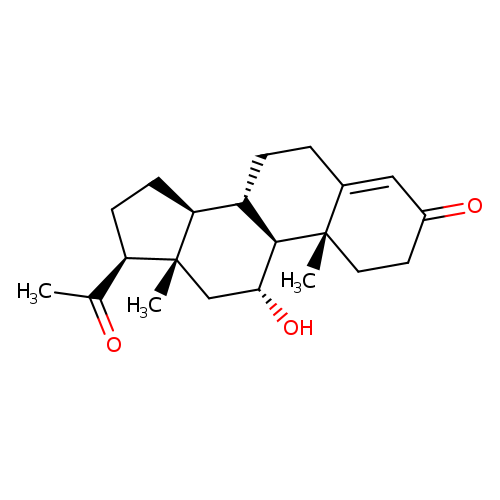
Title: Changing the regioselectivity of a P450 from C15 to C11 hydroxylation of progesterone.
Journal: Chembiochem : a European journal of chemical biology 20120529
Title: Fast-liquid chromatography using columns of different internal diameters packed with sub-2 μm silica particles.
Journal: Journal of chromatography. A 20120309
Title: Synthesis of deuterium labeled NMDA receptor inhibitor - 20-Oxo-5β-[9,12,12-(2)H(3)]pregnan-3α-yl-L-glutamyl 1-ester.
Journal: Steroids 20120201
Title: Microbial Baeyer-Villiger oxidation of steroidal ketones using Beauveria bassiana: Presence of an 11α-hydroxyl group essential to generation of D-homo lactones.
Journal: Biochimica et biophysica acta 20110401
Title: Discovery of a steroid 11α-hydroxylase from Rhizopus oryzae and its biotechnological application.
Journal: Journal of biotechnology 20101101
Title: Biotransformation of some steroids by Aspergillus wentii.
Journal: Zeitschrift fur Naturforschung. C, Journal of biosciences 20100101
Title: Steroid extraction in a microchannel system--mathematical modelling and experiments.
Journal: Lab on a chip 20070701
Title: Amino terminal domains of human UDP-glucuronosyltransferases (UGT) 2B7 and 2B15 associated with substrate selectivity and autoactivation.
Journal: Biochemical pharmacology 20070501
Title: Microsphere-based protease assays and screening application for lethal factor and factor Xa.
Journal: Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501
Title: Glucuronidation of steroidal alcohols using iodosugar and imidate donors.
Journal: Organic & biomolecular chemistry 20050421
Title: Validation of the accuracy of the perturbation peak method for determination of single and binary adsorption isotherm parameters in LC.
Journal: Analytical chemistry 20040815
Title: Specific interactions of steroids, arylhydrocarbons and flavonoids with progesterone receptors from the cytosol of the fungus Rhizopus nigricans.
Journal: The Journal of steroid biochemistry and molecular biology 20040801
Title: Identification of monohydroxy progesterones produced by CYP106A2 using comparative HPLC and electrospray ionisation collision-induced dissociation mass spectrometry.
Journal: Biochemical and biophysical research communications 20040625
Title: Hydroxylation of progesterone by some Trichoderma species.
Journal: Folia microbiologica 20040101
Title: Azasteroids: structure-activity relationships for inhibition of 5 alpha-reductase and of androgen receptor binding.
Journal: Journal of medicinal chemistry 19861101