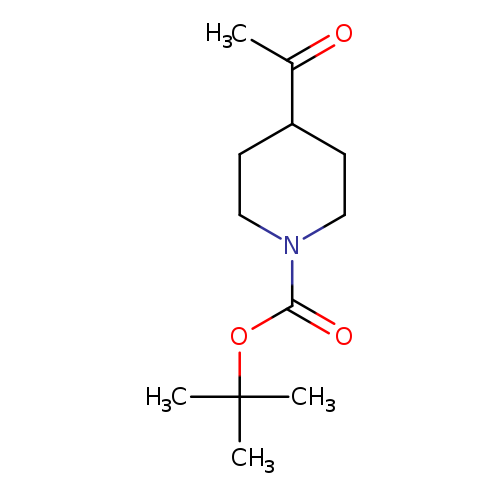
[1]Patent:WO2013/96744,2013,A1,.Locationinpatent:Page/Pagecolumn192
[2]Patent:EP3255042,2017,A2,.Locationinpatent:Paragraph0209;0213
[1]BioorganicandMedicinalChemistryLetters,2004,vol.14,#4,p.935-939
[2]BioorganicandMedicinalChemistryLetters,2011,vol.21,#5,p.1299-1305
[3]Patent:EP3255042,2017,A2,.Locationinpatent:Paragraph0209;0212
[4]Patent:WO2008/17461,2008,A1,.Locationinpatent:Page/Pagecolumn62
[5]Patent:WO2008/42925,2008,A1,.Locationinpatent:Page/Pagecolumn96
[6]Patent:WO2008/42925,2008,A1,.Locationinpatent:Page/Pagecolumn96
[7]Patent:WO2009/150144,2009,A1,.Locationinpatent:Page/Pagecolumn139
[8]Patent:WO2013/96744,2013,A1,.Locationinpatent:Page/Pagecolumn191
[9]Patent:WO2016/40498,2016,A1,.Locationinpatent:Paragraph0269
[10]Patent:US2004/2504,2004,A1,.Locationinpatent:Page/Pagecolumn23;38
[11]AngewandteChemie-InternationalEdition,2017,vol.56,#23,p.6646-6650
[12]Angew.Chem.,2017,vol.56,#23,p.6646-6650,5
[13]Patent:WO2018/33135,2018,A1,.Locationinpatent:Paragraph0102-0103
[14]Patent:TW2018/11794,2018,A,.Locationinpatent:Paragraph0055;0091;0095
[15]Patent:WO2018/137681,2018,A1,.Locationinpatent:Paragraph00137;00138
[1]Patent:KR2017/45749,2017,A,.Locationinpatent:Paragraph0614;0620-0622
[2]Patent:WO2004/41777,2004,A2,.Locationinpatent:Page54-55
[3]Patent:US6140333,2000,A,
[1]Tetrahedron,2015,vol.71,#42,p.8208-8212
[1]Patent:WO2004/99178,2004,A1,.Locationinpatent:Page39
[1]BioorganicandMedicinalChemistryLetters,2004,vol.14,p.935-939
[2]BioorganicandMedicinalChemistryLetters,2011,vol.21,p.1299-1305
[3]Patent:EP3255042,2017,A2.Locationinpatent:Paragraph0209;0212
[4]Patent:WO2008/17461,2008,A1.Locationinpatent:Page/Pagecolumn62
[5]Patent:WO2008/42925,2008,A1.Locationinpatent:Page/Pagecolumn96
[6]Patent:WO2009/150144,2009,A1.Locationinpatent:Page/Pagecolumn139
[7]Patent:WO2013/96744,2013,A1.Locationinpatent:Page/Pagecolumn191
[8]Patent:WO2016/40498,2016,A1.Locationinpatent:Paragraph0269
[9]Patent:US2004/2504,2004,A1.Locationinpatent:Page/Pagecolumn23;38
[10]AngewandteChemie-InternationalEdition,2017,vol.56,p.6646-6650 Angew.Chem.,2017,vol.56,p.6646-6650,5
[11]Patent:WO2018/33135,2018,A1.Locationinpatent:Paragraph0102-0103
[12]Patent:TW2018/11794,2018,A.Locationinpatent:Paragraph0055;0091;0095
[13]Patent:WO2018/137681,2018,A1.Locationinpatent:Paragraph00137;00138
[14]Patent:WO2019/108795,2019,A1.Locationinpatent:Paragraph0109
[1]JournalofMedicinalChemistry,2004,vol.47,p.3111-3130
[1]Patent:WO2009/150144,2009,A1.Locationinpatent:Page/Pagecolumn139
[2]TetrahedronLetters,2012,vol.53,p.852-853
[3]BioorganicandMedicinalChemistryLetters,2004,vol.14,p.3419-3424
[4]Patent:WO2004/41279,2004,A1.Locationinpatent:Page/Pagecolumn70
[5]Patent:WO2015/88565,2015,A1.Locationinpatent:Paragraph00214
[6]Patent:WO2008/42925,2008,A1
[1]ChemicalandPharmaceuticalBulletin,2001,vol.49,p.822-829
[1]CurrentPatentAssignee:MERCK&COINC-WO2005/100344,2005,A1Locationinpatent:Page/Pagecolumn40-41