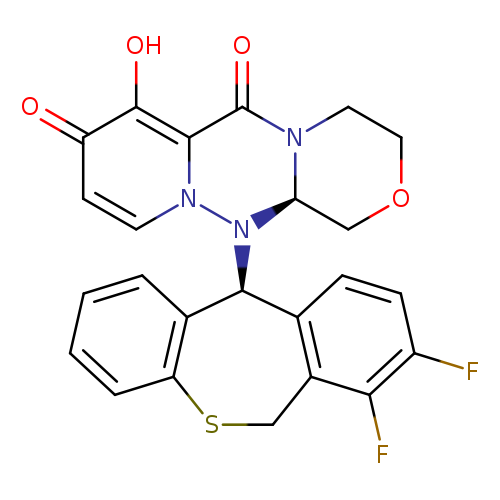
[1]Patent:JP5971830,2016,B1.Locationinpatent:Paragraph0080
[2]Patent:WO2020/58745,2020,A1.Locationinpatent:Page/Pagecolumn18
[3]Patent:JP6212678,2017,B1.Locationinpatent:Paragraph0058
[4]Patent:CN111233891,2020,A.Locationinpatent:Paragraph0076-0079
[5]Patent:JP6267397,2018,B1.Locationinpatent:Paragraph0089
[1]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP5971830,2016,B1Locationinpatent:Paragraph0080
[2]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP6267397,2018,B1Locationinpatent:Page/Pagecolumn0091
[3]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-WO2020/58745,2020,A1Locationinpatent:Page/Pagecolumn18;19
[4]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-US2020/261481,2020,A1Locationinpatent:Paragraph0281-0284
[1]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP5971830,2016,B1Locationinpatent:Paragraph0081
[2]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP6267397,2018,B1Locationinpatent:Paragraph0092
[3]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-US2020/261481,2020,A1Locationinpatent:Paragraph0285;0287-0288
[1]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP5971830,2016,B1Locationinpatent:Paragraph0081
[2]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP6267397,2018,B1Locationinpatent:Paragraph0094
[3]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-US2020/261481,2020,A1Locationinpatent:Paragraph0292-0293
[1]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP5971830,2016,B1Locationinpatent:Paragraph0082
[2]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-JP6267397,2018,B1Locationinpatent:Paragraph0096
[3]CurrentPatentAssignee:SHIONOGI&CO.,LTD.-US2020/261481,2020,A1Locationinpatent:Paragraph0297-0299
Title: Omoto S, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018 Jun 25;8(1):9633.
Title: Noshi T, et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res. 2018 Dec;160:109-117.