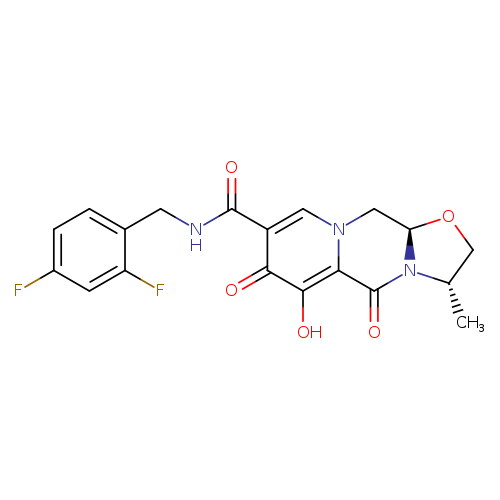
[1]CurrentPatentAssignee:SHIONOGI&CO.,LTD.;GLAXOSMITHKLINEPLC-WO2010/68262,2010,A1Locationinpatent:Page/Pagecolumn26-27
[2]CurrentPatentAssignee:SHIONOGI&CO.,LTD.;GLAXOSMITHKLINEPLC-US9133216,2015,B2Locationinpatent:Page/Pagecolumn17;18;21
[3]CurrentPatentAssignee:GLAXOSMITHKLINEPLC;SHIONOGI&CO.,LTD.-JP5848595,2016,B2Locationinpatent:Paragraph0045
[4]Johns,BrianA.;Kawasuji,Takashi;Weatherhead,JasonG.;Taishi,Teruhiko;Temelkoff,DavidP.;Yoshida,Hiroshi;Akiyama,Toshiyuki;Taoda,Yoshiyuki;Murai,Hitoshi;Kiyama,Ryuichi;Fuji,Masahiro;Tanimoto,Norihiko;Jeffrey,Jerry;Foster,ScottA.;Yoshinaga,Tomokazu;Seki,Takahiro;Kobayashi,Masanori;Sato,Akihiko;Johnson,MatthewN.;Garvey,EdwardP.;Fujiwara,Tamio[JournalofMedicinalChemistry,2013,vol.56,#14,p.5901-5916]
[1]CurrentPatentAssignee:LUPINLIMITED-US2021/9606,2021,A1Locationinpatent:Paragraph0102-0104
[2]Johns,BrianA.;Kawasuji,Takashi;Weatherhead,JasonG.;Taishi,Teruhiko;Temelkoff,DavidP.;Yoshida,Hiroshi;Akiyama,Toshiyuki;Taoda,Yoshiyuki;Murai,Hitoshi;Kiyama,Ryuichi;Fuji,Masahiro;Tanimoto,Norihiko;Jeffrey,Jerry;Foster,ScottA.;Yoshinaga,Tomokazu;Seki,Takahiro;Kobayashi,Masanori;Sato,Akihiko;Johnson,MatthewN.;Garvey,EdwardP.;Fujiwara,Tamio[JournalofMedicinalChemistry,2013,vol.56,#14,p.5901-5916]
[3]CurrentPatentAssignee:CIPLALTD-WO2019/159199,2019,A1Locationinpatent:Page/Pagecolumn40
[4]CurrentPatentAssignee:SHIONOGI&CO.,LTD.;GLAXOSMITHKLINEPLC-WO2010/68262,2010,A1Locationinpatent:Page/Pagecolumn27
[5]CurrentPatentAssignee:SHIONOGI&CO.,LTD.;GLAXOSMITHKLINEPLC-US9133216,2015,B2Locationinpatent:Page/Pagecolumn18;21;22
[6]CurrentPatentAssignee:GLAXOSMITHKLINEPLC;SHIONOGI&CO.,LTD.-JP5848595,2016,B2Locationinpatent:Paragraph0045
[7]CurrentPatentAssignee:KINGLAWRENCE;CIPLALTD-WO2015/177537,2015,A1Locationinpatent:Page/Pagecolumn40
[8]CurrentPatentAssignee:CIPLALTD-WO2018/109786,2018,A1Locationinpatent:Page/Pagecolumn42-44
[9]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2018/149608,2018,A1Locationinpatent:Page/Pagecolumn38-39;40
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2011/119566,2011,A1
[2]CurrentPatentAssignee:ZUNYIMEDICALUNIVERSITY-CN114605437,2022,A
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2011/119566,2011,A1
[2]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[3]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[4]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[5]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[6]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[7]Wang,Huan;Kowalski,MatthewD.;Lakdawala,AmiS.;Vogt,FrederickG.;Wu,Lianming[OrganicLetters,2015,vol.17,#3,p.564-567]
[8]CurrentPatentAssignee:ZUNYIMEDICALUNIVERSITY-CN114605437,2022,A

[1]Patent:WO2011/119566,2011,A1
[2]OrganicLetters,2015,vol.17,p.564-567
[3]OrganicLetters,2015,vol.17,p.564-567
[4]OrganicLetters,2015,vol.17,p.564-567
[5]OrganicLetters,2015,vol.17,p.564-567
[6]OrganicLetters,2015,vol.17,p.564-567
[7]OrganicLetters,2015,vol.17,p.564-567
[8]Patent:WO2019/159199,2019,A1
Title: Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1.
Journal: Antimicrobial agents and chemotherapy 20171201
Title: Impact of HIV-1 Integrase L74F and V75I Mutations in a Clinical Isolate on Resistance to Second-Generation Integrase Strand Transfer Inhibitors.
Journal: Antimicrobial agents and chemotherapy 20170801
Title: Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection.
Journal: Antimicrobial agents and chemotherapy 20150101
Title: Zhou T, et al. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials. 2018 Jan;151:53-65.
Title: Reese MJ, et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica. 2015 Sep 4:1-12.