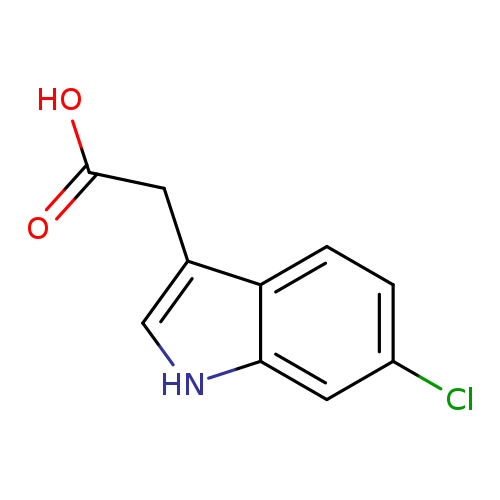
[1]Patent:WO2007/29629,2007,A1,.Locationinpatent:Page/Pagecolumn65
[2]AngewandteChemie-InternationalEdition,2011,vol.50,#35,p.8105-8109
[1]JournaloftheAmericanChemicalSociety,1951,vol.73,p.2756,2758
[1]BioorganicandMedicinalChemistryLetters,2002,vol.12,#18,p.2523-2526
[2]AngewandteChemie-InternationalEdition,2011,vol.50,#35,p.8105-8109
[1]JournaloftheAmericanChemicalSociety,1951,vol.73,p.2756,2758
[1]BioorganicandMedicinalChemistryLetters,2002,vol.12,p.2523-2526
[2]Patent:EP1296676,2004,B1
[3]Patent:US6890948,2005,B1
[1]ChemicalResearchinToxicology,2002,vol.15,p.877-882
[2]ChemicalResearchinToxicology,2002,vol.15,p.877-882
Title: Binding of ring-substituted indole-3-acetic acids to human serum albumin.
Journal: Bioorganic & medicinal chemistry 20070701
Title: Halogenated indole-3-acetic acids as oxidatively activated prodrugs with potential for targeted cancer therapy.
Journal: Bioorganic & medicinal chemistry letters 20020916