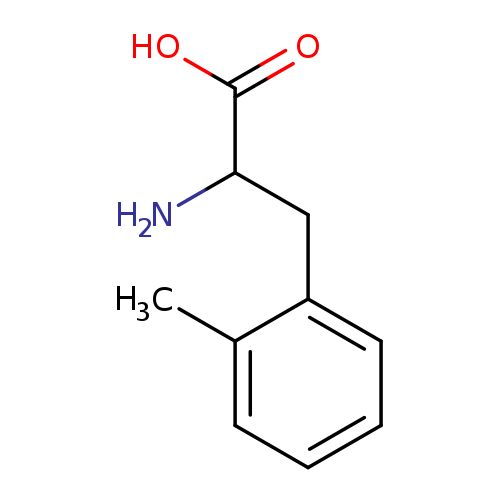
[1]AdvancedSynthesisandCatalysis,2017,vol.359,#9,p.1570-1576
[1]JournalofLabelledCompoundsandRadiopharmaceuticals,2011,vol.54,#2,p.80-85
[1]JournaloftheAmericanChemicalSociety,2015,vol.137,#40,p.12977-12983
[1]JournaloftheAmericanChemicalSociety,2015,vol.137,#40,p.12977-12983
[1]JournalofOrganicChemistry,2009,vol.74,#23,p.9152-9157
[1]TetrahedronLetters,1987,vol.28,p.3745-3746
[1]JournaloftheAmericanChemicalSociety,2007,vol.129,p.6988-6989
[1]JournalofMedicinalChemistry,2007,vol.50,p.2753-2766
[1]JournalofMedicinalChemistry,2007,vol.50,p.2753-2766
[1]JournalofMedicinalChemistry,2007,vol.50,p.2753-2766
Title: Preparative separation and identification of derivatized beta-methylphenylalanine enantiomers by chiral SFC, HPLC and NMR for development of new peptide ligand mimetics in drug discovery.
Journal: Journal of pharmaceutical and biomedical analysis 20060303
Title: Discovery of potent, selective, and orally bioavailable pyridone-based dipeptidyl peptidase-4 inhibitors.
Journal: Bioorganic & medicinal chemistry letters 20060301
Title: Discovery of potent and selective phenylalanine based dipeptidyl peptidase IV inhibitors.
Journal: Bioorganic & medicinal chemistry letters 20050516
Title: Modifications to the N-terminus but not the C-terminus of calcitonin gene-related peptide(8-37) produce antagonists with increased affinity.
Journal: Journal of medicinal chemistry 20030605
Title: Magnesium and biological activity of oxytocin analogues modified on aromatic ring of amino acid in position 2.
Journal: Journal of peptide science : an official publication of the European Peptide Society 20010801