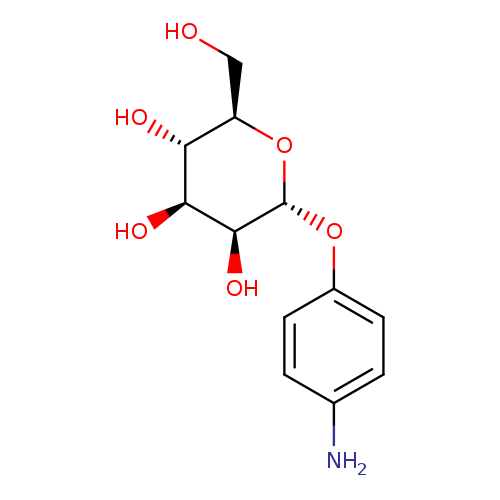
[1]Uzawa,Hirotaka;Ito,Hiroki;Izumi,Masayuki;Tokuhisa,Hideo;Taguchi,Kazuhiro;Minoura,Norihiko[Tetrahedron,2005,vol.61,#24,p.5895-5905]
[2]Nagase;Shinkai;Hamachi[ChemicalCommunications,2001,#3,p.229-230]
[3]CurrentPatentAssignee:HARBINMEDICALUNIVERSITY-CN111249234,2020,ALocationinpatent:Paragraph0072;0077
[4]CurrentPatentAssignee:HARBINMEDICALUNIVERSITY-CN111249235,2020,ALocationinpatent:Paragraph0085;0090
[5]Beiroth,Femke;Koudelka,Tomas;Overath,Thorsten;Knight,StefanD.;Tholey,Andreas;Lindhorst,ThisbeK.[BeilsteinJournalofOrganicChemistry,2018,vol.14,p.1890-1900]
[6]Amaike;Kobayashi;Shinkai[BulletinoftheChemicalSocietyofJapan,2000,vol.73,#11,p.2553-2558]
[7]CurrentPatentAssignee:BAYERAG-US6271342,2001,B1
[8]Downs,FrederickJ.;Carroll,RobertW.[CarbohydrateResearch,1981,vol.88,p.323-325]
[9]Westphal;Feier[ChemischeBerichte,1956,vol.89,p.582,587]
[10]Kieburg,Christoffer;Lindhorst,ThisbeK.[TetrahedronLetters,1997,vol.38,#22,p.3885-3888]
[11]Hartmann,Mirja;Horst,AndreaK.;Klemm,Per;Lindhorst,ThisbeK.[ChemicalCommunications,2010,vol.46,#2,p.330-332]
[12]Locationinpatent:experimentalpartGrabosch,Carsten;Hartmann,Mirja;Schmidt-Lassen,Joern;Lindhorst,ThisbeK.[ChemBioChem,2011,vol.12,#7,p.1066-1074]
[1]Durette,PhilippeL.;Shen,TsungY.[CarbohydrateResearch,1980,vol.81,p.261-274]
[1]Kieburg,Christoffer;Lindhorst,ThisbeK.[TetrahedronLetters,1997,vol.38,#22,p.3885-3888]
[1]Li,Jun;Zacharek,Sima;Chen,Xi;Wang,Jianqiang;Zhang,Wei;Janczuk,Adam;Wang,PengGeorge[BioorganicandMedicinalChemistry,1999,vol.7,#8,p.1549-1558]
[1]Amaike;Kobayashi;Shinkai[BulletinoftheChemicalSocietyofJapan,2000,vol.73,#11,p.2553-2558]
Title: Mannosylated liposomes bearing Amphotericin B for effective management of visceral Leishmaniasis.
Journal: Journal of liposome research 20111201
Title: Pharmacokinetics and tissue distribution of dual-targeting daunorubicin liposomes in mice.
Journal: Pharmacology 20110101
Title: Targeted liposomal drug delivery to monocytes and macrophages.
Journal: Journal of drug delivery 20110101
Title: Structure-based drug design and optimization of mannoside bacterial FimH antagonists.
Journal: Journal of medicinal chemistry 20100624
Title: Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals.
Journal: Journal of controlled release : official journal of the Controlled Release Society 20100125
Title: Effect of surface-mannose modification on aerosolized liposomal delivery to alveolar macrophages.
Journal: Drug development and industrial pharmacy 20100101
Title: Development of mannosylated liposomes for bioadhesive oral drug delivery via M cells of Peyer's patches.
Journal: Drug delivery 20090701
Title: Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections.
Journal: Journal of controlled release : official journal of the Controlled Release Society 20080407
Title: Uptake characteristics of liposomes by rat alveolar macrophages: influence of particle size and surface mannose modification.
Journal: The Journal of pharmacy and pharmacology 20070101
Title: Autocrine and paracrine transcriptional regulation of type IIA secretory phospholipase A2 gene in vascular smooth muscle cells.
Journal: Arteriosclerosis, thrombosis, and vascular biology 20050601
Title: Inhibition of bovine sperm-oocyte fusion by the p-aminophenyl derivative of D-mannose.
Journal: Molecular reproduction and development 20040201
Title: Targeting of antiviral drugs to T4-lymphocytes. Anti-HIV activity of neoglycoprotein-AZTMP conjugates in vitro.
Journal: Biochemical pharmacology 19901215