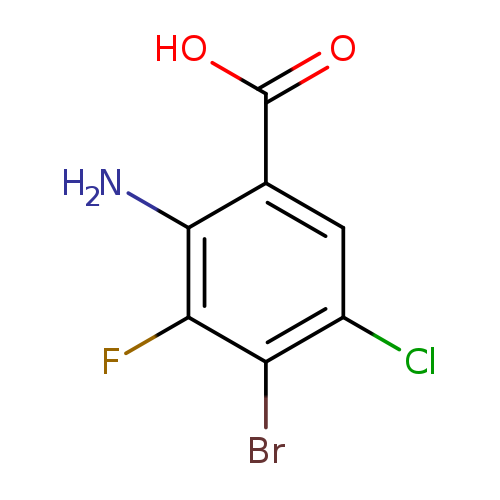
[1]CurrentPatentAssignee:ARAXESPHARMALLC-WO2018/218070,2018,A2Locationinpatent:Page/Pagecolumn173
[2]CurrentPatentAssignee:ARAXESPHARMALLC-WO2020/113071,2020,A1Locationinpatent:Page/Pagecolumn122
[3]CurrentPatentAssignee:ARVINAS;YALEUNIVERSITY-WO2019/195609,2019,A2Locationinpatent:Paragraph00492-00493
[4]CurrentPatentAssignee:CHENGDUBAIYUPHARMACEUTICALCO.,LTD.-WO2022/152313,2022,A1Locationinpatent:Page/Pagecolumn10-11
[5]CurrentPatentAssignee:ROCHEHOLDINGAG-WO2020/97537,2020,A2Locationinpatent:Paragraph0458
[6]CurrentPatentAssignee:YAOYATECHSHANGHAICOLTD-CN115181106,2022,ALocationinpatent:Paragraph0132-0137
[7]CurrentPatentAssignee:BIOTHERYXINC-WO2021/51034,2021,A1Locationinpatent:Paragraph0310
[8]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/164675,2016,A1Locationinpatent:Page/Pagecolumn231-233
[9]CurrentPatentAssignee:ARAXESPHARMALLC-WO2017/172979,2017,A1Locationinpatent:Paragraph0286
[10]CurrentPatentAssignee:ARAXESPHARMALLC-WO2015/54572,2015,A1Locationinpatent:Page/Pagecolumn288
[11]CurrentPatentAssignee:INVENTISBIOCOLTD-WO2022/2102,2022,A1Locationinpatent:Paragraph175;177
[12]CurrentPatentAssignee:SHANGHAIPHARMACEUTICALSHOLDINGCOMPANYLIMITED-WO2022/68921,2022,A1Locationinpatent:Page/Pagecolumn195-196
[1]CurrentPatentAssignee:SHANGHAIPHARMACEUTICALSHOLDINGCOMPANYLIMITED-WO2022/68921,2022,A1Locationinpatent:Page/Pagecolumn694;696
[2]CurrentPatentAssignee:ARAXESPHARMALLC-WO2015/54572,2015,A1Locationinpatent:Page/Pagecolumn289;290
[3]CurrentPatentAssignee:TAILIBIOTECHNOLOGYSHANGHAI;TYLIGANDBIOSCIENCESHANGHAI-WO2022/95960,2022,A1Locationinpatent:Page/Pagecolumn41
[4]CurrentPatentAssignee:SHANGHAIJIYUPHARMACEUTICALTECH;JIANGXIJEMINCAREGROUPCOLTD-CN113321654,2021,ALocationinpatent:Paragraph0135;0149-0153
[5]CurrentPatentAssignee:INCYTECORP-WO2021/211864,2021,A1Locationinpatent:Page/Pagecolumn147-148
[6]CurrentPatentAssignee:INCYTECORP-WO2021/211864,2021,A1Locationinpatent:Page/Pagecolumn147-148
[1]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/164675,2016,A1Locationinpatent:Page/Pagecolumn272
[2]CurrentPatentAssignee:ARVINAS;YALEUNIVERSITY-WO2019/195609,2019,A2Locationinpatent:Paragraph00552-00553
[3]CurrentPatentAssignee:REVOLUTIONMEDICINESINC-WO2021/108683,2021,A1Locationinpatent:Page/Pagecolumn179
[4]CurrentPatentAssignee:ARAXESPHARMALLC-WO2015/54572,2015,A1Locationinpatent:Page/Pagecolumn254
[5]CurrentPatentAssignee:BIOTHERYXINC-WO2021/51034,2021,A1Locationinpatent:Paragraph0196-0197
[6]CurrentPatentAssignee:LEIDOSHOLDINGS,INC.;LAWRENCELIVERMORENATIONALSECURITY,LLC;THERASINC-WO2022/177917,2022,A2Locationinpatent:Paragraph1160-1161
[1]CurrentPatentAssignee:ARAXESPHARMALLC-WO2015/54572,2015,A1
[2]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/49568,2016,A1
[3]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/164675,2016,A1
[4]CurrentPatentAssignee:ARAXESPHARMALLC-WO2017/87528,2017,A1
[5]CurrentPatentAssignee:ARAXESPHARMALLC-WO2017/58915,2017,A1
[6]CurrentPatentAssignee:ARAXESPHARMALLC-US2018/15087,2018,A1
[7]CurrentPatentAssignee:BETTAPHARMACEUTICALSCOLTD-EP3967695,2022,A1
[8]CurrentPatentAssignee:BETTAPHARMACEUTICALSCOLTD-WO2022/83616,2022,A1
[1]CurrentPatentAssignee:ARAXESPHARMALLC-WO2015/54572,2015,A1
[2]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/49568,2016,A1
[3]CurrentPatentAssignee:ARAXESPHARMALLC-WO2016/164675,2016,A1
[4]CurrentPatentAssignee:ARAXESPHARMALLC-WO2017/87528,2017,A1
[5]CurrentPatentAssignee:ARAXESPHARMALLC-WO2017/58915,2017,A1
[6]CurrentPatentAssignee:ARAXESPHARMALLC-US2018/15087,2018,A1
[7]CurrentPatentAssignee:SHANGHAIJIYUPHARMACEUTICALTECH;JIANGXIJEMINCAREGROUPCOLTD-CN113321654,2021,A
[8]CurrentPatentAssignee:BETTAPHARMACEUTICALSCOLTD-EP3967695,2022,A1
[9]CurrentPatentAssignee:BETTAPHARMACEUTICALSCOLTD-WO2022/83616,2022,A1
[10]CurrentPatentAssignee:WUXIAPPTECCO.,LTD.-WO2021/259331,2021,A1