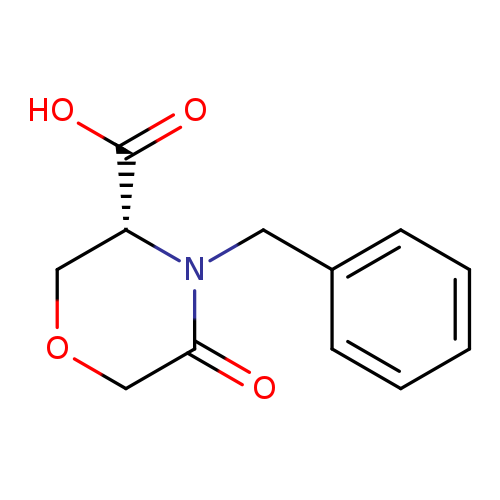
[1]Patent:WO2011/23733,2011,A1,.Locationinpatent:Page/Pagecolumn19-20
[2]Patent:WO2009/61879,2009,A1,.Locationinpatent:Page/Pagecolumn85
[3]Patent:WO2013/82388,2013,A1,.Locationinpatent:Page/Pagecolumn74-75
[4]Patent:US2008/300242,2008,A1,.Locationinpatent:Page/Pagecolumn93
[5]Patent:WO2008/47109,2008,A1,.Locationinpatent:Page/Pagecolumn40
[6]Patent:WO2009/71895,2009,A1,.Locationinpatent:Page/Pagecolumn109-110
[7]Patent:US2011/3785,2011,A1,.Locationinpatent:Page/Pagecolumn11
[8]Patent:WO2007/28654,2007,A1,.Locationinpatent:Page/Pagecolumn64
[9]JournaloftheChemicalSociety,PerkinTransactions1:OrganicandBio-OrganicChemistry(1972-1999),1985,p.2577-2580
[1]Patent:US2008/255114,2008,A1,.Locationinpatent:Page/Pagecolumn31
[1]Patent:WO2009/1089,2008,A1,.Locationinpatent:Page/Pagecolumn28-29
[1]JournaloftheChemicalSociety,PerkinTransactions1:OrganicandBio-OrganicChemistry(1972-1999),1985,p.2577-2580
[2]Patent:WO2013/82388,2013,A1,
[1]Patent:WO2008/47109,2008,A1,.Locationinpatent:Page/Pagecolumn40-41
[2]Patent:WO2009/1089,2008,A1,.Locationinpatent:Page/Pagecolumn29
[3]Patent:WO2009/71895,2009,A1,.Locationinpatent:Page/Pagecolumn110
[4]Patent:US2011/3785,2011,A1,.Locationinpatent:Page/Pagecolumn11
[5]Patent:US2008/255114,2008,A1,.Locationinpatent:Page/Pagecolumn31
[6]JournaloftheChemicalSociety,PerkinTransactions1:OrganicandBio-OrganicChemistry(1972-1999),1985,p.2577-2580
[1]Patent:WO2007/28654,2007,A1.Locationinpatent:Page/Pagecolumn64-65
[2]JournaloftheChemicalSociety.PerkintransactionsI,1985,p.2577-2580
[1]JournaloftheChemicalSociety.PerkintransactionsI,1989,p.1401-1403
[1]Patent:WO2008/47109,2008,A1.Locationinpatent:Page/Pagecolumn40-41
[2]Patent:WO2009/1089,2008,A1.Locationinpatent:Page/Pagecolumn29
[3]Patent:WO2009/71895,2009,A1.Locationinpatent:Page/Pagecolumn110
[4]Patent:US2011/3785,2011,A1.Locationinpatent:Page/Pagecolumn11
[5]JournalofMedicinalChemistry,2019,vol.62,p.8609-8630
[6]Patent:US2008/255114,2008,A1.Locationinpatent:Page/Pagecolumn31
[7]JournaloftheChemicalSociety.PerkintransactionsI,1985,p.2577-2580
[1]Patent:WO2011/23733,2011,A1.Locationinpatent:Page/Pagecolumn19-20
[2]Patent:WO2009/61879,2009,A1.Locationinpatent:Page/Pagecolumn85
[3]Patent:WO2013/82388,2013,A1.Locationinpatent:Page/Pagecolumn74-75
[4]JournalofMedicinalChemistry,2019,vol.62,p.8609-8630
[5]Patent:US2008/300242,2008,A1.Locationinpatent:Page/Pagecolumn93
[6]Patent:WO2008/47109,2008,A1.Locationinpatent:Page/Pagecolumn40
[7]Patent:WO2009/71895,2009,A1.Locationinpatent:Page/Pagecolumn109-110
[8]Patent:US2011/3785,2011,A1.Locationinpatent:Page/Pagecolumn11
[9]Patent:WO2007/28654,2007,A1.Locationinpatent:Page/Pagecolumn64
[10]JournaloftheChemicalSociety.PerkintransactionsI,1985,p.2577-2580
[1]JournaloftheChemicalSociety.PerkintransactionsI,1985,p.2577-2580