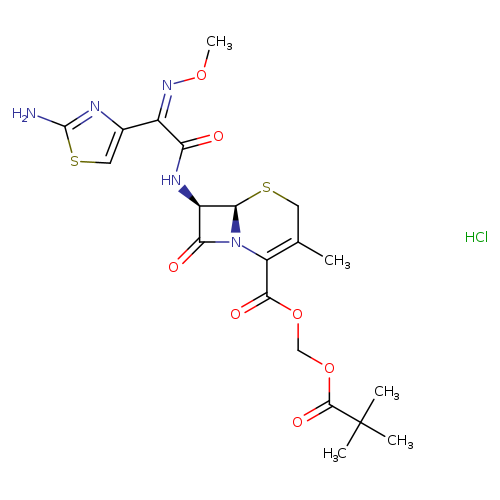
Title: Quantitative determination of cefetamet in human plasma by liquid chromatography-mass spectrometry.
Journal: Biomedical chromatography : BMC 20110701
Title: [The role of the third-generation oral cephalosporin cefditoren pivoxil in the treatment of community-acquired infection in adults].
Journal: Revista espanola de quimioterapia : publicacion oficial de la Sociedad Espanola de Quimioterapia 20050901
Title: The influence of pH, temperature and buffers on the degradation kinetics of cefetamet pivoxil hydrochloride in aqueous solutions.
Journal: Journal of pharmaceutical and biomedical analysis 20040903
Title: Esters of cephalosporins part IX. New method of isolation of cefetamet pivoxil.
Journal: Acta poloniae pharmaceutica 20040101
Title: A multicentric study to evaluate efficacy and safety of cefetamet pivoxyl in lower respiratory tract infections in Indian patients.
Journal: Journal of the Indian Medical Association 20030701
Title: Kinetics of degradation of cefetamet pivaloyloxymethyl ester and its hydrochloride in solid phase.
Journal: Acta poloniae pharmaceutica 20030101
Title: LC method for the analysis of cefetamet pivoxil hydrochloride in drug substance and powder for oral suspension.
Journal: Journal of pharmaceutical and biomedical analysis 20021015
Title: Sateesha S, et al. Formulation development and rheological studies of palatable cefetamet pivoxil hydrochloride dry powdersuspension. Daru. 2011;19(2):118-125.