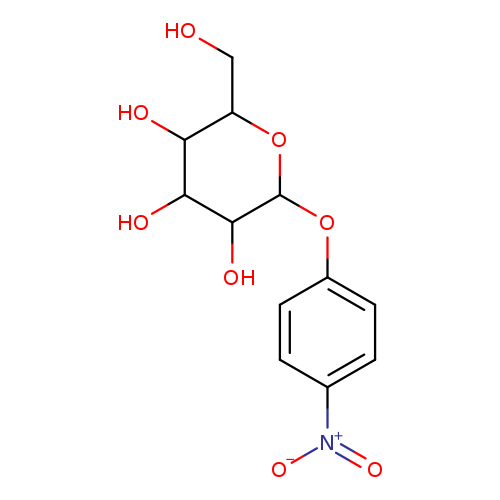
 2106-10-7
2106-10-7



[1]JournaloftheAmericanChemicalSociety,1998,vol.120,p.5583-5584
[1]CarbohydrateResearch,2006,vol.341,p.2055-2065
 35599-02-1
35599-02-1
 2106-10-7
2106-10-7
 2001-96-9
2001-96-9
 2492-87-7
2492-87-7
 3150-24-1
3150-24-1




 26255-70-9
26255-70-9


 2713-54-4
2713-54-4
[1]EuropeanJournalofOrganicChemistry,2013,p.2414-2419
[1]EuropeanJournalofOrganicChemistry,2013,p.2414-2419
Title: Expression and characterization of an extremely thermostable β-glycosidase (mannosidase) from the hyperthermophilic archaeon Pyrococcus furiosus DSM3638.
Journal: New biotechnology 20111001
Title: [A procedure for determination of acid mannosidase activities in biological fluids].
Journal: Klinicheskaia laboratornaia diagnostika 20090301
Title: Sensitive and rapid electrochemical bioassay of glycosidase activity.
Journal: The Analyst 20060801
Title: Purification and characterization of a beta-D-mannosidase from the marine anaspidean Aplysia fasciata.
Journal: Journal of biotechnology 20050922
Title: Synthesis of beta-mannosides using the transglycosylation activity of endo-beta-mannosidase from Lilium longiflorum.
Journal: The FEBS journal 20050401