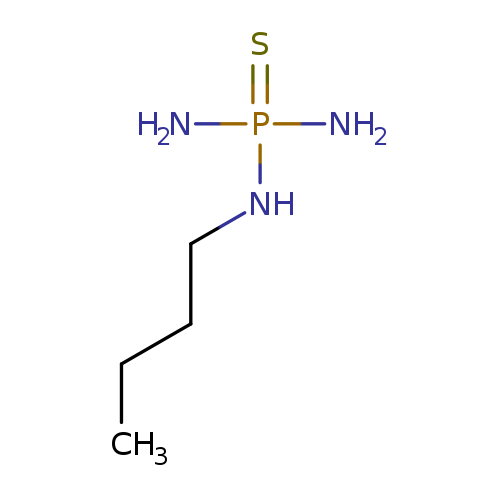
[1]Patent:CN105399767,2016,A,.Locationinpatent:Paragraph0045;0047
[2]Patent:US2011/196172,2011,A1,.Locationinpatent:Page/Pagecolumn4
[3]ChemischeBerichte,1956,vol.89,p.1768,1770
[4]Patent:US2011/28761,2011,A1,.Locationinpatent:Page/Pagecolumn4
[5]Patent:CN105440073,2016,A,.Locationinpatent:Paragraph0038;0041
[1]Patent:CN105254664,2016,A,.Locationinpatent:Paragraph0062;0063
[2]Patent:US2011/196172,2011,A1,.Locationinpatent:Page/Pagecolumn3
[1]Patent:WO2010/45895,2010,A2,.Locationinpatent:Page/Pagecolumn11
[1]Patent:CN105131031,2017,B,.Locationinpatent:Paragraph0033;0035;0036;0037;0045;0048;0050
[1]Patent:US2008/287709,2008,A1,.Locationinpatent:Page/Pagecolumn7
[2]Patent:US2008/287709,2008,A1,.Locationinpatent:Page/Pagecolumn7
[1]Patent:CN105399767,2016,A.Locationinpatent:Paragraph0045;0047
[2]Patent:US2011/196172,2011,A1.Locationinpatent:Page/Pagecolumn4
[3]ChemischeBerichte,1956,vol.89,p.1768,1770
[4]Patent:US2011/28761,2011,A1.Locationinpatent:Page/Pagecolumn4
[5]Patent:CN105440073,2016,A.Locationinpatent:Paragraph0038;0041
[1]Patent:US2008/287709,2008,A1.Locationinpatent:Page/Pagecolumn7
[2]Patent:US2008/287709,2008,A1.Locationinpatent:Page/Pagecolumn7
[1]Patent:WO2010/45895,2010,A2.Locationinpatent:Page/Pagecolumn8
[1]Patent:WO2010/45895,2010,A2.Locationinpatent:Page/Pagecolumn11
Title: Effectiveness of urease inhibition on the abatement of ammonia, nitrous oxide and nitric oxide emissions in a non-irrigated Mediterranean barley field.
Journal: Chemosphere 20120901
Title: The physiological implications of urease inhibitors on N metabolism during germination of Pisum sativum and Spinacea oleracea seeds.
Journal: Journal of plant physiology 20120501
Title: Ammonia volatilization from urea-application influenced germination and early seedling growth of dry direct-seeded rice.
Journal: TheScientificWorldJournal 20120101
Title: Efficiency of urease and nitrification inhibitors in reducing ammonia volatilization from diverse nitrogen fertilizers applied to different soil types and wheat straw mulching.
Journal: Journal of the science of food and agriculture 20110701
Title: Short term physiological implications of NBPT application on the N metabolism of Pisum sativum and Spinacea oleracea.
Journal: Journal of plant physiology 20110301
Title: Ureic nitrogen transformation in multi-layer soil columns treated with urease and nitrification inhibitors.
Journal: Journal of agricultural and food chemistry 20090610
Title: Effect of N-(n-butyl) thiophosphoric triamide and 3,4 dimethylpyrazole phosphate on gaseous emissions from grasslands under different soil water contents.
Journal: Journal of environmental quality 20090101
Title: Structural characteristics of phosphoramide derivatives as urease inhibitors. Requirements for activity.
Journal: Journal of agricultural and food chemistry 20080924
Title: Viability of zoonotic pathogens Escherichia coli and Salmonella in swine manure slurries with and without a urease inhibitor and thymol.
Journal: Letters in applied microbiology 20080401
Title: Fabrication of molecular nanotemplates in self-assembled monolayers by extreme-ultraviolet-induced chemical lithography.
Journal: Small (Weinheim an der Bergstrasse, Germany) 20071201
Title: Combination of a urease inhibitor and a plant essential oil to control coliform bacteria, odour production and ammonia loss from cattle waste.
Journal: Journal of applied microbiology 20070201
Title: Determination of urease activity in soils by carbon dioxide release for ecotoxicological evaluation of contaminated soils.
Journal: Ecotoxicology (London, England) 20021001
Title: Inhibition of jack bean urease by N-(n-butyl) thiophosphorictriamide and N-(n-butyl) phosphorictriamide: determination of the inhibition mechanism.
Journal: Journal of enzyme inhibition 20011201