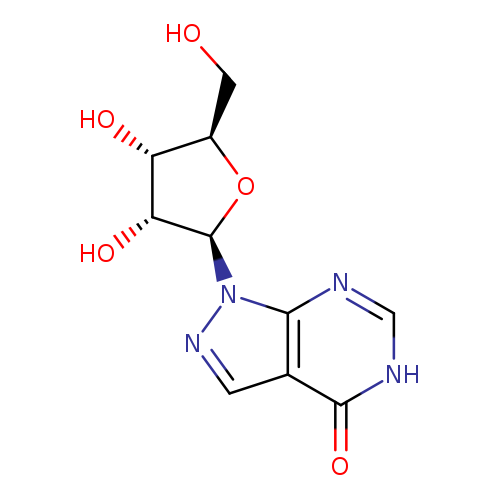
[1]JournalofMedicinalChemistry,1982,vol.25,p.1040-1044
Title: New drug developments for opportunistic infections in immunosuppressed patients: Pneumocystis carinii.
Journal: Journal of medicinal chemistry 19951124
Title: Activity of inosine analogs against Pneumocystis carinii in culture.
Journal: Antimicrobial agents and chemotherapy 19860701
Title: Synthesis and antiviral activity of certain carbamoylpyrrolopyrimidine and pyrazolopyrimidine nucleosides.
Journal: Journal of medicinal chemistry 19821101
Title: Nishida Y, et al. Inhibition of purine nucleoside phosphorylase activity and of T-cell function with allopurinol-riboside. Agents Actions. 1979 Dec;9(5-6):549-52.
Title: Pacher P, et al. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006 Mar;58(1):87-114.
Title: Shapiro TA, et al. Pharmacokinetics and metabolism of allopurinol riboside. Clin Pharmacol Ther. 1991 May;49(5):506-14.
Title: Were JB, et al. Effects of probenecid on the pharmacokinetics of allopurinol riboside. Antimicrob Agents Chemother. 1993 May;37(5):1193-6.