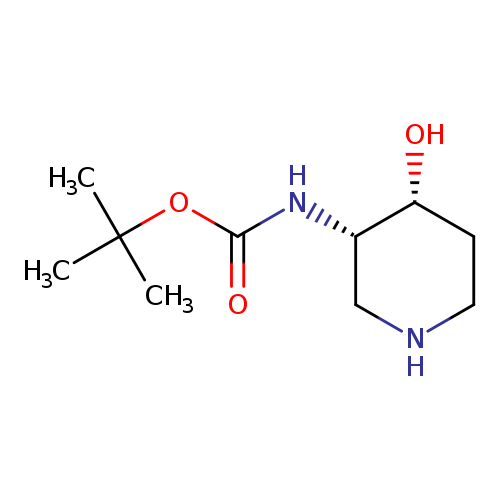
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2014/15905,2014,A1
[2]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-US2015/175600,2015,A1
[3]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2016/185279,2016,A1
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2014/15905,2014,A1
[2]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-US2015/175600,2015,A1
[3]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2016/185279,2016,A1
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2014/15905,2014,A1
[2]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2016/185279,2016,A1
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2014/15905,2014,A1
[2]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2016/185279,2016,A1
[1]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2014/15905,2014,A1Locationinpatent:Page/Pagecolumn61;62
[2]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-US2015/175600,2015,A1Locationinpatent:Paragraph0469;0470;0471
[3]CurrentPatentAssignee:GLAXOSMITHKLINEPLC-WO2016/185279,2016,A1Locationinpatent:Page/Pagecolumn103
Title: Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation.
Journal: Nature chemical biology 20150301