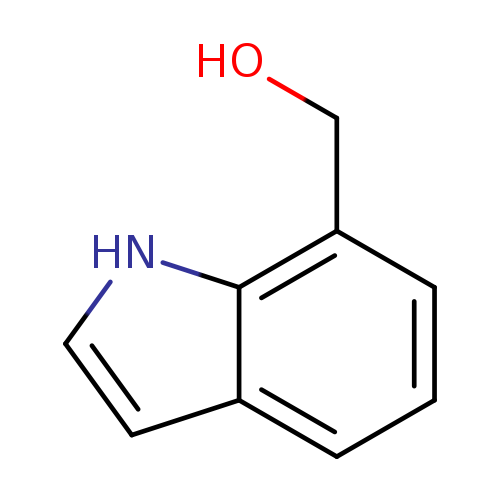
Indole-7-methanolCatalog No.:AA008R31 CAS No.:1074-87-9 MDL No.:MFCD02179597 MF:C9H9NO MW:147.1739 |
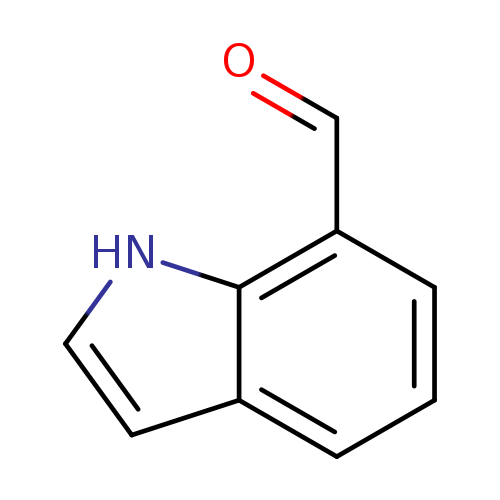
7-FormylindoleCatalog No.:AA0037XP CAS No.:1074-88-0 MDL No.:MFCD01318152 MF:C9H7NO MW:145.1580 |
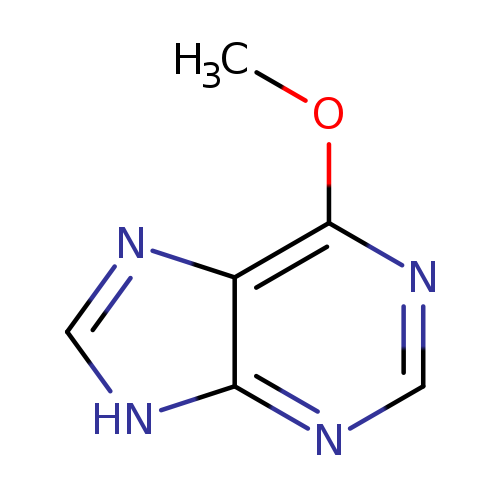
6-MethoxypurineCatalog No.:AA003N6Z CAS No.:1074-89-1 MDL No.:MFCD16878999 MF:C6H6N4O MW:150.1380 |
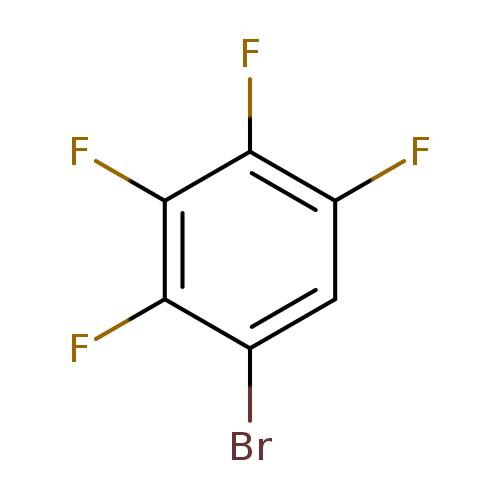
1-Bromo-2,3,4,5-tetrafluorobenzeneCatalog No.:AA003DZX CAS No.:1074-91-5 MDL No.:MFCD00017900 MF:C6HBrF4 MW:228.9698 |

1-tert-Butyl-2-methylbenzeneCatalog No.:AA00325R CAS No.:1074-92-6 MDL No.:MFCD00059209 MF:C11H16 MW:148.2447 |
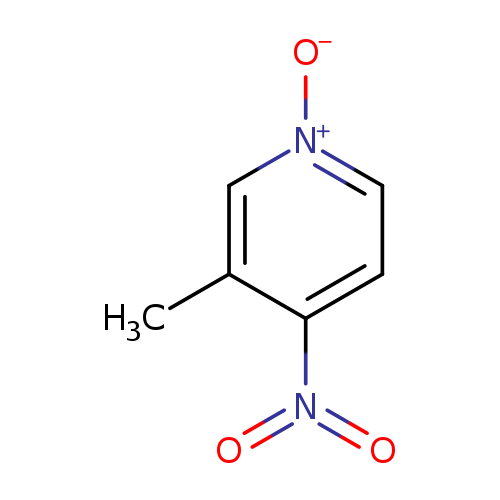
3-Methyl-4-nitropyridine 1-oxideCatalog No.:AA003LS2 CAS No.:1074-98-2 MDL No.:MFCD00014626 MF:C6H6N2O3 MW:154.1234 |
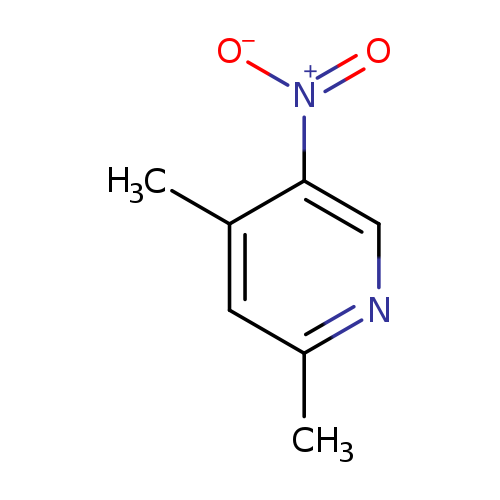
2,4-Dimethyl-5-nitropyridineCatalog No.:AA007W2U CAS No.:1074-99-3 MDL No.:MFCD05982051 MF:C7H8N2O2 MW:152.1506 |
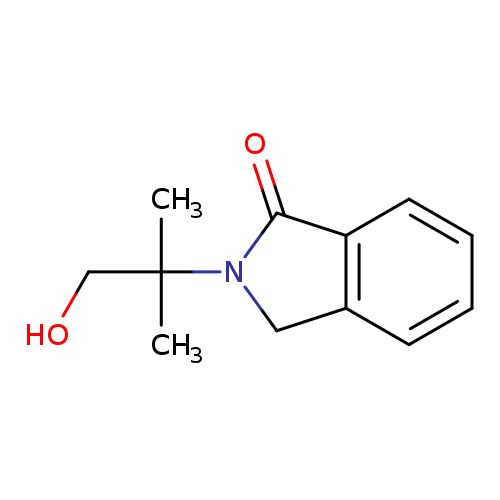
2-(1-hydroxy-2-methylpropan-2-yl)-2,3-dihydro-1H-isoindol-1-oneCatalog No.:AA00INFI CAS No.:107400-33-9 MDL No.:MFCD02082781 MF:C12H15NO2 MW:205.2530 |
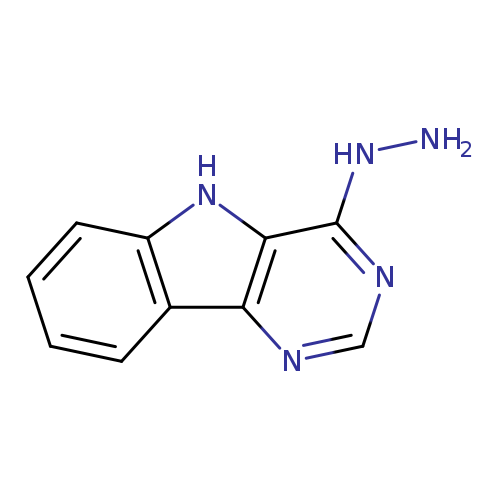
{5H-pyrimido[5,4-b]indol-4-yl}hydrazineCatalog No.:AA00IWZ9 CAS No.:107401-01-4 MDL No.:MFCD00141066 MF:C10H9N5 MW:199.2120 |
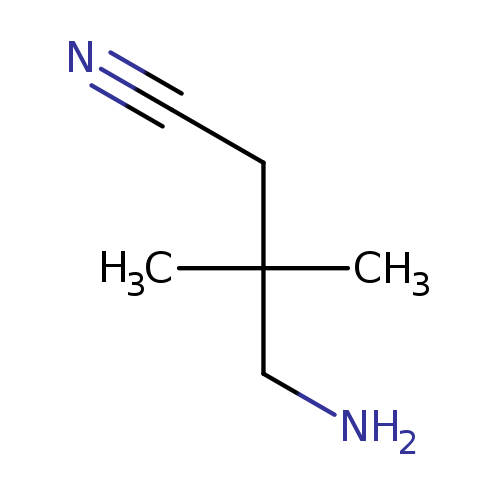
4-amino-3,3-dimethylbutanenitrileCatalog No.:AA01C2JE CAS No.:1074021-90-1 MDL No.:MFCD29055053 MF:C6H12N2 MW:112.1729 |
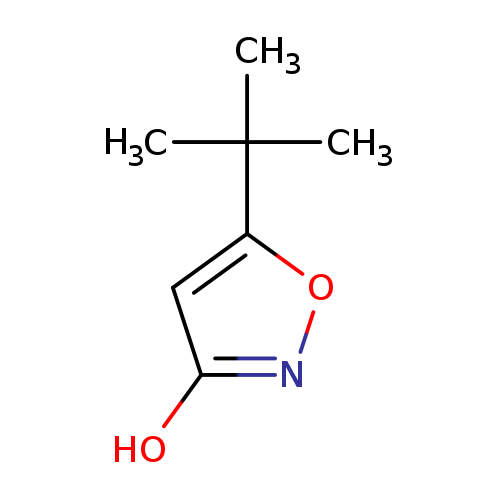
3(2H)-Isoxazolone, 5-(1,1-dimethylethyl)-Catalog No.:AA01DX59 CAS No.:107403-12-3 MDL No.:MFCD25955187 MF:C7H11NO2 MW:141.1677 |
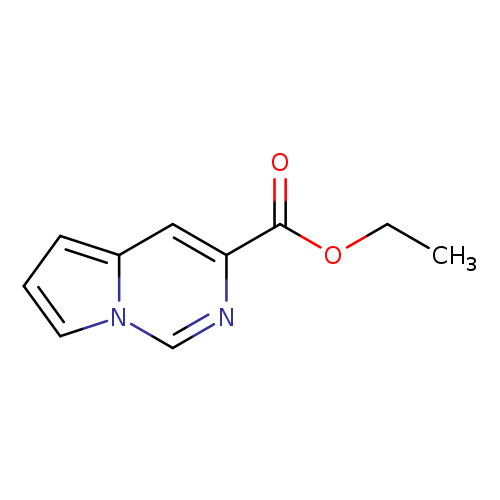
Ethyl pyrrolo[1,2-c]pyrimidine-3-carboxylateCatalog No.:AA007EEP CAS No.:107407-80-7 MDL No.:MFCD02082826 MF:C10H10N2O2 MW:190.1986 |
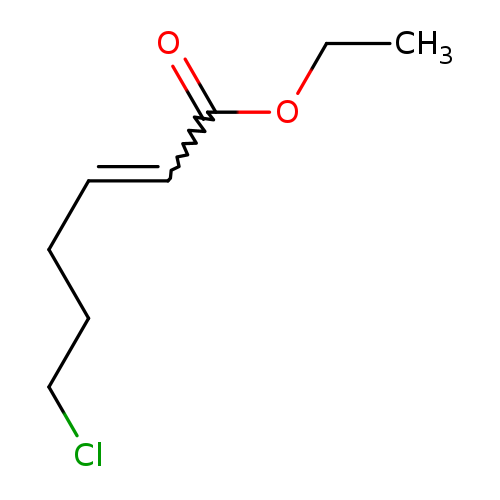
6-CHLORO-TRANS-2-HEXENOIC ACID ETHYL ESTERCatalog No.:AA01DKRR CAS No.:107408-34-4 MDL No.:MFCD05863887 MF:C8H13ClO2 MW:176.6406 |
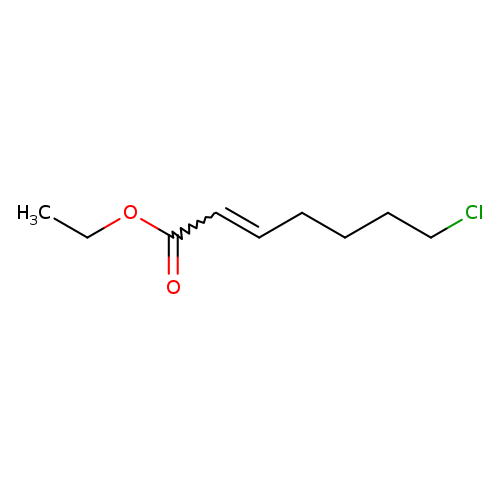
7-CHLORO-TRANS-2-HEPENOIC ACID ETHYL ESTERCatalog No.:AA01DMAW CAS No.:107408-35-5 MDL No.:MFCD05863885 MF:C9H15ClO2 MW:190.6672 |
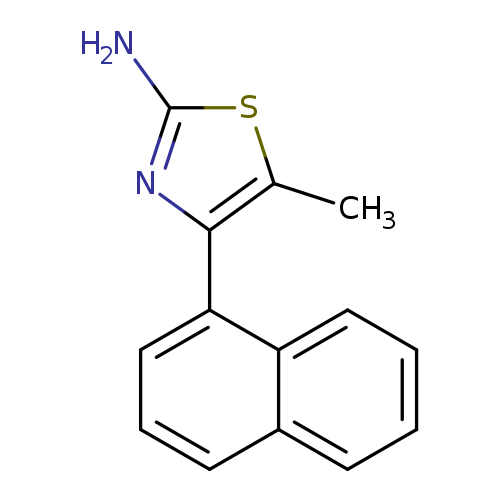
2-Thiazolamine, 5-methyl-4-(1-naphthalenyl)-Catalog No.:AA007EEM CAS No.:107411-05-2 MDL No.:MFCD20171325 MF:C14H12N2S MW:240.3235 |
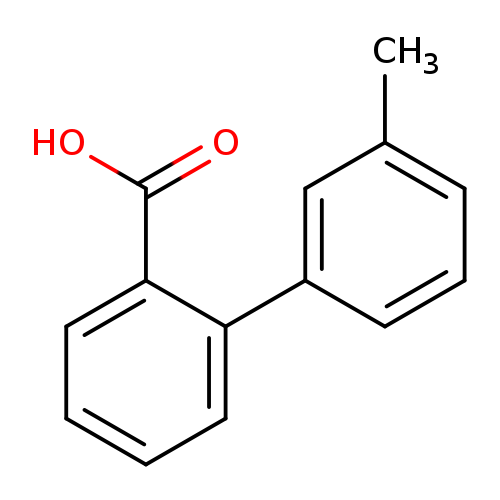
2-(3-Methylphenyl)benzoic acidCatalog No.:AA008SSR CAS No.:107412-71-5 MDL No.:MFCD04039113 MF:C14H12O2 MW:212.2439 |
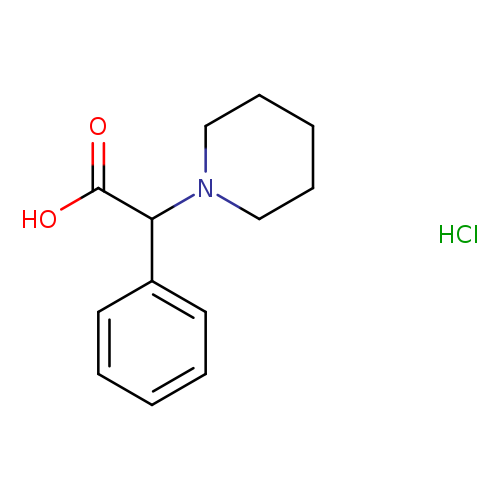
2-Phenyl-2-(piperidin-1-yl)acetic acid hydrochlorideCatalog No.:AA007W7T CAS No.:107416-50-2 MDL No.:MFCD06801198 MF:C13H18ClNO2 MW:255.7405 |
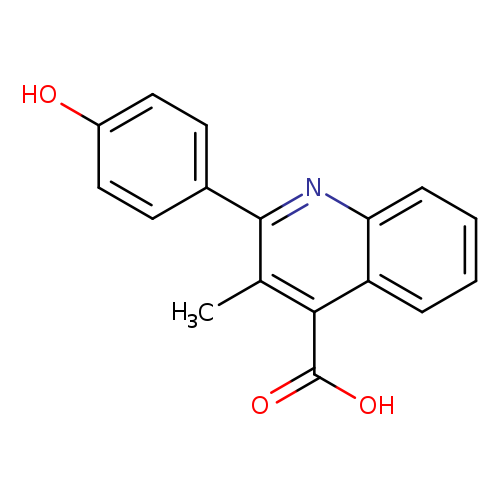
2-(4-Hydroxyphenyl)-3-methyl-4-quinolinecarboxylic acidCatalog No.:AA0084ML CAS No.:107419-49-8 MDL No.:MFCD06200949 MF:C17H13NO3 MW:279.2900 |
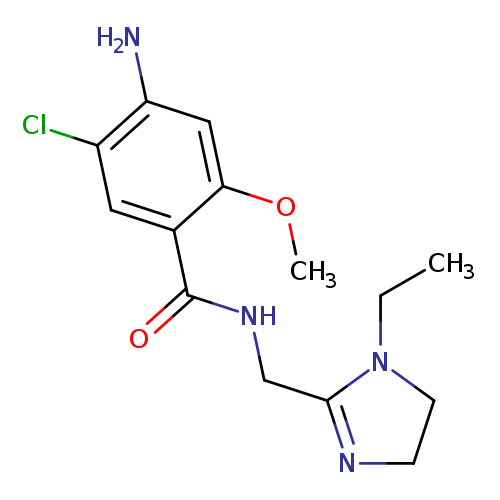
LintoprideCatalog No.:AA008Z8L CAS No.:107429-63-0 MDL No.:MFCD00868099 MF:C14H19ClN4O2 MW:310.7793 |
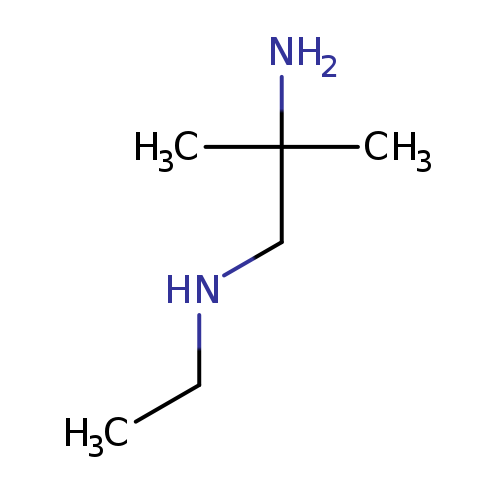
(2-amino-2-methylpropyl)(ethyl)amineCatalog No.:AA01FP8M CAS No.:107429-97-0 MDL No.:MFCD14609232 MF:C6H16N2 MW:116.2046 |
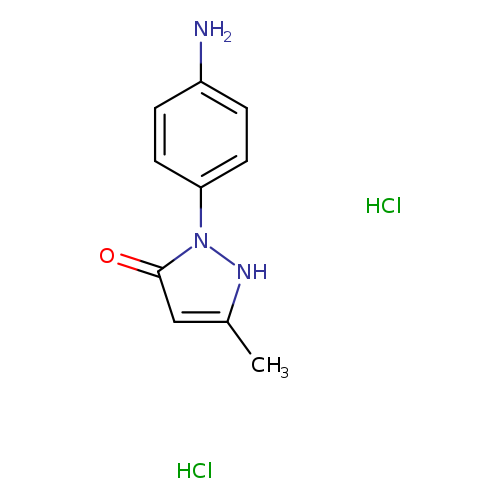
2-(4-aminophenyl)-5-methyl-2,4-dihydro-3H-pyrazol-3-one dihydrochlorideCatalog No.:AA00J3BD CAS No.:107430-44-4 MDL No.:MFCD13186342 MF:C10H13Cl2N3O MW:262.1357 |
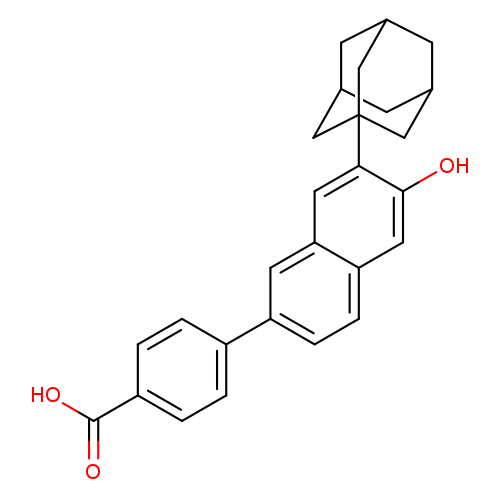
CD 1530Catalog No.:AA007EEJ CAS No.:107430-66-0 MDL No.:MFCD09263615 MF:C27H26O3 MW:398.4935 |
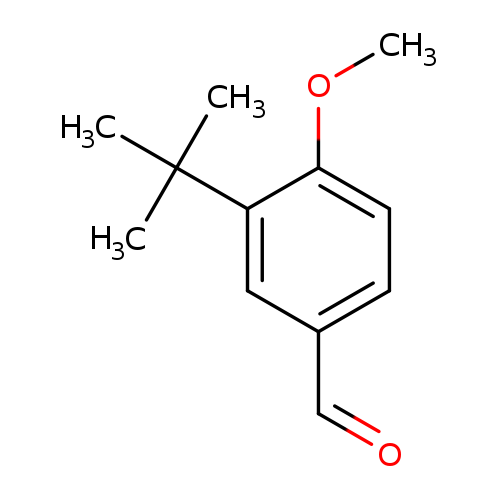
3-(tert-Butyl)-4-methoxybenzaldehydeCatalog No.:AA007W6I CAS No.:107430-92-2 MDL No.:MFCD01104342 MF:C12H16O2 MW:192.2542 |
1-[(9Z)-1-oxo-9-octadecen-1-yl]-L-prolineCatalog No.:AA01EPL2 CAS No.:107432-37-1 MDL No.: MF:C23H41NO3 MW:379.5765 |
Ginkgolide JCatalog No.:AA0084MF CAS No.:107438-79-9 MDL No.:MFCD03093737 MF:C20H24O10 MW:424.3986 |
GLUCAGON-LIKE PEPTIDE I FRAGMENT 7-36 AMIDE HUMANCatalog No.:AA008R2T CAS No.:107444-51-9 MDL No.:MFCD00133373 MF:C149H226N40O45 MW:3297.6297 |
Ethanone, 1-(4-pyridinyl)-, oxime, (1E)- (9CI)Catalog No.:AA00906V CAS No.:107445-21-6 MDL No.:MFCD00185708 MF:C7H8N2O MW:136.1512 |
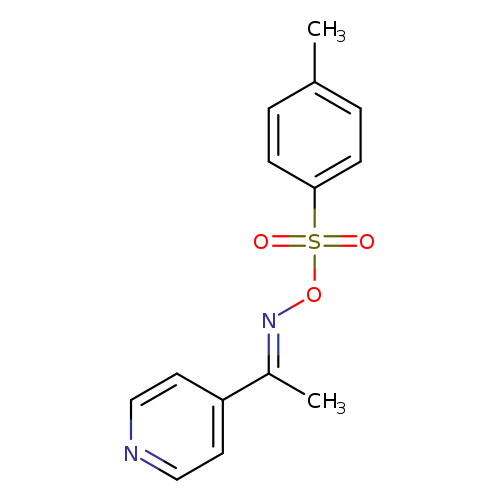
(E)-[1-(pyridin-4-yl)ethylidene]amino 4-methylbenzene-1-sulfonateCatalog No.:AA01EJJV CAS No.:107445-22-7 MDL No.:MFCD28990331 MF:C14H14N2O3S MW:290.3376 |
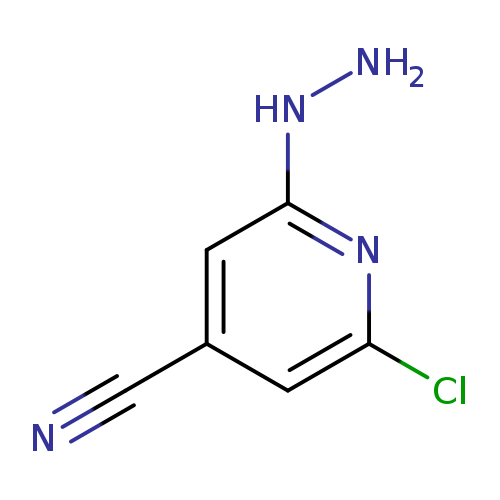
2-chloro-6-hydrazinylpyridine-4-carbonitrileCatalog No.:AA01A3QS CAS No.:107445-56-7 MDL No.:MFCD10000008 MF:C6H5ClN4 MW:168.5837 |
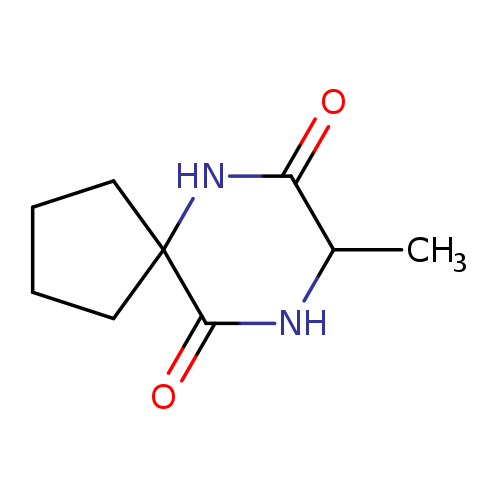
6,9-Diazaspiro[4.5]decane-7,10-dione,8-methyl-Catalog No.:AA007EEE CAS No.:107447-22-3 MDL No.:MFCD00792713 MF:C9H14N2O2 MW:182.2197 |
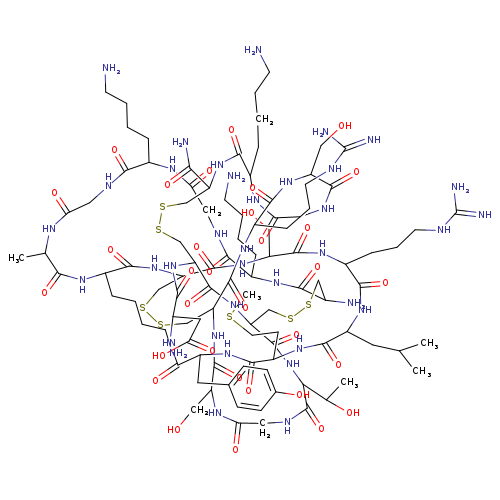
w-Conotoxin M VIIACatalog No.:AA01CCO4 CAS No.:107452-89-1 MDL No.:MFCD00145036 MF:C102H172N36O32S7 MW:2639.1341 |
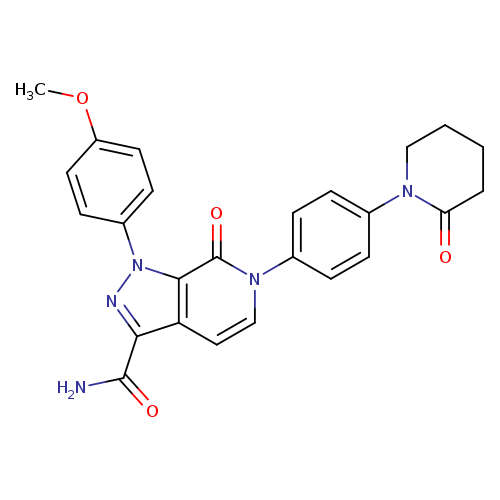
4,5-Dehydro ApixabanCatalog No.:AA01CBA4 CAS No.:1074549-89-5 MDL No.: MF:C25H23N5O4 MW:457.4812 |
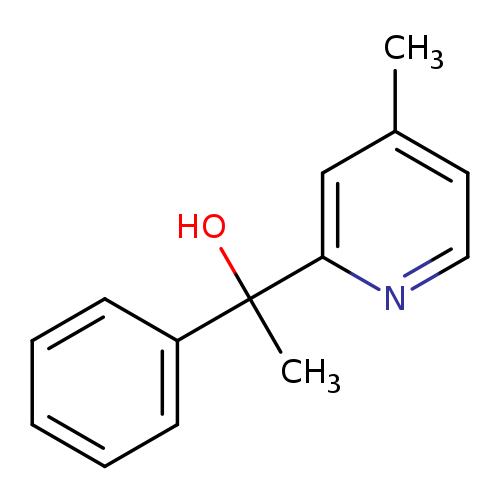
1-(4-Methylpyridin-2-yl)-1-phenylethan-1-olCatalog No.:AA01EQMH CAS No.:107455-67-4 MDL No.:MFCD02929412 MF:C14H15NO MW:213.2750 |
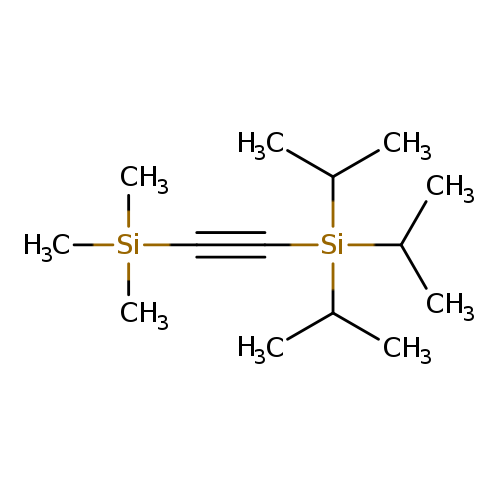
Triisopropyl[(trimethylsilyl)ethynyl]silaneCatalog No.:AA00943E CAS No.:107474-02-2 MDL No.:MFCD28975120 MF:C14H30Si2 MW:254.5590 |
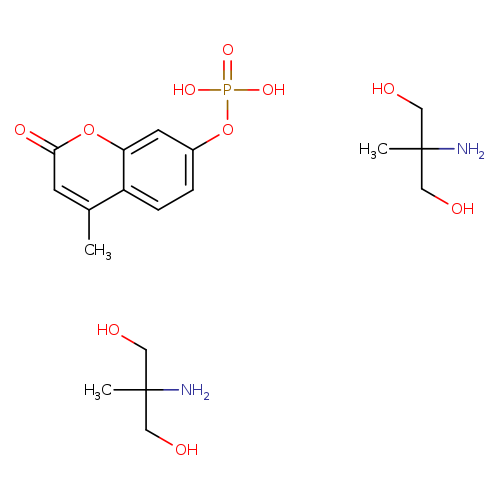
4-Methylumbelliferyl phosphate di-(2-amino-2-methyl-1,3-propanediol)saltCatalog No.:AA00HAXB CAS No.:107475-10-5 MDL No.:MFCD00044932 MF:C18H31N2O10P MW:466.4199 |
8-Chloro-2,3,4,5-tetrahydro-1h-benzo[e][1,4]diazepineCatalog No.:AA008S55 CAS No.:107479-55-0 MDL No.:MFCD07369929 MF:C9H11ClN2 MW:182.6500 |
1,9-BIS(ACRYLOYLOXY)NONANECatalog No.:AA003DPO CAS No.:107481-28-7 MDL No.:MFCD00080592 MF:C15H24O4 MW:268.3487 |
5-Chloro-8-iodo-1,6-naphthyridineCatalog No.:AA00HAXC CAS No.:107484-69-5 MDL No.:MFCD16250553 MF:C8H4ClIN2 MW:290.4882 |
(Z)-2-(TERT-BUTYLIMINO)-3-ISOPROPYL-5-PHENYL-1,3,5-THIADIAZINAN-4-ONECatalog No.:AA007EE1 CAS No.:107484-86-6 MDL No.:MFCD11870859 MF:C16H23N3OS MW:305.4383 |
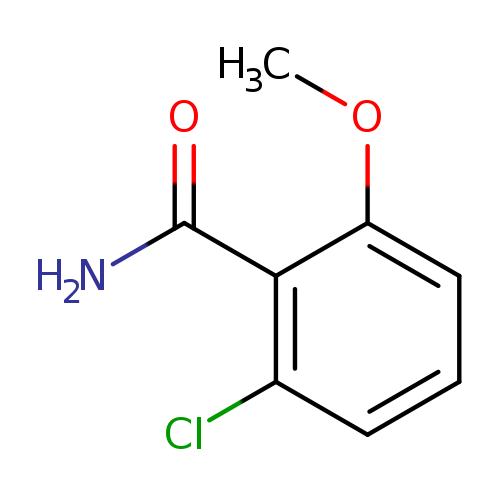
2-Chloro-6-methoxybenzamideCatalog No.:AA008UFE CAS No.:107485-43-8 MDL No.:MFCD11846167 MF:C8H8ClNO2 MW:185.6076 |
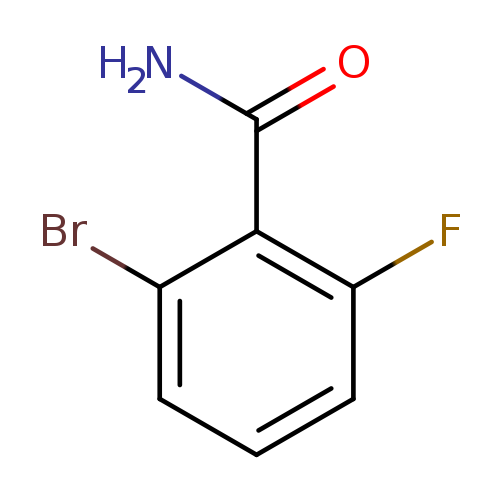
2-Bromo-6-fluorobenzamideCatalog No.:AA008TQO CAS No.:107485-63-2 MDL No.:MFCD07368694 MF:C7H5BrFNO MW:218.0231 |
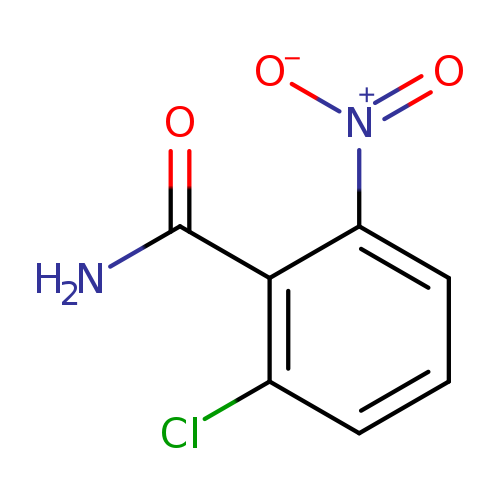
2-Chloro-6-nitrobenzamideCatalog No.:AA003GYA CAS No.:107485-64-3 MDL No.:MFCD02093931 MF:C7H5ClN2O3 MW:200.5792 |
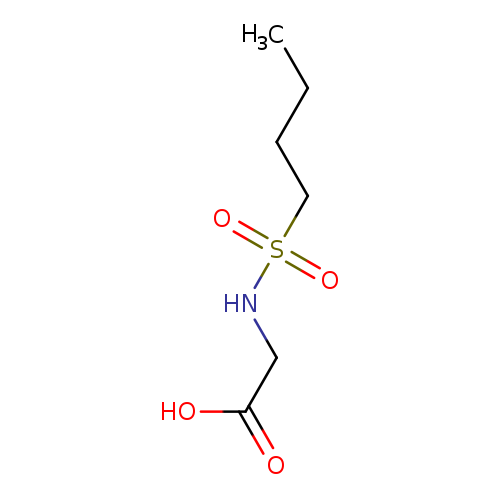
2-(butane-1-sulfonamido)acetic acidCatalog No.:AA01A2I0 CAS No.:107491-00-9 MDL No.:MFCD08443421 MF:C6H13NO4S MW:195.2367 |
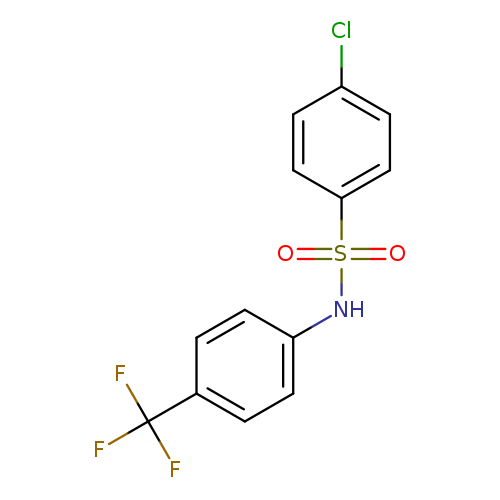
4-chloro-N-[4-(trifluoromethyl)phenyl]benzene-1-sulfonamideCatalog No.:AA00ITVL CAS No.:107491-59-8 MDL No.:MFCD01315649 MF:C13H9ClF3NO2S MW:335.7293 |
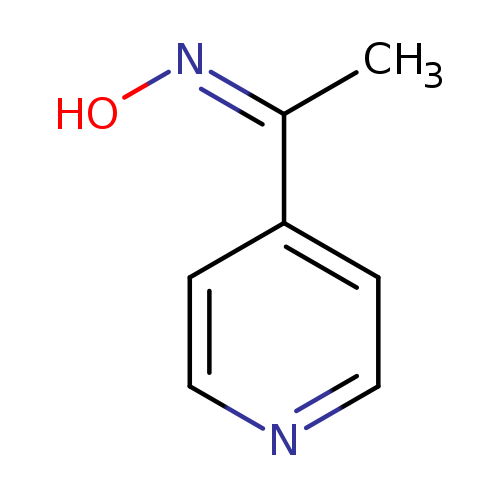
Ethanone, 1-(4-pyridinyl)-, oxime, (1Z)- (9CI)Catalog No.:AA009NOX CAS No.:107492-79-5 MDL No.:MFCD00180593 MF:C7H8N2O MW:136.1512 |
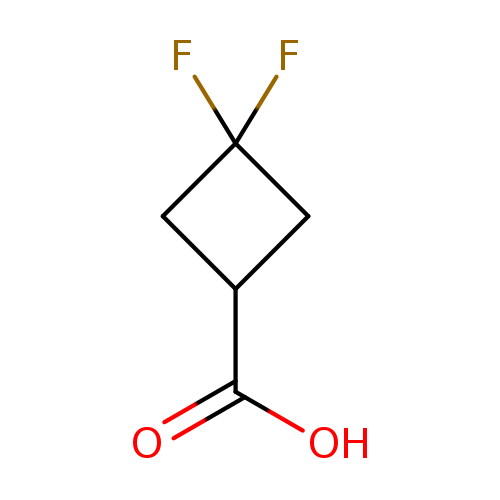
3,3-Difluorocyclobutanecarboxylic acidCatalog No.:AA008TN4 CAS No.:107496-54-8 MDL No.:MFCD08705857 MF:C5H6F2O2 MW:136.0967 |
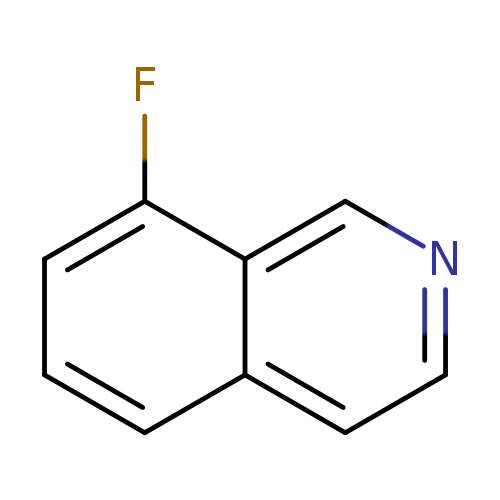
8-FluoroisoquinolineCatalog No.:AA008SR5 CAS No.:1075-00-9 MDL No.:MFCD09878369 MF:C9H6FN MW:147.1490 |
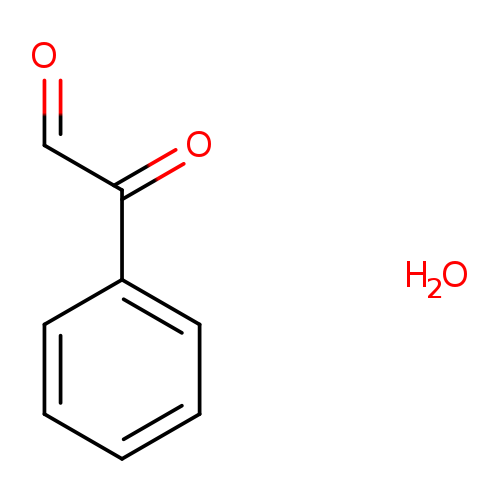
Phenylglyoxal monohydrateCatalog No.:AA0035EF CAS No.:1075-06-5 MDL No.:MFCD00006959 MF:C8H8O3 MW:152.1473 |
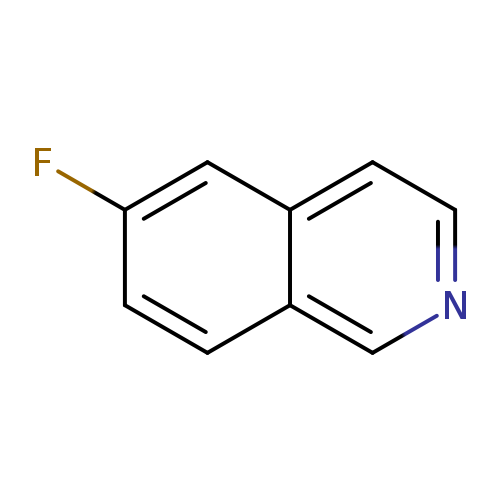
6-FluoroisoquinolineCatalog No.:AA008YYF CAS No.:1075-11-2 MDL No.:MFCD11226829 MF:C9H6FN MW:147.1490 |
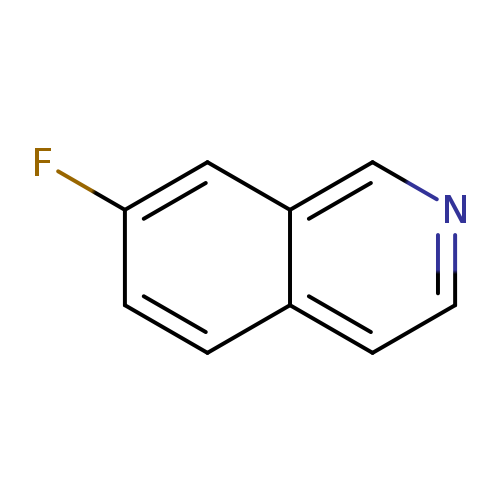
7-FluoroisoquinolineCatalog No.:AA00921T CAS No.:1075-12-3 MDL No.:MFCD11226905 MF:C9H6FN MW:147.1490 |
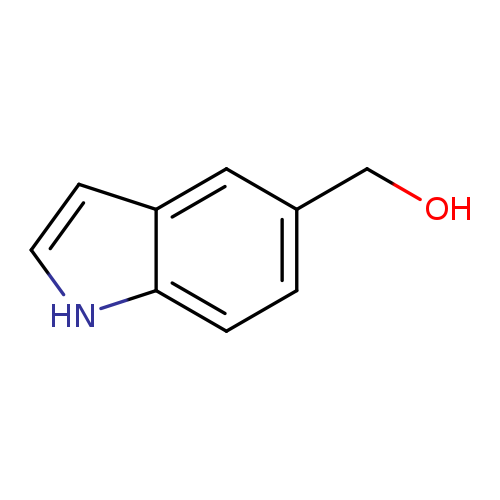
Indole-5-methanolCatalog No.:AA008R30 CAS No.:1075-25-8 MDL No.:MFCD02179594 MF:C9H9NO MW:147.1739 |
Indole-6-methanolCatalog No.:AA007EB4 CAS No.:1075-26-9 MDL No.:MFCD02179595 MF:C9H9NO MW:147.1739 |
Bicyclo[4.2.0]octa-1,3,5-trien-7-yl(methyl) ketoneCatalog No.:AA009L53 CAS No.:1075-30-5 MDL No.:MFCD01716591 MF:C10H10O MW:146.1858 |
5-Bromo-2-methylindoleCatalog No.:AA007W2P CAS No.:1075-34-9 MDL No.:MFCD01863677 MF:C9H8BrN MW:210.0705 |
5-Chloro-2-methylindoleCatalog No.:AA007W2O CAS No.:1075-35-0 MDL No.:MFCD00005619 MF:C9H8ClN MW:165.6195 |
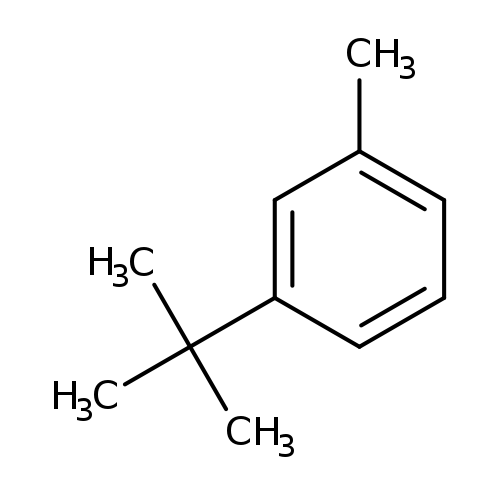
1-tert-Butyl-3-methylbenzeneCatalog No.:AA003JU0 CAS No.:1075-38-3 MDL No.:MFCD00059210 MF:C11H16 MW:148.2447 |
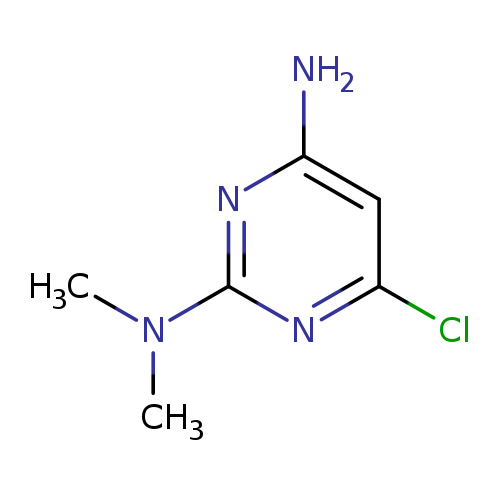
6-Chloro-N2,N2-dimethylpyrimidine-2,4-diamineCatalog No.:AA007W2M CAS No.:1075-39-4 MDL No.:MFCD09864563 MF:C6H9ClN4 MW:172.6155 |
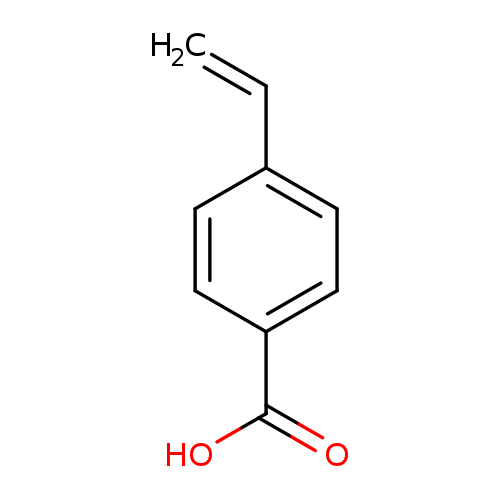
4-Vinylbenzoic acidCatalog No.:AA003M01 CAS No.:1075-49-6 MDL No.:MFCD00002569 MF:C9H8O2 MW:148.1586 |
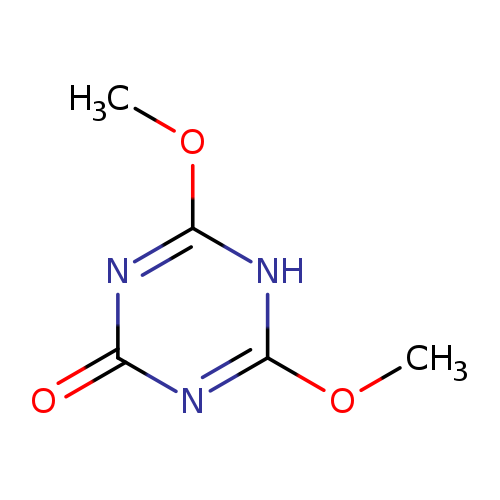
4,6-Dimethoxy-1,3,5-triazin-2(1H)-oneCatalog No.:AA0033SR CAS No.:1075-59-8 MDL No.:MFCD02932731 MF:C5H7N3O3 MW:157.1274 |
N-(6-aminopyridin-2-yl)acetamideCatalog No.:AA003G7Q CAS No.:1075-62-3 MDL No.:MFCD01099052 MF:C7H9N3O MW:151.1659 |
3-AnilinopropionitrileCatalog No.:AA0037XM CAS No.:1075-76-9 MDL No.:MFCD00001953 MF:C9H10N2 MW:146.1891 |
3,3-TetramethyleneglutarimideCatalog No.:AA00327I CAS No.:1075-89-4 MDL No.:MFCD00023871 MF:C9H13NO2 MW:167.2050 |
2,2'-(Cyclobutane-1,1-diyl)diacetic acidCatalog No.:AA008XTW CAS No.:1075-98-5 MDL No.:MFCD17012080 MF:C8H12O4 MW:172.1785 |
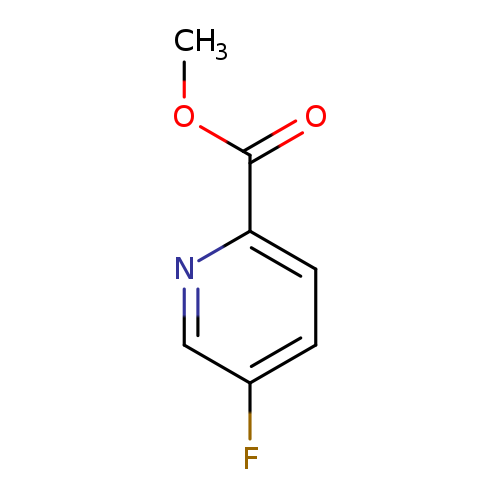
Methyl 5-fluoropicolinateCatalog No.:AA00389D CAS No.:107504-07-4 MDL No.:MFCD11845630 MF:C7H6FNO2 MW:155.1264 |
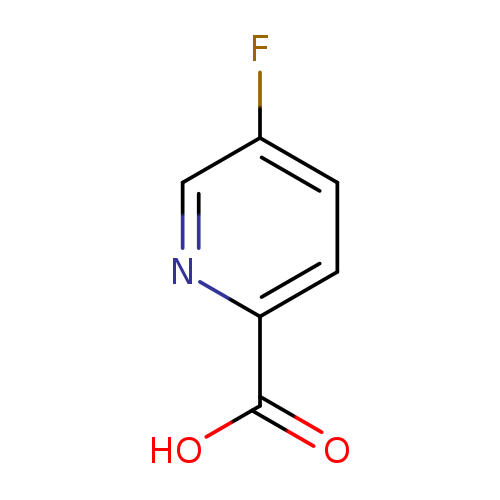
5-Fluoropicolinic acidCatalog No.:AA003472 CAS No.:107504-08-5 MDL No.:MFCD04114196 MF:C6H4FNO2 MW:141.0999 |
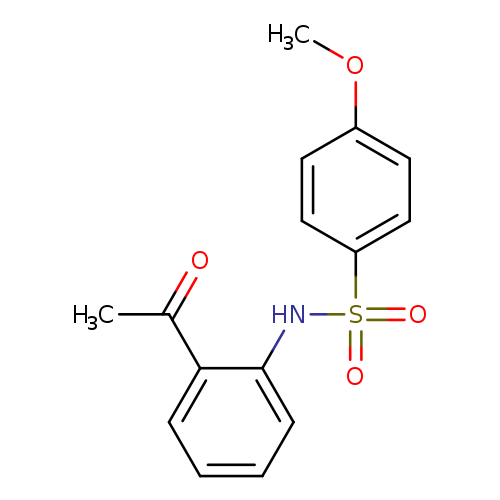
N-(2-acetylphenyl)-4-methoxybenzene-1-sulfonamideCatalog No.:AA00INFA CAS No.:107506-24-1 MDL No.:MFCD00215397 MF:C15H15NO4S MW:305.3489 |
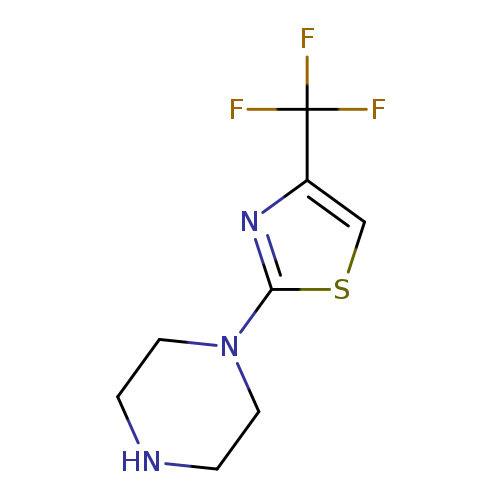
1-[4-(trifluoromethyl)-1,3-thiazol-2-yl]piperazineCatalog No.:AA007EDV CAS No.:107507-53-9 MDL No.:MFCD09910391 MF:C8H10F3N3S MW:237.2453 |