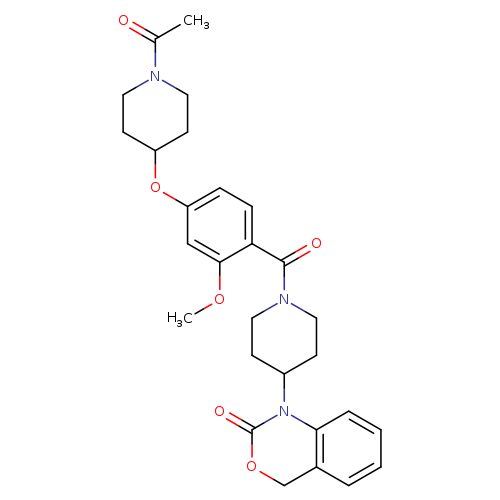
Title: Oral oxytocin antagonists.
Journal: Journal of medicinal chemistry 20100923
Title: Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist.
Journal: British journal of pharmacology 20051101
Title: A Gly/Ala switch contributes to high affinity binding of benzoxazinone-based non-peptide oxytocin receptor antagonists.
Journal: FEBS letters 20050117
Title: Identification of potent and selective oxytocin antagonists. Part 1: indole and benzofuran derivatives.
Journal: Bioorganic & medicinal chemistry letters 20020520
Title: Identification of potent and selective oxytocin antagonists. Part 2: further investigation of benzofuran derivatives.
Journal: Bioorganic & medicinal chemistry letters 20020520
Title: Spontaneous contractions of myometrium from humans, non-human primate and rodents are sensitive to selective oxytocin receptor antagonism in vitro.
Journal: BJOG : an international journal of obstetrics and gynaecology 20010901
Title: Development of orally active oxytocin antagonists: studies on 1-(1-[4-[1-(2-methyl-1-oxidopyridin-3-ylmethyl)piperidin-4-yloxy]-2- methoxybenzoyl]piperidin-4-yl)-1,4-dihydrobenz[d][1,3]oxazin-2-one (L-372,662) and related pyridines.
Journal: Journal of medicinal chemistry 19980604
Title: Williams PD, et al. 1-(1--[(N-acetyl-4-piperidinyl)oxy-2-methoxybenzoylpiperidin-4- yl)-4H-3,1-benzoxazin-2(1H)-one (L-371,257): a new, orally bioavailable, non-peptide oxytocin antagonist. J Med Chem. 1995 Nov 10;38(23):4634-6.
Title: Tunstall BJ, et al. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 2019 Apr 16;17(4):e2006421.
Title: Jacqueline M Ho, et al. Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology. 2014 Aug;155(8):2845-57.