2020-03-11 21:42:02
Tian-Tian Wang, Qi-Chun Wei, Zhen-Tao Zhang, Meng-Ting Lin,
Jie-Jian Chen, Yi Zhou, Ning-Ning Guo, Xin-Cheng Zhong, Wen-Hong Xu,b Zhan-Xiang Liu, Min Han and Jian-Qing Gao
Results and discussion
The amphipathic polymers PEG-Pep-TPE were synthesized according to the synthetic route. S1† and were first confirmed by LC-MS. Fig. 1a displays a peak at 3078.187, which is in good agreement with the MW of the product PEG-Pep-TPE, and the difference between the two adjacent peaks was 44, which happened to be the molecular weight of one PEG cycle unit inconsistency with the structure of PEG-Pep-TPE. Besides, the successful fabrication of PEG-Pep- TPE was further confirmed by the FT-IR spectra. As shown in Fig. 1b, the stretching vibration band of C–H(–CHO) at 2728 cm−1 and its bending vibration band at 2843 cm−1 in the TPE-CHO spectrum disappeared in the FT-IR spectrum of PEG-Pep and PEG-Pep-TPE, while the characteristic peak at 1663 cm−1 (CvN) appeared in the spectrum of PEG-Pep-TPE, evidencing the introduction of AIEgen in PEG-Pep.
Polyethylene glycol (PEG) is a kind of nonionic water-soluble polymer equipped with excellent properties such as water solubility and biocompatible ability. In this work, the amphiphilic copolymers PEG-Pep-TPE with the hydrophilic PEG and hydrophobic Pep-TPE could self-assemble into nanoparticles in aqueous solutions. As shown in the top panel of Fig. 1c, dynamic light scattering (DLS) revealed that PEG-Pep- TPE nanoparticles had an average hydrodynamic diameter of
137 nm with the polydispersity index of 0.249. Meanwhile, TEM was used to observe the morphology and size of PEG-Pep- TPE nanoparticles, and the results suggested a spherical morphology with a size of about 100 nm, convincingly evidencing the formation of the nanoparticle structure.
In order to systematically study the pH/GSH-responsive characteristic of PEG-Pep-TPE NPs, we completed a detailed investigation of its behavior in a physical stimulation and in A549 cells. The bottom panel of Fig. 1c, displayed that PEG-Pep-TPE NPs pretreated with pH 5.0 and 10 mM GSH for 12 h disintegrated responsively and the peptide fragments assembled to form fibers. In contrast to the results at 24 h (Fig. S3, ESI†), there was no significant change in the degree of fracture. Such phenomena supported the hypothesis that PEG-Pep-TPE NPs have a pH/GSH-responsive characteristic, and had broken completely under physical conditions for 12 h. Besides, the DLS results suggested that the size of pre-treated preparation was bigger than that of untreated nanoparticles, which could be explained in terms of aggregation of hydrophobic fragments (TPE-CHO molecules) and self-assembling peptides. Subsequently, after A549 cells (Institute of Biochemistry and Cell Biology of Chinese Academy of Science (Shanghai, China)) were incubated with the PEG-Pep-TPE NPs for 12 h, the reticulated self-assembled fibers were observed in the cytoplasm (Fig. 1d) via TEM compared with the control group. Relying on obtained results, it could be assumed that the pH/GSH-responsiveness of PEG-Pep-TPE NPs happened whether in the physical stimulation or in A549 cells and implied that PEG-Pep-TPE NPs would remain stable core-shell architectures in blood circulation, and disintegrate responsively in tumor cells.
The structure of the TPE-CHO molecules was similar to
typical AIE luminous such as TPE, and its AIE behavior could be evaluated by fluorescence assay. As shown in Fig. 2a, TPE-CHO dissolved in THF showed weak fluorescence; yet, the fluorescence intensity increased dramatically when the water fraction added to the THF solution exceeded 90% vol% under the same concentration and excitation condition, which was ascribed to the restriction of their intramolecular rotations induced by the aggregation of the hydrophobic TPE-CHO molecules. It was worth mentioning that the fluorescence intensity of the TPE-CHO molecules in water increased linearly with the increase of water fraction over 90% vol% and reached up to the maximum in water theoretically (Fig. 2b). Because TPE-CHO, as the hydrophobic moieties were trapped in the core of PEG-Pep-TPE NPs, the aggregation state of the TPE-CHO molecules, was expected to excite the AIE behavior. As stated before, the AIE behavior of PEG-Pep-TPE polymer could also be examined by fluorescence assay, and the same phenomenon occurred in Fig. 2c, which could also provide sufficient evidence to prove successful self-assembly of PEG-Pep-TPE polymers to PEG-Pep-TPE NPs, and the noticeable AIE effect of PEG-Pep-TPE NPs can be further employed in cell imaging.
Although the AIE effect can be used to trace the intracellular uptake of PEG-Pep-TPE NPs in the nanocarriers, the exploration of the drug-release behavior of the nanoparticles was still limited. Therefore, we further introduced the fluorescence resonance energy transfer (FRET) effect, which may open a route to the realization of investigating the intracellular drug release from nanoparticles. Furthermore, the maximum emission wavelength of the TPE-CHO molecules was located at 490 nm (as seen from Fig. 2d), same as the maximum absorption wavelength of the antitumor drug of doxorubicin (DOX), suggesting that the AIE group and DOX could become a FRET pair to monitor the drug-release process dynamically.
As shown in Fig. 2e, further insight could be gained by comparing the fluorescence spectrum of PEG-Pep-TPE NPs with that of PEG-Pep-TPE/DOX NPs only excited by UV lamp at 350 nm; the intensity of PEG-Pep-TPE/DOX NPs located at 490 nm was much lower than that of the PEG-Pep-TPE NPs (fluorescence quenching), whereas the fluorescence intensity at 600 nm emitted by the receptor DOX was greatly enhanced (sensitized fluorescence). This result presented a detailed analysis and discussion that FRET happened in PEG-Pep-TPE/DOX NPs. With the expectation to further verify the FRET effect of PEG-Pep-TPE/DOX NPs and monitor the release of DOX molecules from PEG-Pep-TPE/DOX NPs at the same time, we took advantage of their pH/GSH-triggered disintegration to treat PEG-Pep-TPE/DOX with pH 5.0 and 10 mM GSH and measured the changes of the fluorescence intensity at different time points in Fig. S4.† We could find out that the fluorescence intensity located at 490 nm increased gradually as time went on, which reflected the disintegration of the nanoparticles and the disappearance of the FRET effect between AIE and DOX. However, the fluorescence intensity of DOX was not weakened as expected but was enhanced continuously to some extent, which may be inconsistent with the partial overlap of the DOX emission spectrum and the AIE emission spectrum. To eliminate the effects of the spectral overlap, we adopted the ratio of the maximum fluorescence intensity of AIE to DOX as a measurement of the FRET. As shown in Fig. 2f, we could observe that the ratio increased over time and reached a constant value after 3 h, which indicated a gradual disappearance of FRET between TPE-CHO and DOX and visually reflected the release of DOX from PEG-Pep-TPE/DOX NPs. At the same time, this experiment could be invoked to prove the pH/GSH-responsiveness of PEG-Pep-TPE NPs in turn, and further explain the phenomenon that PEG-Pep-TPE NPs broke completely under physical conditions for 12 h without an obvious difference for 24 h, as shown in the bottom panel of Fig. 1c.
The effective release of drugs is one of the significant preconditions for their efficacy. To explore the impact of release behavior on efficacy, we set up four drug groups with different release abilities, including free DOX solution, PEG-Pep-TPE NPs + DOX, PEG-Pep-AIE/DOX NPs + GSH-OEt, and PEG-Pep-AIE/DOX NPs. Accordingly, the DOX cumulative release curve was respectively 94.49%, 93.59%, 77.16%, and 34.08% at 6 h, which implied that the drug release ability was declining gradually. In the meantime, the attractive results displayed in Fig. S5a† showed that the killing effects on tumor cells of four drug groups were gradually reduced as expected, and the IC50 values increased in turn. Besides, the impact of release ability on the efficacy was increasingly significant with the increase of drug concentration. Additionally, a significant correlation could be observed between IC50 values and in vitro release (Fig. S5b and c†). Therefore, we concluded that the ability of drug release from carriers would have a certain influence on the performance of drug efficacy, which may provide a novel guide for developing a drug delivery strategy in the future.
To further intuitively image drug delivery in cells and trace intracellular release from PEG-Pep-TPE/DOX NPs, we employed CLSM to determine the change of FRET signals occurring in AIE probes and DOX, which could visually reflect the release of the drug. At present, the detection of the FRET signal by a method only stays at the qualitative level, which easily leads to accidental experimental results. Therefore, here we adopted three general approaches to measure FRET based on practical considerations: (i) acceptor photobleaching (Fig. 3a); (ii) spectral imaging (Fig. 3c); and (iii) sensitized emission (Fig. 4a). As shown in Fig. 3a, after 3 h incubation with PEG-Pep-TPE/DOX NPs, blue and green fluorescence were directly observed, suggesting that almost all of PEG-Pep-TPE/DOX NPs were internalized by cells and mainly concentrated in the cytoplasm. Besides, the fluorescence of AIE probes was weak and even at the “OFF” status, indicating that the DOX molecules were mostly entrapped in the core of the nanoparticles. Along with the prolonged time, the blue fluorescence intensities tended to be stronger and more DOX molecules were observed in the nucleus, which indicated that the FRET effect gradually disappeared, and the drug was continuously released from the nanoparticles and then entered the nucleus. The average FRET efficiency measured by the acceptor photobleaching method in Fig. 3b could be employed to further explain this phenomenon and the FRET efficiency at a single site could be up to 25% at 3 h (avi1); yet, the average FRET efficiency reflecting the release behaviors, on the whole, was generally low in cells, which would increase the measurement error. Another confusing object was that the green fluorescence intensities should remain the same in theory, but the fluorescence intensities for DOX at 6 h was slightly weaker than the other groups, which may be associated with the defects of this method that photobleaching the acceptor can also cause photo-damage to the sample and induce incomplete recovery of the acceptor fluorochrome.
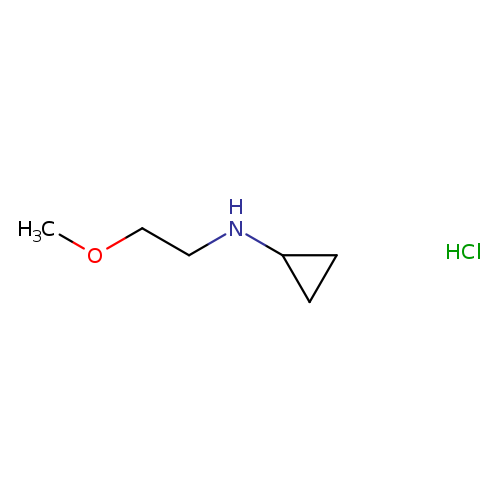
N-(2-Methoxyethyl)cyclopropanamine hydrochlorideCatalog No.:AA01ACQK CAS No.:1116589-38-8 MDL No.:MFCD26936119 MF:C6H14ClNO MW:151.6345 |
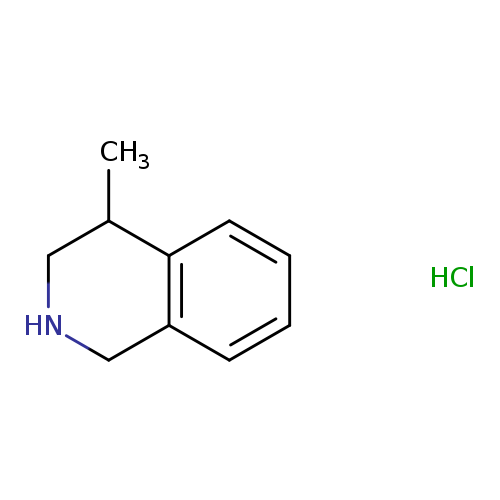
4-Methyl-1,2,3,4-tetrahydroisoquinoline hclCatalog No.:AA0092G9 CAS No.:111661-47-3 MDL No.:MFCD11501478 MF:C10H14ClN MW:183.6779 |
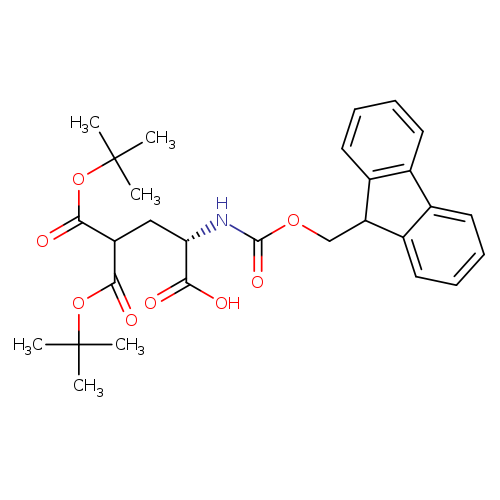
Fmoc-gla(otbu)2-ohCatalog No.:AA0081WW CAS No.:111662-64-7 MDL No.:MFCD00038770 MF:C29H35NO8 MW:525.5901 |
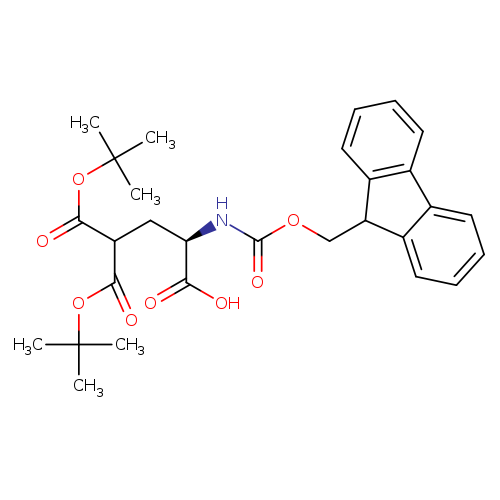
Fmoc-d-gla(otbu)2-ohCatalog No.:AA00HC3P CAS No.:111662-65-8 MDL No.:MFCD00672341 MF:C29H35NO8 MW:525.5901 |

Dehydroevodiamine hydrochlorideCatalog No.:AA01DPKC CAS No.:111664-82-5 MDL No.:MFCD06636629 MF:C19H16ClN3O MW:337.8028 |
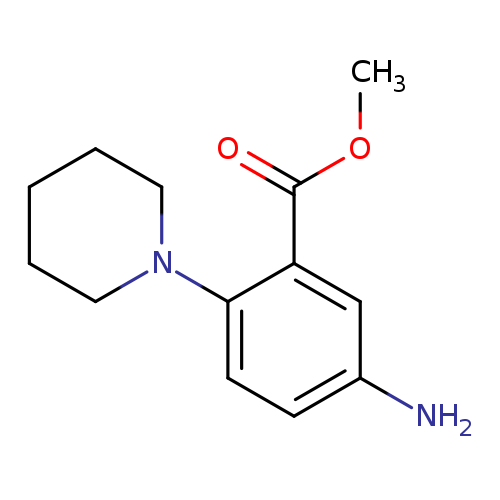
methyl 5-amino-2-(piperidin-1-yl)benzoateCatalog No.:AA01C15Y CAS No.:1116689-33-8 MDL No.:MFCD29988521 MF:C13H18N2O2 MW:234.2942 |
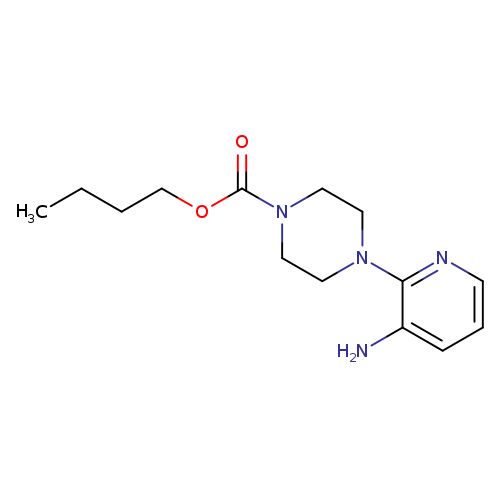
3-Amino-2-[4-butoxycarbonyl(piperazino)]pyridineCatalog No.:AA0079B3 CAS No.:111669-25-1 MDL No.:MFCD03407996 MF:C14H22N4O2 MW:278.3501 |
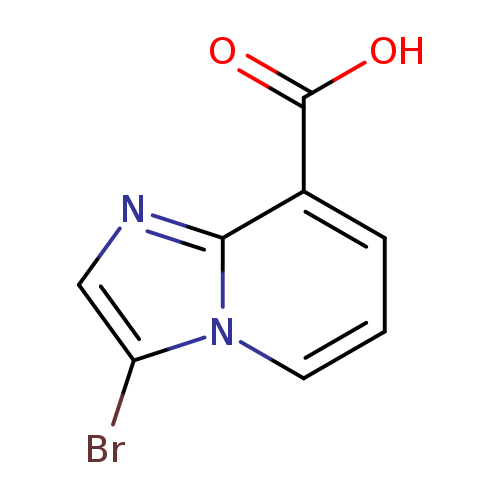
3-bromoimidazo[1,2-a]pyridine-8-carboxylic acidCatalog No.:AA0093KY CAS No.:1116691-26-9 MDL No.:MFCD17016090 MF:C8H5BrN2O2 MW:241.0415 |
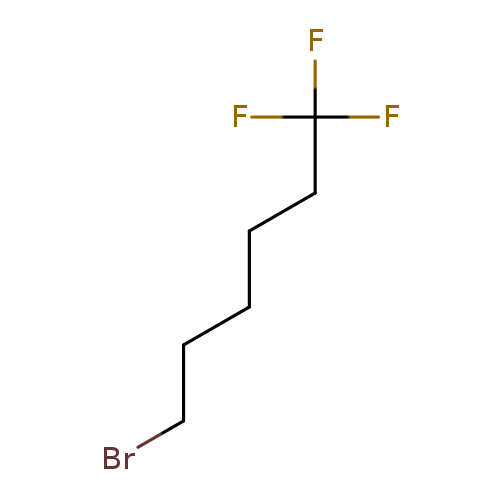
Hexane, 6-bromo-1,1,1-trifluoro-Catalog No.:AA0081WV CAS No.:111670-37-2 MDL No.:MFCD08062428 MF:C6H10BrF3 MW:219.0428 |
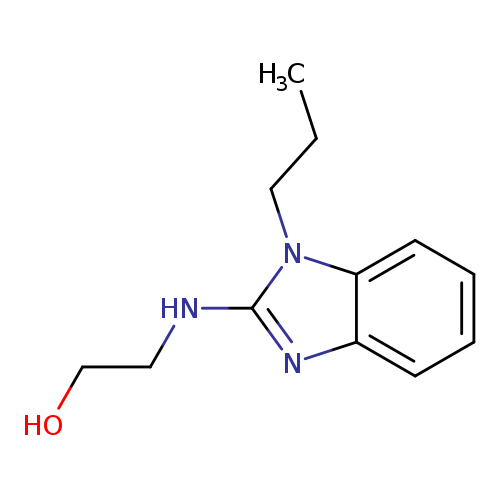
2-[(1-Propyl-1h-benzimidazol-2-yl)amino]ethanolCatalog No.:AA009743 CAS No.:111678-86-5 MDL No.:MFCD01136089 MF:C12H17N3O MW:219.2829 |
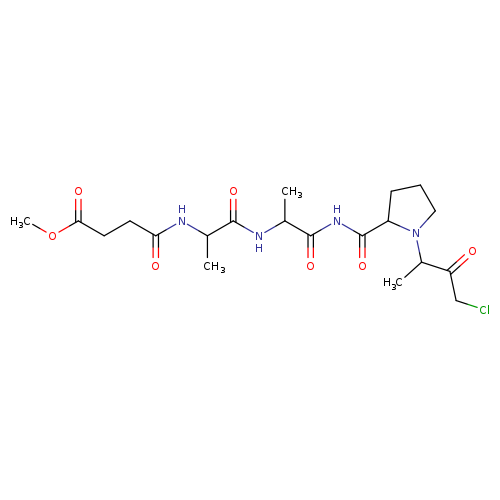
MEOSUC-ALA-ALA-PRO-ALA-CHLOROMETHYLKETONECatalog No.:AA008RQ7 CAS No.:111682-13-4 MDL No.:MFCD00238296 MF:C20H31ClN4O7 MW:474.9357 |
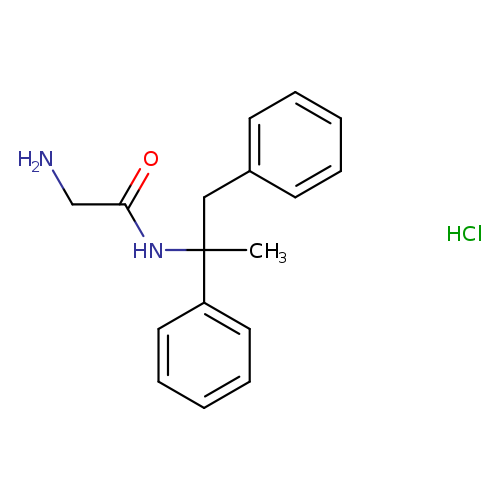
2-AMINO-N-(1-METHYL-1,2-DIPHENYLETHYL)ACETAMIDE HYDROCHLORIDECatalog No.:AA008Y16 CAS No.:111686-79-4 MDL No.:MFCD00882179 MF:C17H21ClN2O MW:304.8144 |
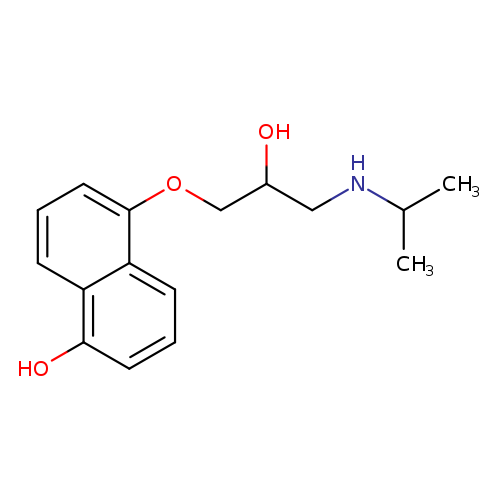
1-Naphthalenol, 5-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]-Catalog No.:AA0081WK CAS No.:111691-89-5 MDL No.:MFCD00869519 MF:C16H21NO3 MW:275.3428 |
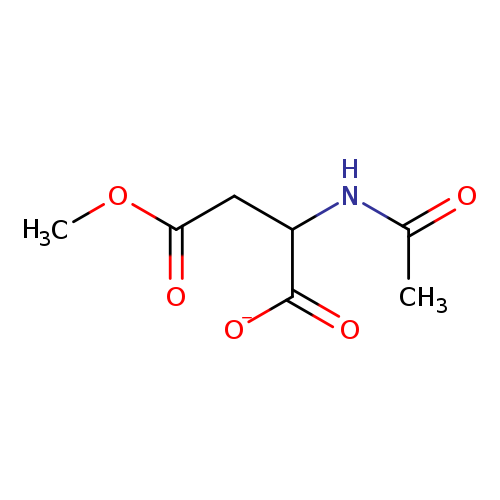
2-Acetamido-4-methoxy-4-oxobutanoic acidCatalog No.:AA007SBY CAS No.:111691-92-0 MDL No.:MFCD20643449 MF:C7H10NO5- MW:188.1580 |
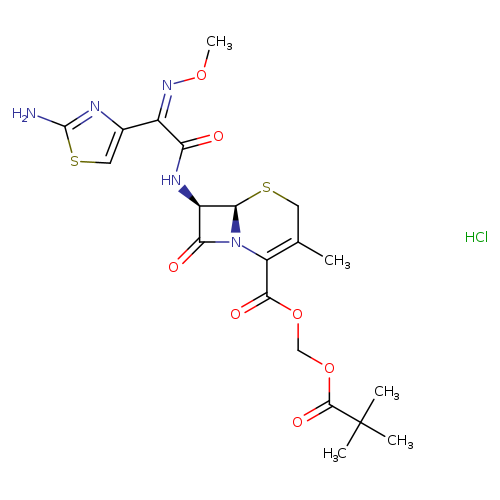
Cefetamet pivoxil hydrochlorideCatalog No.:AA00HC3T CAS No.:111696-23-2 MDL No.:MFCD00864931 MF:C20H26ClN5O7S2 MW:548.0327 |
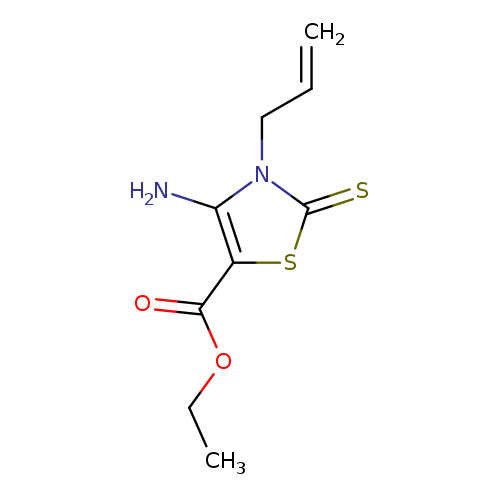
5-Thiazolecarboxylicacid, 4-amino-2,3-dihydro-3-(2-propen-1-yl)-2-thioxo-, ethyl esterCatalog No.:AA0081WE CAS No.:111698-89-6 MDL No.:MFCD01072107 MF:C9H12N2O2S2 MW:244.3338 |
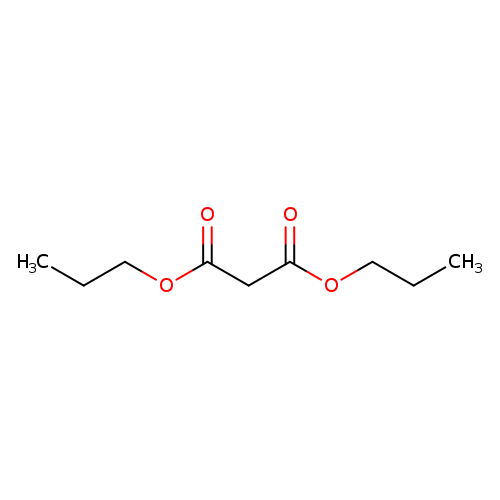
Dipropyl malonateCatalog No.:AA003PNM CAS No.:1117-19-7 MDL No.:MFCD00059406 MF:C9H16O4 MW:188.2209 |
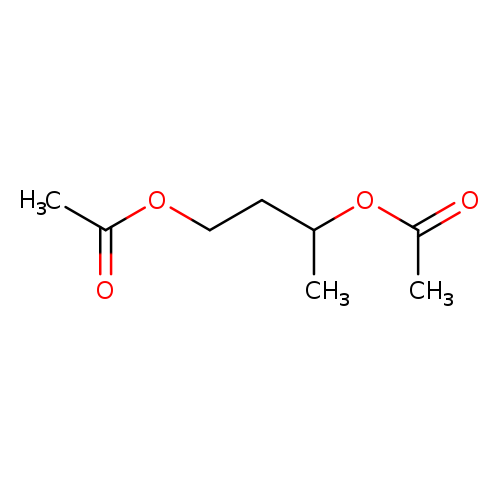
1,3-BUTANEDIOL DIACETATECatalog No.:AA003DFK CAS No.:1117-31-3 MDL No.:MFCD00008710 MF:C8H14O4 MW:174.1944 |
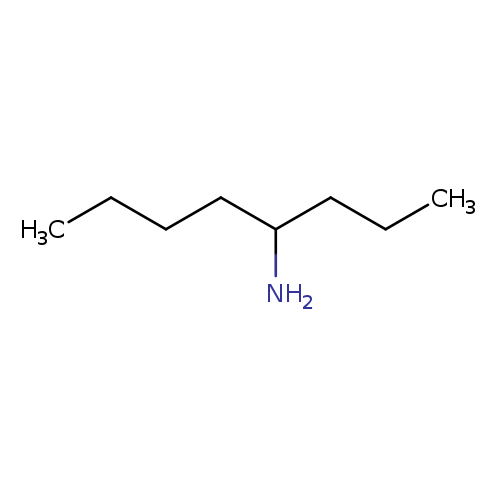
octan-4-amineCatalog No.:AA019TX8 CAS No.:1117-33-5 MDL No.:MFCD11655388 MF:C8H19N MW:129.2432 |
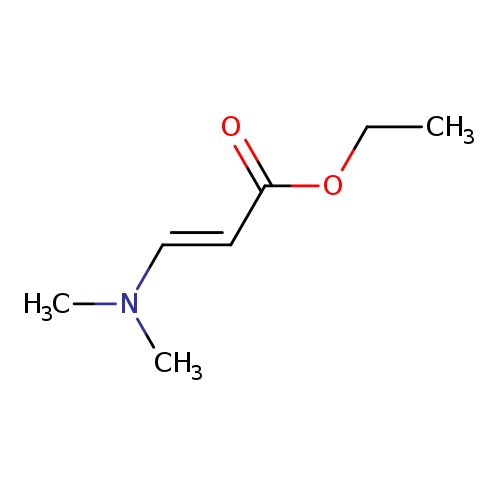
Ethyl trans-3-(N,N-dimethylamino)acrylateCatalog No.:AA008RAM CAS No.:1117-37-9 MDL No.:MFCD00144269 MF:C7H13NO2 MW:143.1836 |
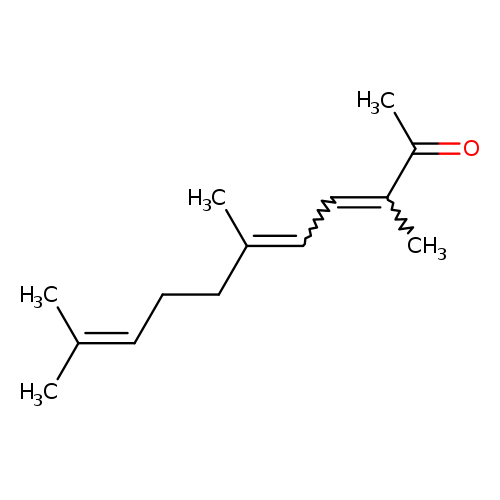
3,6,10-trimethylundeca-3,5,9-trien-2-oneCatalog No.:AA008WFO CAS No.:1117-41-5 MDL No.:MFCD01677196 MF:C14H22O MW:206.3239 |
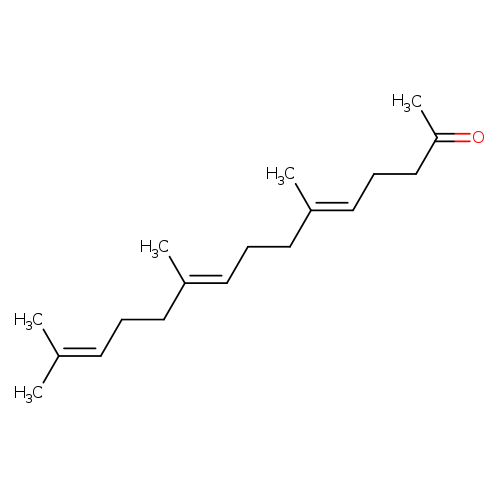
FarnesylacetoneCatalog No.:AA00HC3U CAS No.:1117-52-8 MDL No.:MFCD00036517 MF:C18H30O MW:262.4302 |
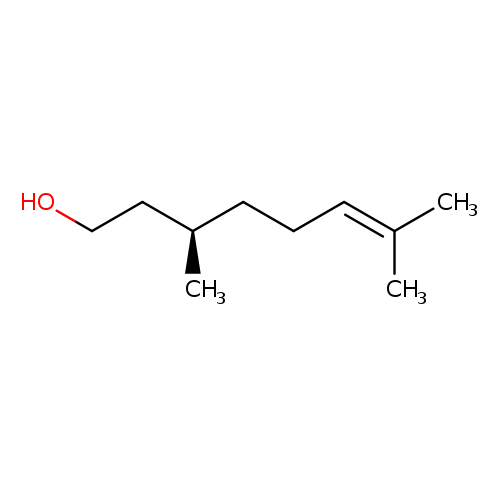
6-Octen-1-ol, 3,7-dimethyl-, (3R)-Catalog No.:AA00HC3V CAS No.:1117-61-9 MDL No.:MFCD00063215 MF:C10H20O MW:156.2652 |
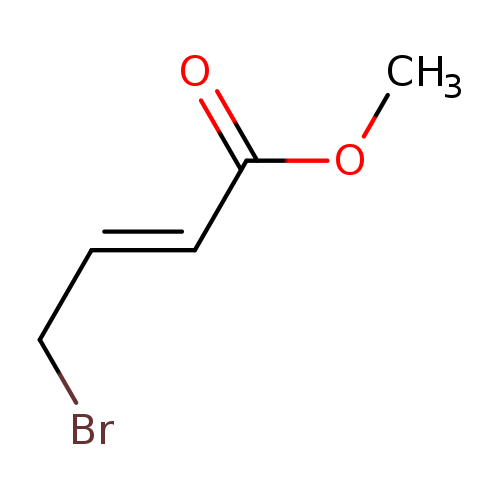
Methyl 4-bromocrotonate, tech gradeCatalog No.:AA00390G CAS No.:1117-71-1 MDL No.:MFCD00000246 MF:C5H7BrO2 MW:179.0119 |
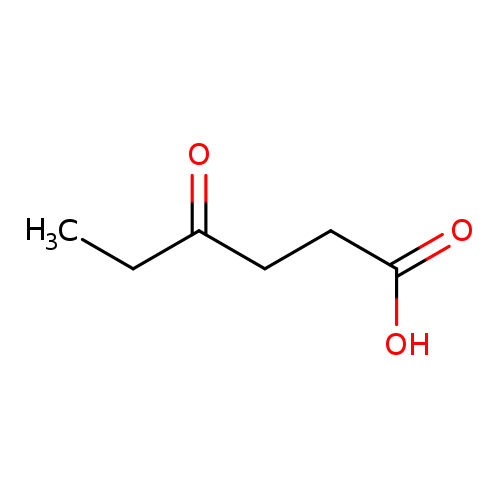
4-Oxohexanoic acidCatalog No.:AA007S5E CAS No.:1117-74-4 MDL No.:MFCD00068854 MF:C6H10O3 MW:130.1418 |
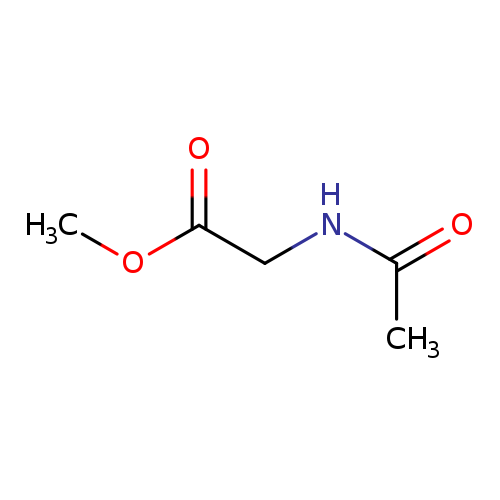
Methyl 2-acetamidoacetateCatalog No.:AA003RYD CAS No.:1117-77-7 MDL No.:MFCD00090795 MF:C5H9NO3 MW:131.1299 |
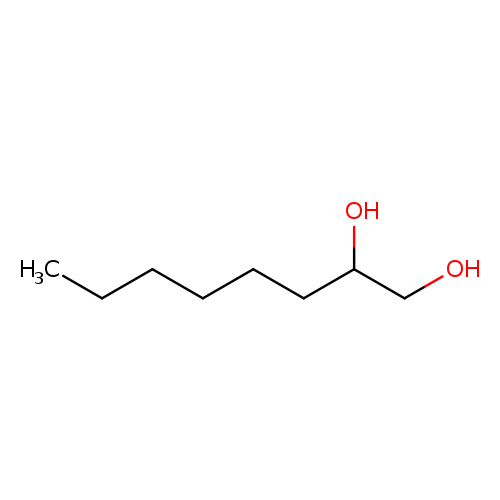
1,2-OctanediolCatalog No.:AA003DCN CAS No.:1117-86-8 MDL No.:MFCD00010738 MF:C8H18O2 MW:146.2273 |
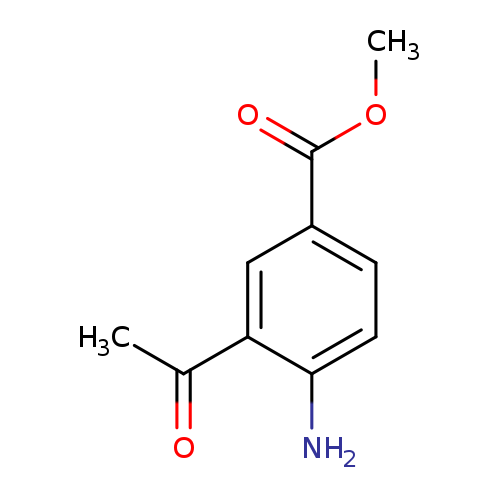
Methyl 3-acetyl-4-aminobenzoateCatalog No.:AA007SBG CAS No.:111714-47-7 MDL No.:MFCD11656627 MF:C10H11NO3 MW:193.1992 |
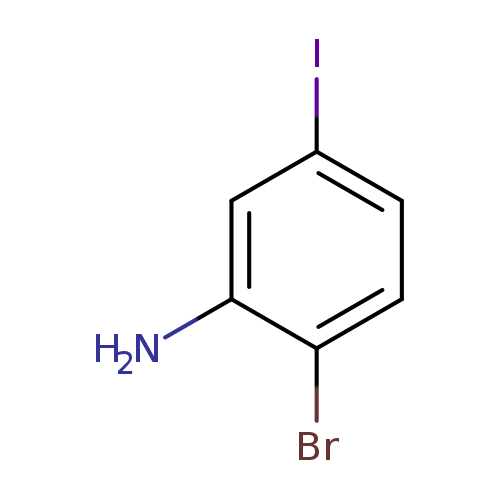
2-Bromo-5-iodoanilineCatalog No.:AA008TZK CAS No.:111721-74-5 MDL No.:MFCD16038675 MF:C6H5BrIN MW:297.9191 |
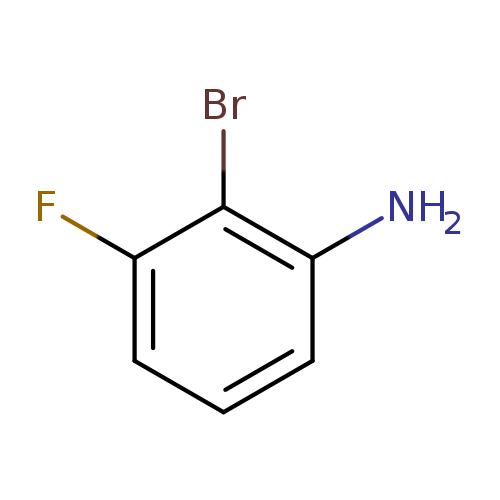
2-Bromo-3-fluoroanilineCatalog No.:AA003GJZ CAS No.:111721-75-6 MDL No.:MFCD07369915 MF:C6H5BrFN MW:190.0130 |
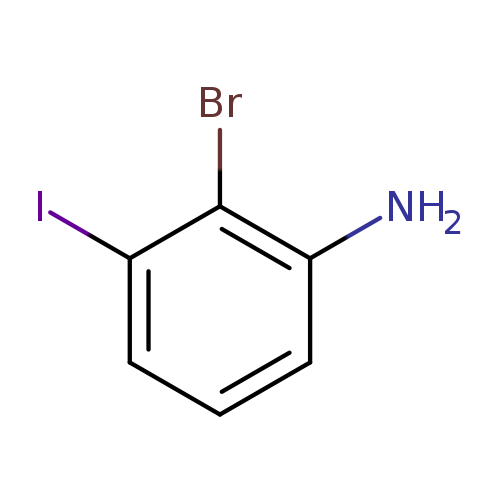
2-bromo-3-iodoanilineCatalog No.:AA01FHDB CAS No.:111721-76-7 MDL No.:MFCD18391614 MF:C6H5BrIN MW:297.9191 |
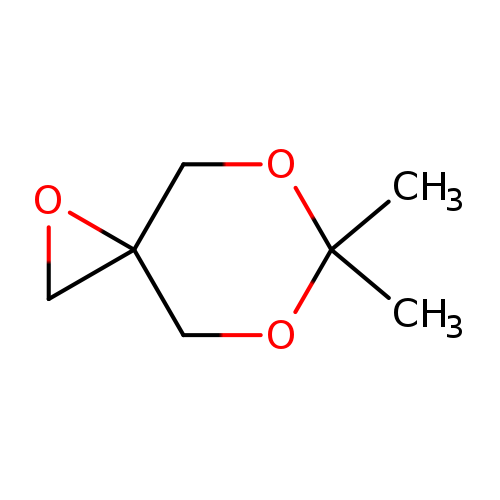
6,6-dimethyl-1,5,7-trioxaspiro[2.5]octaneCatalog No.:AA01EJYN CAS No.:111722-48-6 MDL No.:MFCD29085101 MF:C7H12O3 MW:144.1684 |
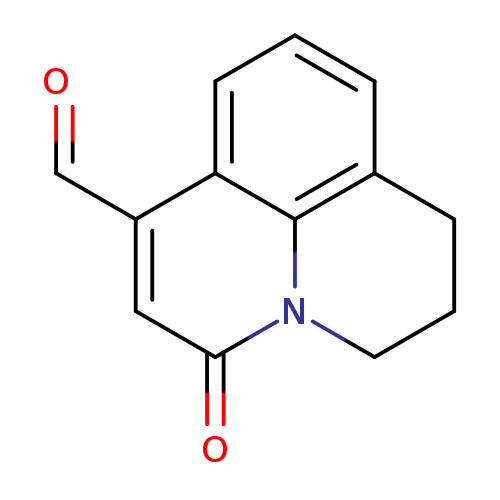
5-Oxo-2,3-dihydro-1h,5h-pyrido[3,2,1-ij]quinoline-7-carbaldehydeCatalog No.:AA0078UI CAS No.:111724-62-0 MDL No.:MFCD03198261 MF:C13H11NO2 MW:213.2319 |
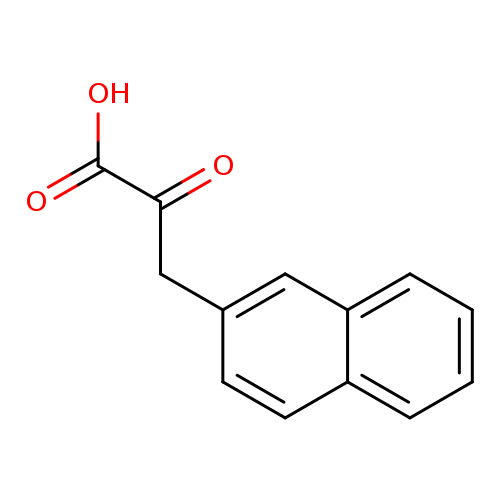
3-(naphthalen-2-yl)-2-oxopropanoic acidCatalog No.:AA019UUC CAS No.:111726-64-8 MDL No.:MFCD00969473 MF:C13H10O3 MW:214.2167 |
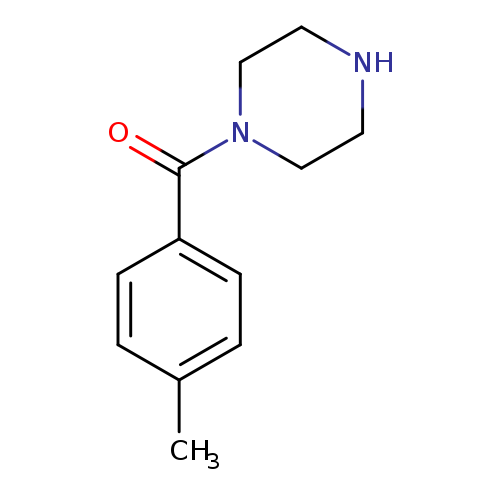
1-(4-Methylbenzoyl)piperazineCatalog No.:AA003CXP CAS No.:111752-26-2 MDL No.:MFCD04116566 MF:C12H16N2O MW:204.2682 |
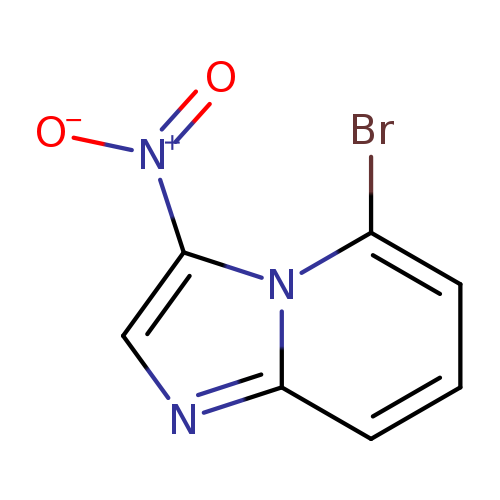
5-Bromo-3-nitroimidazo[1,2-a]pyridineCatalog No.:AA0096V9 CAS No.:111753-05-0 MDL No.:MFCD26383555 MF:C7H4BrN3O2 MW:242.0296 |
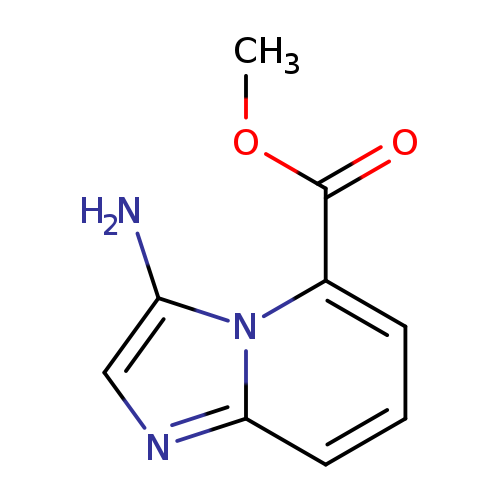
Methyl 3-aminoimidazo[1,2-a]pyridine-5-carboxylateCatalog No.:AA0093KW CAS No.:111753-15-2 MDL No.:MFCD18830504 MF:C9H9N3O2 MW:191.1867 |
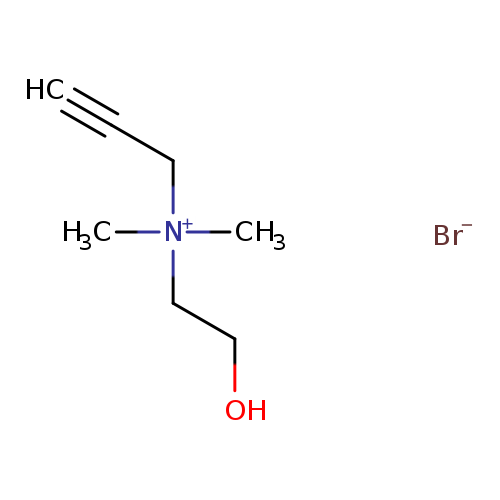
N-(2-hydroxyethyl)-N,N-dimethyl-2-propyn-1-aminium,monobromideCatalog No.:AA01EQ9F CAS No.:111755-76-1 MDL No.:MFCD22199535 MF:C7H14BrNO MW:208.0962 |
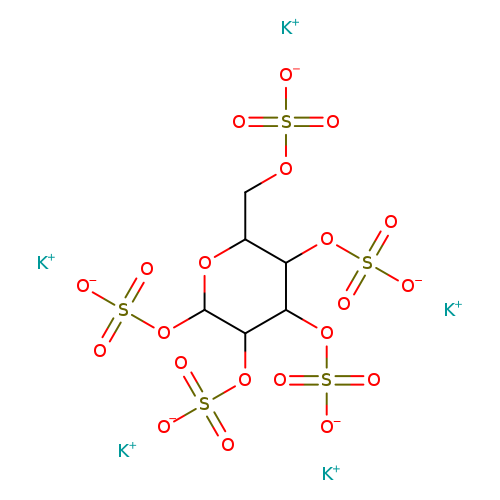
D-MannopyransepentasulfatepotassiumsaltCatalog No.:AA008YJ3 CAS No.:111757-61-0 MDL No.:MFCD09750784 MF:C6H7K5O21S5 MW:770.9237 |
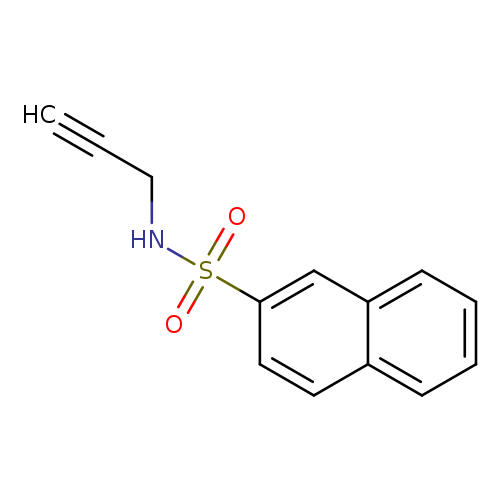
N-(prop-2-yn-1-yl)naphthalene-2-sulfonamideCatalog No.:AA00IXAM CAS No.:111758-62-4 MDL No.:MFCD01567653 MF:C13H11NO2S MW:245.2969 |
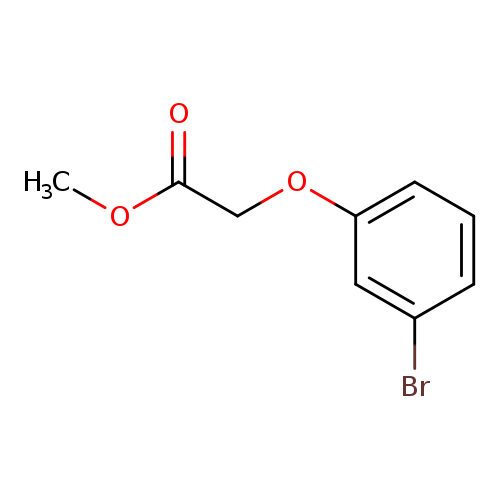
2-(3-Bromophenoxy)-acetic acid methyl esterCatalog No.:AA00HC3W CAS No.:111758-64-6 MDL No.:MFCD00461100 MF:C9H9BrO3 MW:245.0700 |
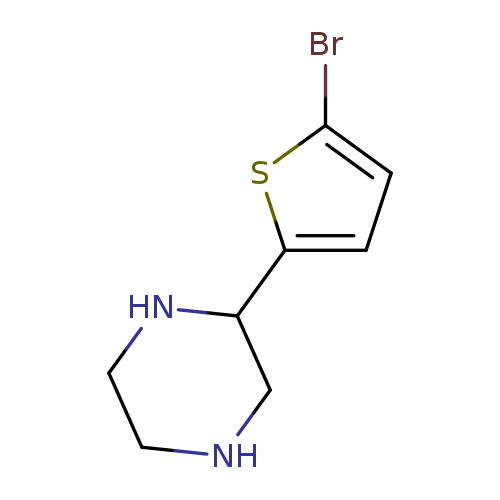
2-(5-Bromothiophen-2-yl)piperazineCatalog No.:AA008SDX CAS No.:111760-29-3 MDL No.:MFCD05864680 MF:C8H11BrN2S MW:247.1553 |
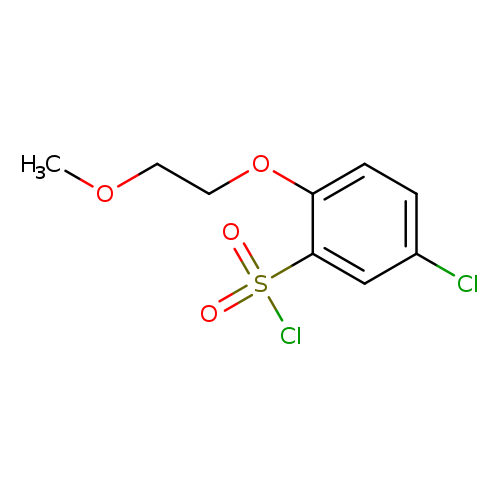
5-Chloro-2-(2-methoxyethoxy)benzene-1-sulfonyl chlorideCatalog No.:AA0078RG CAS No.:111762-21-1 MDL No.:MFCD16681756 MF:C9H10Cl2O4S MW:285.1443 |
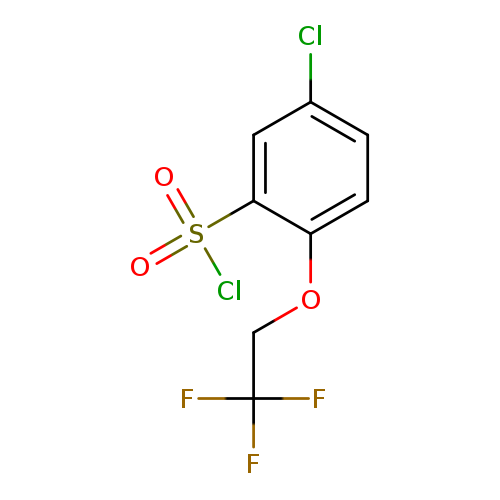
"5-chloro-2-(2,2,2-trifluoroethoxy)benzene-1-sulfonyl chloride"Catalog No.:AA01FMXU CAS No.:111762-22-2 MDL No.:MFCD17301616 MF:C8H5Cl2F3O3S MW:309.0897 |
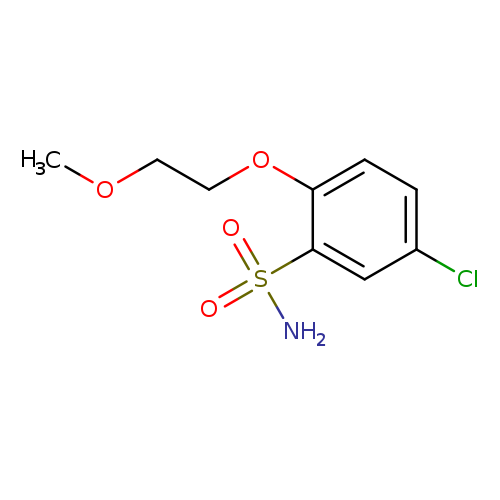
5-Chloro-2-(2-methoxyethoxy)benzene-1-sulfonamideCatalog No.:AA01A3G6 CAS No.:111762-24-4 MDL No.:MFCD16681772 MF:C9H12ClNO4S MW:265.7139 |
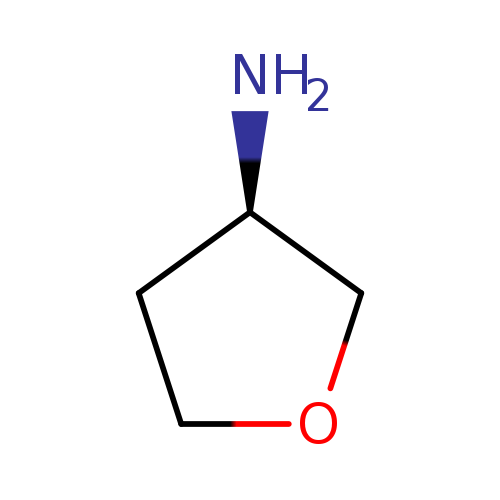
(R)-3-AminotetrahydrofuranCatalog No.:AA0038W0 CAS No.:111769-26-7 MDL No.:MFCD08234424 MF:C4H9NO MW:87.1204 |
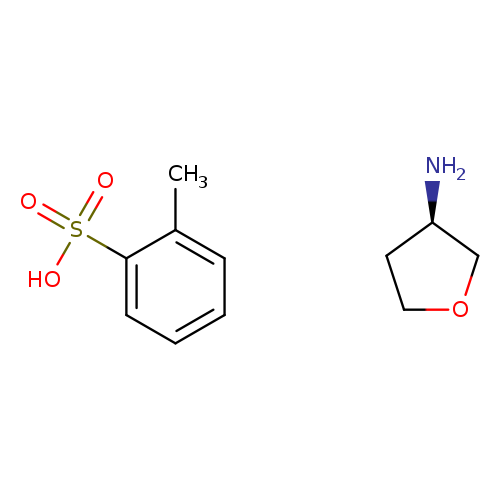
(R)-(+)-Tetrahydro-3-furylamine p-toluenesulfonate saltCatalog No.:AA0081Q3 CAS No.:111769-27-8 MDL No.:MFCD00040622 MF:C11H17NO4S MW:259.3220 |
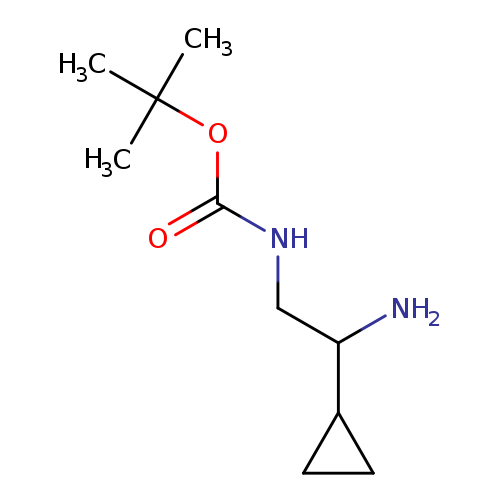
tert-Butyl n-(2-amino-2-cyclopropylethyl)carbamateCatalog No.:AA01AKIM CAS No.:1117693-58-9 MDL No.:MFCD18658759 MF:C10H20N2O2 MW:200.2780 |
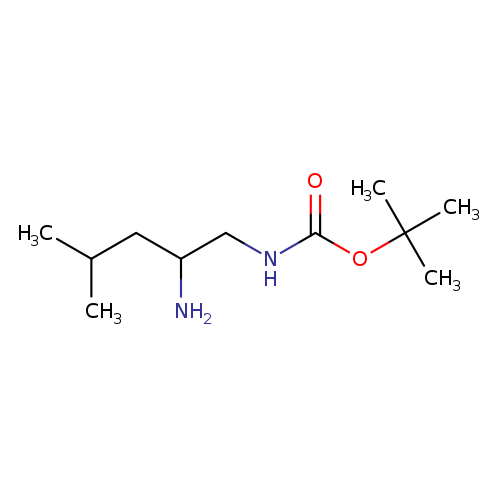
tert-butyl N-(2-amino-4-methylpentyl)carbamateCatalog No.:AA01AH83 CAS No.:1117693-62-5 MDL No.:MFCD14584431 MF:C11H24N2O2 MW:216.3205 |
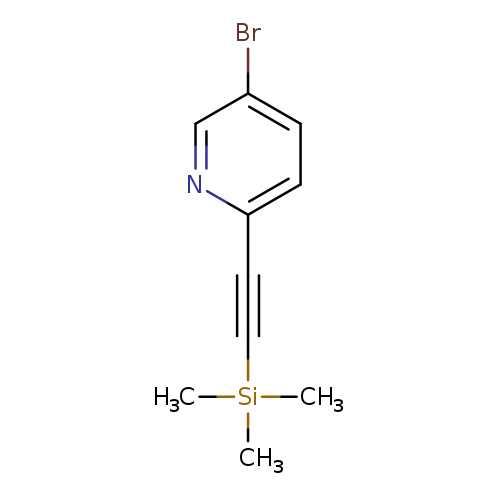
5-Bromo-2-[2-(trimethylsilyl)ethynyl]pyridineCatalog No.:AA008RY6 CAS No.:111770-80-0 MDL No.:MFCD03095275 MF:C10H12BrNSi MW:254.1985 |
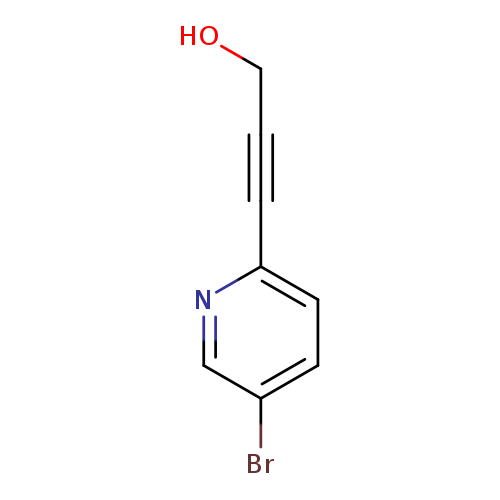
3-(5-Bromopyridin-2-yl)prop-2-yn-1-olCatalog No.:AA01B4SE CAS No.:111770-82-2 MDL No.:MFCD09836528 MF:C8H6BrNO MW:212.0433 |
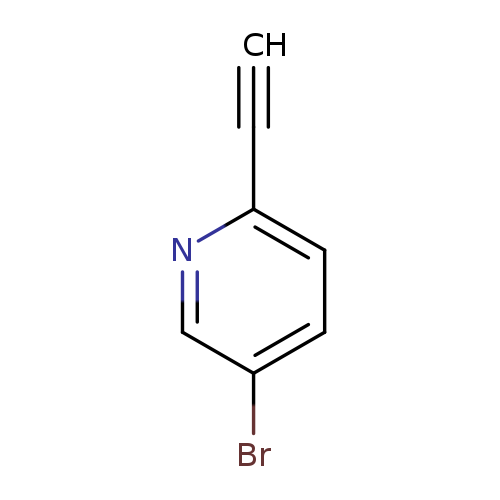
5-Bromo-2-ethynylpyridineCatalog No.:AA00HC3X CAS No.:111770-86-6 MDL No.:MFCD03095276 MF:C7H4BrN MW:182.0174 |