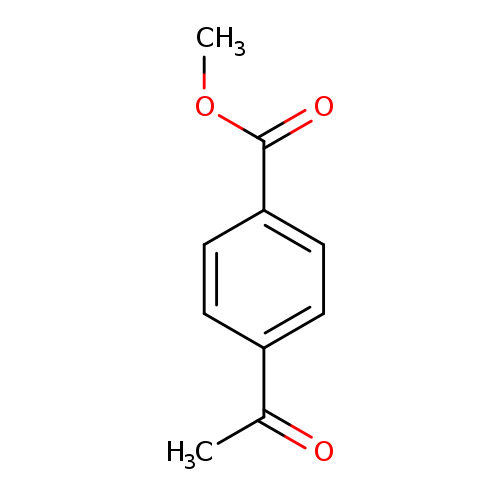
[1]JournalofMedicinalChemistry,2012,vol.55,#6,p.2899-2903
[2]JournalofOrganicChemistry,2015,vol.80,#14,p.7212-7218
[1]JournaloftheAmericanChemicalSociety,1959,vol.81,p.257,259
[2]JournaloftheAmericanChemicalSociety,1959,vol.81,p.257,259
[1]JournaloftheAmericanChemicalSociety,2000,vol.122,#39,p.9361-9366
[2]Patent:WO2006/78698,2006,A1,.Locationinpatent:Page/Pagecolumn64
[3]Patent:US2012/196869,2012,A1,.Locationinpatent:Paragraph0467;0468;0469
[4]InorganicChemistry,2018,vol.57,#1,p.120-128
[5]Patent:WO2008/33745,2008,A2,.Locationinpatent:Page/Pagecolumn70-71
[6]Patent:WO2008/33747,2008,A2,.Locationinpatent:Page/Pagecolumn255
[7]Patent:EP2210891,2010,A1,.Locationinpatent:Page/Pagecolumn19
[8]JournalofMedicinalChemistry,2012,vol.55,#6,p.2899-2903
[9]Patent:WO2015/89327,2015,A1,.Locationinpatent:Paragraph0414
[10]Patent:US6525042,2003,B1,
[11]Patent:EP1104754,2001,A1,
[12]Patent:EP1577302,2005,A1,.Locationinpatent:Page/Pagecolumn109
[13]Patent:WO2007/6547,2007,A1,.Locationinpatent:Page/Pagecolumn43-45
[14]Patent:US2009/23748,2009,A1,.Locationinpatent:Page/Pagecolumn24-25
[15]Patent:WO2010/34788,2010,A1,.Locationinpatent:Page/Pagecolumn38
[16]Patent:WO2010/34789,2010,A1,.Locationinpatent:Page/Pagecolumn33
[17]JournalofMedicinalChemistry,1987,vol.30,#8,p.1497-1502
[18]Patent:WO2006/17124,2006,A2,.Locationinpatent:Page/Pagecolumn66
[19]Patent:EP1975149,2008,A1,.Locationinpatent:Page/Pagecolumn64
[20]Patent:US2004/248949,2004,A1,.Locationinpatent:Page35-36
[21]BioorganicandMedicinalChemistry,2010,vol.18,#6,p.2317-2326
[22]Patent:EP2172462,2010,A1,.Locationinpatent:Page/Pagecolumn87
[23]Patent:WO2006/28970,2006,A1,.Locationinpatent:Page/Pagecolumn68-69
[24]Patent:US2011/189794,2011,A1,
[25]Patent:EP2353613,2011,A1,
[26]JournalofMedicinalChemistry,2015,vol.58,#15,p.6058-6080
[27]OrganicLetters,2017,vol.19,#8,p.1994-1997
[28]OrganicLetters,2018,vol.20,#8,p.2257-2260
[29]ChemicalCommunications(Cambridge,UnitedKingdom),2018,vol.54,#86,p.12182-12185
[1]Patent:WO2004/91610,2004,A1,.Locationinpatent:Page/Pagecolumn67
[1]OrganicLetters,2012,vol.14,#9,p.2414-2417
[2]OrganicLetters,2016,vol.18,#4,p.784-787
[1]Tavares;Penna;Amaral[BollettinoChimicoFarmaceutico,1997,vol.136,#3,p.244-249]
[2]CurrentPatentAssignee:CENTRENATIONALDELARECHERCHESCIENTIFIQUE;CENTREINTERNATIONALDERECHERCHEAUXFRONTIERESDELACHIMIE;UNIVERSITYOFPARISSACLAY;UNIVERSITYOFSTRASBOURG;NATIONALINSTITUTEOFHEALTHANDMEDICALRESEARCH-WO2018/11376,2018,A1Locationinpatent:Page/Pagecolumn44
[3]Tavares;Chiste;Santos;Penna[BollettinoChimicoFarmaceutico,1999,vol.138,#8,p.432-436]
[4]CurrentPatentAssignee:DAIICHISANKYOCOMPANY,LIMITED-EP1577302,2005,A1Locationinpatent:Page/Pagecolumn108
[5]CurrentPatentAssignee:NOVARTISAG;Novartis(w/oSandoz)-WO2015/48507,2015,A1Locationinpatent:Paragraph0154
[6]CurrentPatentAssignee:HALLYMUNIVERSITY;EWHAWOMANSUNIVERSITY-KR2021/89017,2021,ALocationinpatent:Paragraph0088;0090-0092;0094-0096
[7]CurrentPatentAssignee:UNIVERSITYOFWASHINGTON-WO2020/123855,2020,A1Locationinpatent:Page/Pagecolumn36
[8]Meyer,R.[JustusLiebigsAnnalenderChemie,1883,vol.219,p.253]
[9]Masunari,Andrea;Tavares,LeobertoCosta[BioorganicandMedicinalChemistry,2007,vol.15,#12,p.4229-4236]
[10]Locationinpatent:experimentalpartJorge,SalomaoDoria;Masunari,Andrea;Rangel-Yagui,CarlotaOliveira;Pasqualoto,KerlyFernandaMesquita;Tavares,LeobertoCosta[BioorganicandMedicinalChemistry,2009,vol.17,#8,p.3028-3036]
[11]Locationinpatent:experimentalpartNakamura,Ryota;Obora,Yasushi;Ishii,Yasutaka[AdvancedSynthesisandCatalysis,2009,vol.351,#10,p.1677-1684]
[12]Hou,Zengye;Nakanishi,Isao;Kinoshita,Takayoshi;Takei,Yoshinori;Yasue,Misato;Misu,Ryosuke;Suzuki,Yamato;Nakamura,Shinya;Kure,Tatsuhide;Ohno,Hiroaki;Murata,Katsumi;Kitaura,Kazuo;Hirasawa,Akira;Tsujimoto,Gozoh;Oishi,Shinya;Fujii,Nobutaka[JournalofMedicinalChemistry,2012,vol.55,#6,p.2899-2903]
[13]CurrentPatentAssignee:CHINAPHARMACEUTICALUNIVERSITY;YAOKANGZHONGTUOJIANGSUPHARMACEUTICALTECH-CN112321513,2021,ALocationinpatent:Paragraph0120-0125
[1]Synthesis,2017,vol.49,p.4007-4016
[2]OrganicLetters,2017,vol.19,p.5593-5596
[3]TetrahedronLetters,2010,vol.51,p.2063-2066
[4]ChemCatChem,2013,vol.5,p.126-129
[5]JournaloftheAmericanChemicalSociety,1946,vol.68,p.674
[1]TetrahedronLetters,2008,vol.49,p.110-113
[2]Synlett,2012,p.433-437
[3]Patent:US5019298,1991,A
[4]TetrahedronLetters,2012,vol.53,p.3686-3688
[5]Tetrahedron,2013,vol.69,p.6399-6403
[6]Chemistry-AnAsianJournal,2010,vol.5,p.1687-1691
[7]EuropeanJournalofOrganicChemistry,2013,p.5439-5444
[8]Tetrahedron,2014,vol.70,p.2088-2095
[9]Chemistry-AEuropeanJournal,2014,vol.20,p.4242-4245
[10]ChemicalCommunications,2007,p.760-762
[11]AppliedOrganometallicChemistry,2011,vol.25,p.748-752
[12]JournaloftheAmericanChemicalSociety,2016,vol.138,p.8809-8814
[13]JournalofOrganicChemistry,2020,vol.85,p.2242-2249
[14]AdvancedSynthesisandCatalysis,2012,vol.354,p.217-222
[15]Patent:WO2013/75083,2013,A1.Locationinpatent:Paragraph00219
[16]Patent:US9206128,2015,B2.Locationinpatent:Page/Pagecolumn122;123
[17]TetrahedronLetters,1982,vol.23,p.4585-4588
[18]EuropeanJournalofOrganicChemistry,2019,vol.2019,p.995-998
[19]Chemistry-AEuropeanJournal,2018,vol.24,p.12259-12263
[20]AngewandteChemie-InternationalEdition,2016,vol.55,p.11806-11809 Angew.Chem.,2016,vol.128,p.11984-11988,5
[21]Patent:CN106146401,2016,A.Locationinpatent:Paragraph0134;0135
[22]JournaloftheAmericanChemicalSociety,1946,vol.68,p.674
[23]JournalofOrganicChemistry,1959,vol.24,p.549
[24]AngewandteChemie-InternationalEdition,2013,vol.52,p.5120-5124 Angew.Chem.,2013,vol.125,p.5224-5228
[25]JournaloftheAmericanChemicalSociety,2016,vol.138,p.6940-6943
[26]Patent:WO2017/142883,2017,A1.Locationinpatent:Paragraph0179-0180
[27]OrganicandBiomolecularChemistry,2019,vol.17,p.4230-4233
[28]Chem,2019,vol.5,p.1552-1566
[29]AngewandteChemie-InternationalEdition,2019,vol.58,p.17567-17571 Angew.Chem.,2019,vol.131,p.17731-17735,5
[30]ChemicalCommunications,2020,vol.56,p.1203-1206
[1]TetrahedronLetters,1992,vol.33,p.5499-5502
[1]ChemistryLetters,1993,p.925-928
Title: Metal-free carbon-carbon bond-forming reductive coupling between boronic acids and tosylhydrazones.
Journal: Nature chemistry 20090901