Title: Assessment of the effect of mirodenafil on the hemodynamics of healthy male Korean volunteers administered tamsulosin: a randomized, double-blind, placebo-controlled, 2-period crossover study.
Journal: Clinical therapeutics 20120901
Title: Validated LC-MS/MS method for the determination of mirodenafil in rat plasma and its application to a comparative pharmacokinetic study of the free base and hydrochloride salt forms of mirodenafil.
Journal: Arzneimittel-Forschung 20120701
Title: Analysis of prescriptions of alpha-blockers and phosphodiesterase 5 inhibitors from the urology department and other departments.
Journal: International neurourology journal 20111201
Title: cGMP Signaling, Phosphodiesterases and Major Depressive Disorder.
Journal: Current neuropharmacology 20111201
Title: Erectile response to type 5 phosphodiesterase inhibitor could be preserved with the addition of simvastatin to conventional insulin treatment in rat model of diabetes.
Journal: International journal of andrology 20111001
Title: Novel phosphodiesterase type 5 modulators: a patent survey (2008 - 2010).
Journal: Expert opinion on therapeutic patents 20111001
Title: Effects of Ginkgo biloba extracts with mirodenafil on the relaxation of corpus cavernosal smooth muscle and the potassium channel activity of corporal smooth muscle cells.
Journal: Asian journal of andrology 20110901
Title: Prevalence and medical management of erectile dysfunction in Asia.
Journal: Asian journal of andrology 20110701
Title: The management of erectile dysfunction: innovations and future perspectives.
Journal: Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica 20110301
Title: Pharmacokinetics of mirodenafil and its two metabolites, SK3541 and SK3544, in spontaneously or DOCA-salt-induced hypertensive rats.
Journal: Biopharmaceutics & drug disposition 20110101
Title: Efficacy and safety of combination therapy with mirodenafil and α1-blocker for benign prostatic hyperplasia-induced lower urinary tract symptoms accompanied by erectile dysfunction: a multicenter, open-label, prospective study.
Journal: International journal of impotence research 20110101
Title: Future prospects in the treatment of erectile dysfunction: focus on avanafil.
Journal: Drug design, development and therapy 20110101
Title: Crystal forms of SK-3530.
Journal: Archives of pharmacal research 20101201
Title: Efficacy and safety of mirodenafil in men taking antihypertensive medications.
Journal: The journal of sexual medicine 20100901
Title: Efficacy and Safety of Tadalafil 5 mg Administered Once Daily in Korean Men with Erectile Dysfunction: A Prospective, Multicenter Study.
Journal: Korean journal of urology 20100901
Title: The effect of mirodenafil on the penile erection and corpus cavernosum in the rat model of cavernosal nerve injury.
Journal: International journal of impotence research 20100901
Title: Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: a multicenter, randomized, double-blind, placebo-controlled clinical trial.
Journal: The journal of sexual medicine 20100801
Title: Pharmacokinetics of mirodenafil and its two metabolites, SK3541 and SK3544, after intravenous and oral administration of mirodenafil to streptozotocin-induced diabetes mellitus rats.
Journal: Xenobiotica; the fate of foreign compounds in biological systems 20100201
Title: Pharmacokinetics of mirodenafil, a new erectogenic, and its metabolite, SK3541, in rats: involvement of CYP1A1/2, 2B1/2, 2D subfamily, and 3A1/2 for the metabolism of both mirodenafil and SK3541.
Journal: Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques 20100101
Title: The effects of ketoconazole and rifampicin on the pharmacokinetics of mirodenafil in healthy Korean male volunteers: an open-label, one-sequence, three-period, three-treatment crossover study.
Journal: Clinical therapeutics 20091201
Title: Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians.
Journal: Annals of internal medicine 20091103
Title: Faster clearance of mirodenafil in rats with acute renal failure induced by uranyl nitrate: contribution of increased protein expression of hepatic CYP3A1 and intestinal CYP1A1 and 3A1/2.
Journal: The Journal of pharmacy and pharmacology 20091001
Title: Dose-dependent pharmacokinetics and first-pass effects of mirodenafil, a new erectogenic, in rats.
Journal: Biopharmaceutics & drug disposition 20090901
Title: Influence of alcohol on the hemodynamic effects and pharmacokinetic properties of mirodenafil: a single-dose, randomized-sequence, open-label, crossover study in healthy male volunteers in Korea.
Journal: Clinical therapeutics 20090601
Title: Determination of mirodenafil and sildenafil in the plasma and corpus cavernous of SD male rats.
Journal: Journal of pharmaceutical and biomedical analysis 20090220
Title: Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction.
Journal: The journal of sexual medicine 20081101
Title: The penile erection efficacy of a new phosphodiesterase type 5 inhibitor, mirodenafil (SK3530), in rabbits with acute spinal cord injury.
Journal: The Journal of veterinary medical science 20081101
Title: Efficacy and safety of oral SK3530 for the treatment of erectile dysfunction in Korean men: a multicenter, randomized, double-blind, placebo-controlled, fixed dose, parallel group clinical trial.
Journal: Asian journal of andrology 20080901
Title: Chronic treatment with a type 5 phosphodiesterase inhibitor suppresses apoptosis of corporal smooth muscle by potentiating Akt signalling in a rat model of diabetic erectile dysfunction.
Journal: European urology 20080601
Title: Editorial comment on: chronic treatment with a type 5 phosphodiesterase inhibitor suppresses apoptosis of corporal smooth muscle by potentiating Akt signalling in a rat model of diabetic erectile dysfunction.
Journal: European urology 20080601
Title: Identification of cytochrome P450 enzymes responsible for N -dealkylation of a new oral erectogenic, mirodenafil.
Journal: Xenobiotica; the fate of foreign compounds in biological systems 20080101
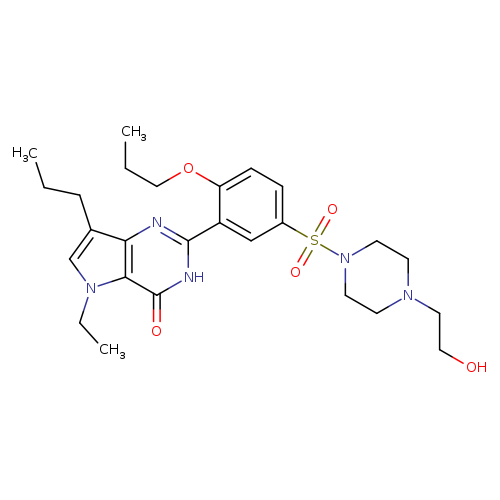
Title: Development of LC/MS/MS assay for the determination of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one (SK3530) in human plasma: application to a clinical pharmacokinetic study.
Journal: Journal of pharmaceutical and biomedical analysis 20070921
Title: Pharmacokinetics and tissue distribution of a novel PDE5 inhibitor, SK-3530, in rats.
Journal: Acta pharmacologica Sinica 20070801
Title: Formation of supramolecular hydrogels with controlled microstructures and stability via molecular assembling in a two-component system.
Journal: Journal of colloid and interface science 20070301
Title: Validation of a HPLC method for the quantification and purity determination of SK3530 in drug substance and tablet.
Journal: Journal of pharmaceutical and biomedical analysis 20070219
Title: Metabolism and excretion of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydropyrrolo[3,2-d]-pyrimidin-4-one (SK3530) in rats.
Journal: Rapid communications in mass spectrometry : RCM 20070101
Title: Paick, J.S., et al., Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med, 2008. 5(11): p. 2672-80.
Title: Kim, B.H., et al., Influence of alcohol on the hemodynamic effects and pharmacokinetic properties of mirodenafil: a single-dose, randomized-sequence, open-label, crossover study in healthy male volunteers in Korea. Clin Ther, 2009. 31(6): p. 1234-43.
Title: Shin, K.H., et al., The effects of ketoconazole and rifampicin on the pharmacokinetics of mirodenafil in healthy Korean male volunteers: an open-label, one-sequence, three-period, three-treatment crossover study. Clin Ther, 2009. 31(12): p. 3009-20.