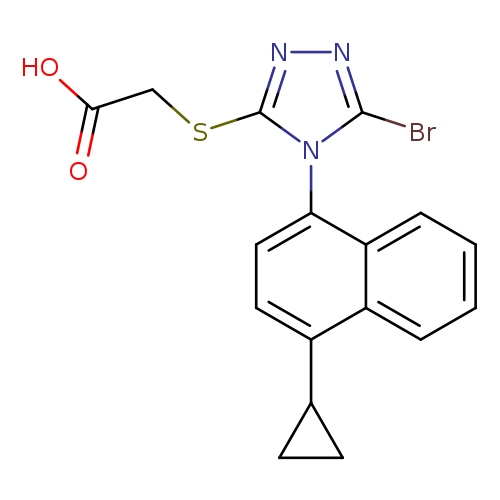
[1]CurrentPatentAssignee:ASTRAZENECAPLC-WO2009/70740,2009,A2Locationinpatent:Page/Pagecolumn91
[2]CurrentPatentAssignee:ASTRAZENECAPLC-WO2006/26356,2006,A2Locationinpatent:Page/Pagecolumn24
[3]CurrentPatentAssignee:ASTRAZENECAPLC-WO2011/85009,2011,A2Locationinpatent:Page/Pagecolumn38-39
[4]Lei,Xiaofang;Wang,Yuanyuan;Fan,Erkang;Sun,Zhihua[OrganicLetters,2019,vol.21,#5,p.1484-1487]
[5]CurrentPatentAssignee:SHENZHENDONGYANGGUANGINDUSTRIALDEVELOPMENTCOLTD-WO2014/198241,2014,A1Locationinpatent:Paragraph077-078
[6]CurrentPatentAssignee:SHANDONGUNIVERSITY-CN105566237,2016,ALocationinpatent:Paragraph0075;0076;0077
[7]CurrentPatentAssignee:CHENGDUMIRACLEPHARMACEUTICAL-CN107955029,2018,ALocationinpatent:Paragraph0064-0066;0080-0082;0096-0098
[8]Li,Yaoqi;Sun,Zhihua[Heterocycles,2020,vol.100,#7]
[9]CurrentPatentAssignee:ASTRAZENECAPLC-WO2014/8295,2014,A1Locationinpatent:Paragraph0340-0344
[10]CurrentPatentAssignee:ASTRAZENECAPLC-WO2014/8295,2014,A1
[11]CurrentPatentAssignee:CHINARESOURCESNATIONALCORPORATION-CN105985295,2016,ALocationinpatent:Paragraph0045;0046;0047
[12]CurrentPatentAssignee:SHANGHAIJINGXINBIOLOGICALMEDICAL;XINCHANGKANGLECHEMICALSCO.,LTD.-CN107098866,2017,A
[13]CurrentPatentAssignee:HUNANOUYABIOLOGICAL-CN106632108,2017,ALocationinpatent:Paragraph0057;0069;0079;0080
[14]CurrentPatentAssignee:ZhaoTiantian-CN107915689,2018,ALocationinpatent:Paragraph0011;0012;0013
[15]CurrentPatentAssignee:BEIJINGXINKAIYUANPHARMACEUTICALSTECHHAINANBRANCH-CN111153862,2020,ALocationinpatent:Paragraph0032-0033
[16]CurrentPatentAssignee:ZHEJIANGHUAHAIPHARMACEUTICALCO.,LTD.-CN111320588,2020,ALocationinpatent:Paragraph0034-0037
[17]Zhao,Tong;Meng,Qing;Sun,Zhuosen;Chen,Yanyu;Ai,Wei;Zhao,Zean;Kang,Dongwei;Dong,Yue;Liang,Ruipeng;Wu,Ting;Pang,Jianxin;Liu,Xinyong;Zhan,Peng[JournalofMedicinalChemistry,2020,vol.63,#19,p.10829-10854]
[18]CurrentPatentAssignee:CHINARESOURESSAIKEPHARMACEUTICAL;CHINARESOURCESNATIONALCORPORATION-US2021/53928,2021,A1
[1]Patent:WO2009/70740,2009,A2.Locationinpatent:Page/Pagecolumn91-92
[2]Patent:WO2011/85009,2011,A2.Locationinpatent:Page/Pagecolumn34-35
[3]Patent:WO2018/85932,2018,A1.Locationinpatent:Paragraph0079
[4]Patent:CN105985295,2016,A.Locationinpatent:Paragraph0070;0071
[5]Patent:CN104447589,2017,B
[1]CurrentPatentAssignee:ASTRAZENECAPLC-WO2009/70740,2009,A2Locationinpatent:Page/Pagecolumn92
[2]CurrentPatentAssignee:CRYSTALPHARMATECHCO.,LTD.;SUZHOUPENGXUPHARMATECHCO.,LTD.-WO2015/95703,2015,A1Locationinpatent:Page/Pagecolumn12;13
[3]CurrentPatentAssignee:CHINARESOURCESNATIONALCORPORATION-CN105985295,2016,ALocationinpatent:Paragraph0072;0073
[1]CurrentPatentAssignee:ASTRAZENECAPLC-WO2009/70740,2009,A2Locationinpatent:Page/Pagecolumn92
[1]CurrentPatentAssignee:ASTRAZENECAPLC-WO2009/70740,2009,A2Locationinpatent:Page/Pagecolumn79-80
[2]CurrentPatentAssignee:ASTRAZENECAPLC-WO2011/85009,2011,A2Locationinpatent:Page/Pagecolumn35
Title: Lesinurad: First Global Approval.
Journal: Drugs 20160301
Title: Gout therapeutics: new drugs for an old disease.
Journal: Lancet (London, England) 20110108
Title: Shen Z, et al. In Vitro and In Vivo Interaction Studies Between Lesinurad, a Selective Urate Reabsorption Inhibitor, and Major Liver or Kidney Transporters. Clin Drug Investig. 2016 Jun;36(6):443-52.
Title: Sattui SE, et al. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis. 2016 Aug;8(4):145-59.
Title: L.Yeh, et al. RDEA594, a potential uric acid lowering agent througn inhibition of uric acid reuptake ,shows better pharmacokinetics rhan its prodrug RDEA806. 2008 ACR/ARHP Annual Scientific Meeting, 24-29 October 2008, USA.