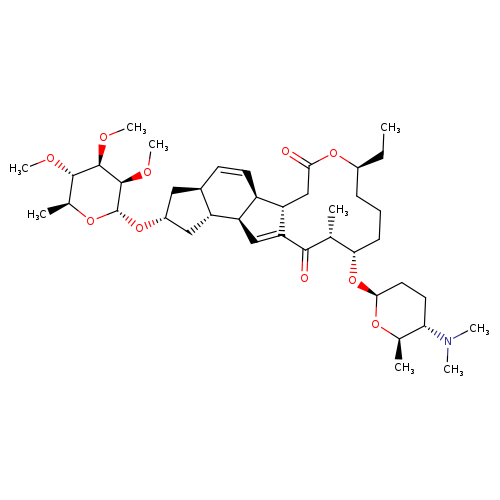
Title: Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A.
Journal: Nature 20110505
Title: A spinosyn-sensitive Drosophila melanogaster nicotinic acetylcholine receptor identified through chemically induced target site resistance, resistance gene identification, and heterologous expression.
Journal: Insect biochemistry and molecular biology 20100501
Title: Biosynthesis of spinosyn in Saccharopolyspora spinosa: synthesis of permethylated rhamnose and characterization of the functions of SpnH, SpnI, and SpnK.
Journal: Journal of the American Chemical Society 20100310
Title: Glycosylation engineering of spinosyn analogues containing an L-olivose moiety.
Journal: Organic & biomolecular chemistry 20090421
Title: SpnH from Saccharopolyspora spinosa encodes a rhamnosyl 4'-O-methyltransferase for biosynthesis of the insecticidal macrolide, spinosyn A.
Journal: Journal of industrial microbiology & biotechnology 20081201
Title: Total synthesis of (-)-spinosyn A: examination of structural features that govern the stereoselectivity of the key transannular Diels-Alder reaction.
Journal: The Journal of organic chemistry 20080307
Title: A new family of ATP-dependent oligomerization-macrocyclization biocatalysts.
Journal: Nature chemical biology 20071001
Title: Discovery, synthesis, and insecticidal activity of cycloaspeptide E.
Journal: Journal of natural products 20061001
Title: Spinosyn g: proof of structure by semisynthesis.
Journal: The Journal of organic chemistry 20050318
Title: Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons.
Journal: Journal of insect physiology 20041001
Title: The stereochemical outcome of electrophilic addition reactions on the 5,6-double bond in the spinosyns.
Journal: The Journal of organic chemistry 20011214
Title: Hak Joong Kim, et al. Biosynthesis of spinosyn in Saccharopolyspora spinosa: synthesis of permethylated rhamnose and characterization of the functions of SpnH, SpnI, and SpnK. J Am Chem Soc. 2010 Mar 10;132(9):2901-3.
Title: D T Vo, et al. Insect nicotinic acetylcholine receptor agonists as flea adulticides in small animals. J Vet Pharmacol Ther. 2010 Aug;33(4):315-22.