Title: Dual Role of Epidermal Growth Factor Receptor in Liver Injury and Regeneration after Acetaminophen Overdose in Mice.
Journal: Toxicological sciences : an official journal of the Society of Toxicology 20170201
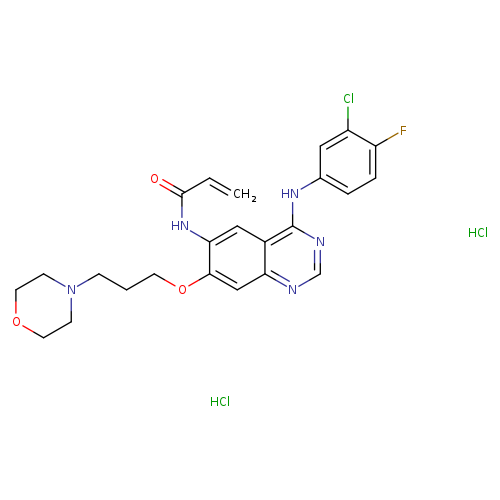
Title: Tyrosine Kinase Inhibitors. 20. Optimization of Substituted Quinazoline and Pyrido[3,4-d]pyrimidine Derivatives as Orally Active, Irreversible Inhibitors of the Epidermal Growth Factor Receptor Family.
Journal: Journal of medicinal chemistry 20160908
Title: Discovery, synthesis, and investigation of the antitumor activity of novel piperazinylpyrimidine derivatives.
Journal: European journal of medicinal chemistry 20110601
Title: Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors.
Journal: Journal of medicinal chemistry 20110310
Title: Suppression of proliferation of two independent NF1 malignant peripheral nerve sheath tumor cell lines by the pan-ErbB inhibitor CI-1033.
Journal: Cancer biology & therapy 20081201
Title: Tyrosine kinase inhibitors. 19. 6-Alkynamides of 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as irreversible inhibitors of the erbB family of tyrosine kinase receptors.
Journal: Journal of medicinal chemistry 20060223
Title: Plasma vascular endothelial growth factor and interleukin-8 as biomarkers of antitumor efficacy of a prototypical erbB family tyrosine kinase inhibitor.
Journal: Molecular cancer therapeutics 20050601
Title: A phase II study of CI-980 in previously untreated extensive small cell lung cancer: an Ohio State University phase II research consortium study.
Journal: Cancer investigation 20020101
Title: Tyrosine kinase inhibitors. 18. 6-Substituted 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as soluble, irreversible inhibitors of the epidermal growth factor receptor.
Journal: Journal of medicinal chemistry 20010201
Title: Smaill JB, et al. Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido,2-dpyrimidine-6-acrylamides bearing additional solubilizing functions. J Med Chem. 2000 Apr 6;43(7):1380-97.
Title: Djerf Severinsson EA, et al. The pan-ErbB receptor tyrosine kinase inhibitor canertinib promotes apoptosis of malignant melanoma in vitro and displays anti-tumor activity in vivo. Biochem Biophys Res Commun. 2011 Oct 28;414(3):563-8.
Title: McAndrews KM, et, al. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019 Mar 30;18(1):52.
Title: Smee DF, et, al. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother. 2008;19(3):115-24.