2019-11-21 16:13:42
Brandon Quillian1 · Will E. Lynch1 · Clifford W. Padgett1 · Alexis Lorbecki1 · Anthony Petrillo1 · Michael Tran1
Received: 23 May 2018 / Accepted: 22 August 2018 / Published online: 27 August 2018
© Springer Science+Business Media, LLC, part of Springer Nature 2018
Introduction
In the nearly 20 years since their discovery [1], the facially coordinating bis(pyrazolyl)acetic acid ligands have been used to prepare numerous transition metal coordination compounds [2]. Many of these coordination compounds were studied for their unique structural, electrochemical, and catalytic properties. The study of copper with bis(pyrazol-1-yl)acetate ligands has largely been inspired by biomimicry of metalloenzymes such as the bis-histidine-carboxylate N,N,O-bonding motif [2–7]. However, recently they have been implicated as possible replacements for cis-platin as anti-cancer drugs due to copper’s endogenous properties in biological systems [8]. The literature presents a trove of structurally characterized copper(II) complexes supported by substituted bis(pyrazol-1-yl) acetate ligands (typically substituted at the 3,5-position of the pyrazolyl ligand) and other derivatives [9–11]; however, there are only a few with the parent, base, unsubstituted ligand [6] and to our knowledge a discrete, structurally characterized complex without a complementary co-crystalized species within the outer coordination sphere is unknown. Herein, we present the synthesis, characterization and single-crystal X-ray structures of [Cu(L-H)Br(µ-Br)]2 (1) and [Cu(L)2].4H2O (2). Compound 1 represents the first structurally characterized [12] dimer of a copper(II) dibromide complex supported by bis(pyrazolyl) acetate, while compound 2 represents the first structurally characterized, well-defined, bis(pyrazolyl)acetate copper (II) complex.
Experimental General
All reactions were performed in air. All commercially available reagents and solvents were used as received with no further purifications. Bis(pyrazolyl)acetic acid (L-H) was prepared by a slightly modified procedure [13], 1,1-dichloroacetic acid was used in place of 1,1-dibromoacetic acid and the reaction was refluxed overnight rather than for 6 h.
Physical Measurements
Elemental analyses were performed by Atlantic Microlab, Inc. FT-IR spectra were obtained on a Perkin Elmer Spectrum Two as KBr dispersions via diffuse reflectance. UV–Vis were recorded as solutions on a Hewlett Packard 8453 in quartz cells. Single-crystal X-ray structure determination was performed on a Rigaku MiniFlex.
Syntheses Synthesis of [Cu(L‑H)Br(µ‑Br)]2 (1)
In separate vessels, copper(II) bromide, CuBr2 (0.0510 g, 0.229 mmol) and L-H (0.0439 g, 0.229 mmol) were dissolved in a minimal amount of THF (30 mL and 20 mL, respectively). The two solutions were combined, resulting in a brown solution, and stirred for 5 min at room temperature. The solution was allowed to evaporate at room temperature to afford green/brown X-ray quality crystals of 1. The solid is found to be moderately hygroscopic. Yield: (0.0720 g, 75.9%). Anal. Calcd. (%) C16H16N8O4Cu2Br4 (FW=831.08 g mol−1) C, 23.12; H, 1.94; N, 13.48. Found (%): C, 24.08; H, 1.91; N, 13.95. IR (KBr, cm−1): 3129 (w), 2979 (w), 1744 (s), 1601 (w), 1454 (m), 1399 (m), 1382 (m), 1287 (s), 1062 (s), 989 (m), 815 (m), 756 (s). UV–Vis (methanol, nm, cm−1 M−1) 303 (222,000), 749 (8200).
Synthesis of [Cu(L)2]·4H2O (2)
Method A [Cu(L-H)Br(µ-Br)]2 (1) (0.0500 g, 0.060 mmol) was dissolved in methanol (10 mL) with stirring. The solvent was slowly evaporated, causing the brown solution to become aqua blue in color. Aqua blue crystals (2) of X-ray quality were obtained after a period of 4–5 days. The material in the solid state is highly hygroscopic. Yield (0.0231 g, 72.0%).
Method B In separate reaction vessels, copper(II) bromide, CuBr2 (0.0510 g, 0.229 mmol) and L-H (0.0880 g, 0.229 mmol) were dissolved in a minimal amount of methanol (20 mL and 10 mL, respectively). The two solutions were combined and the resulting green solution was stirred for 5 min at room temperature. The solution was slowly evaporated over 2 h, turning from green to aqua blue in color. X-ray quality aqua blue crystals of 2 precipitate within 2 h of slow evaporation of the solvent. The solid material is found to be highly hydroscopic. Yield: (0.0820 g, 68.3%). Anal. Calcd. (%) C16H23N8O8.5Cu: (FW=527.01 g mol−1) C, 36.47; H, 4.39; N, 21.26. Found (%): C, 36.31; H, 4.01; N, 20.33. IR (KBr, cm−1): 3114 (w), 2922 (m), 2853 (m), 1659 (m), 1644 (s), 1594 (w), 1452 (m), 1404 (m), 1348 (m), 1285 (m), 1075 (m), 859 (m), 754 (s). UV–Vis (methanol, nm, cm−1 M−1) 311 (39,700) 730 (1590).
Crystal Structure Determination
Crystals suitable for single-crystal X-ray analysis for (1) and (2) were grown by slow evaporation of the appropriate solvent at room temperature under aerobic conditions. Singlecrystal X-ray diffraction data for compounds (1) and (2) were collected at 173 K on a Rigaku XtaLAB mini diffractometer equipped with Mo-Kα radiation (λ=0.71073 Å) and processed using CrystalClear (Rigaku) software [14, 15]. The structure was solved using direct methods and refined by using full matrix least squares refinement using SHELXT and SHELXL2017 [16, 17]. All non-hydrogen atoms were refined anisotropically. H-atoms were placed in calculated positions and refined with riding model approximations.
Results and Discussion Synthesis
Bis(pyrazol-1-yl)acetic acid (L-H) was synthesized by a modified literature method [13], using 1,1-dichloroacetic acid and refluxing the reaction overnight. The result of the synthesis was an analytically pure compound that matched all spectroscopic parameters of the literature compound. The bromo-bridged dimer copper(II) complex (1) was synthesized by the reaction of L-H with CuBr2 in tetrahydrofuran in ambient air (Scheme 1). The solution turned dark brown immediately and after a period, brownishgreen X-ray quality crystals were collected. Prolonged exposure of 1 to ambient air causes it to slowly collect water, presumably initiating the transformation to the bisligated copper complex (2). The conversion of the methyl ester of bis(3,5-dimethylpyrazol-1-yl) acetic copper(II) dichloride complex into a bis-ligated copper(II) complex has similarly been reported [11].
The bis-ligated copper complex 2 can be prepared directly by reaction of H-L with CuBr2 in methanol or dissolving complex 1 in methanol (Scheme 1). As complex 2 forms, there is a noticeable change in the color from brownish-green (presumably 1) to a blueish-green solution. The solid product of 2 is isolated as a fairly intractable pale-blue species that is only sparingly soluble in common organic solvents (methanol, THF, methylene chloride, and chloroform). Both methods afford a deprotonated ligand and a complex absent of bromide anions (confirmed by X-ray crystallography, vide infra). The two
fac-coordinating, tridentate anionic bis(pyrazolyl) acetate ligands compensate for the + 2 charge on the copper ion. Complex 2 is highly hygroscopic, quickly collecting water from air to deliquesce as a dissolved aqueous solution.
Spectroscopy
The IR data presented in the "Experimental" section are consistent with a complexed ligand. The most striking feature of the spectrum is the carbonyl stretch at 1744 cm−1 in [Cu(LH)Br(µ-Br)]2 (1) due to the uncoordinated carboxylic acid. There is also a broad absorption from 3500 to 2800 cm−1consistent with overlapping stretches of the carboxylic acid OH and residual water. The UV–vis spectrum of 1 indicates a strong ligand absorption at 303 nm with an extinction coefficient of 222,000 cm−1 M−1. The d–d transitions are much weaker at 749 nm with a coefficient of 8200 cm−1 M−1.
In complex 2, formulated as [Cu(L)2]·4H2O, the carbonyl stretch is shifted to lower energy (1644 cm−1) as compared to 1, consistent with a coordinated carboxylate ligand (L). The color of the solution for complex 2 diminishes in its intensity changing from dark brown to an aqua color. The UV–vis spectrum for 2 shows a slight change in the energy of the bands with absorptions at 311 nm (ε=39,700 cm−1 M−1) and the d–d transitions observed at 730 nm (ε=1590 cm−1 M−1). The color change is likely a consequence of the five-fold change in extinction coefficient observed between the two
complexes.
Crystal Structure Description
The molecular structures of the bridged copper complex [Cu(L-H)Br(µ-Br)]2 (1) and the bis-ligated complex Cu(L)2(2) were determined by single-crystal X-ray analysis as shown in Figs. 1 and 3. Selected bond lengths and angles are presented in Tables 1, 2 and 3, while parameters related to data collection and refinement are shown in Table 4. The ellipsoid displacement plot of [Cu(L-H)Br(µ-Br)]2(1) is shown in Fig. 1. Selected bond distances and angles are given in Table 1. To our knowledge, compound 1 constitutes the first bis(pyrazol-1-yl)acetate copper(II) bromide complex, including those with substituted pyrazolyl ligands. The complex crystalizes in the monoclinic C2/c space group, a=15.749 (14) Å, b=9.854 (9) Å, c=15.880 (14) Å, β=102.629 (11)° and Z=4. The copper metal center is five-coordinate with one bridging (Br1) and one terminal (Br2) bromide. The structure adopts an intermediate geometry between square pyramidal and trigonal bipyramidal with a τ value of 0.42, slightly favoring square pyramidal.
The Cu1–Br1 bond distance is 2.418 (2) Å, whereas the terminal distance between Cu1–Br2 is 2.403 (3) Å. The remainder of the metal coordination environment is constituted by a symmetry-related bridging bromide (Br1i) and two nitrogen atoms from the ligand (L) (N1 and N3). The Cu1–Br1i bond distance is 2.721 (3) Å, whereas the Cu1–N1 and Cu1–N3 bond lengths are 2.010 (7) Å is 2.044 (6) Å, respectively. There is a long range Cu1–O1 interaction at 3.174 (6) Å, which is substantially longer than the sum of their covalent radii (1.98 Å) [19]. The corresponding metal center angles are Br1–Cu1–Br2 92.22 (5)°, N1–Cu1–Br1 178.76 (19)°, N1–Cu1–Br2 88.8 (2)°, N3–Cu1–Br1 91.91(18)°, N3–Cu1–Br2 153.5 (2)°, N3–Cu1–N1 86.9 (3)° and Br1–Cu1–Br1i 87.52 (4)°. Compound 1 packs in a herringbone pattern with the zigzag running along the c-axis, the herringbone layer-to-layer distance is 5.822 (4) Å. The herringbone bend angle is 72.44 (7)°, see Fig. 2.
The ORTEP view of Cu(L)2 (2) is presented in Fig. 3. Selected bond distances and angles are given in Table 2. The molecule crystalizes in the triclinic space group P-1 with two molecules per unit cell, however, Fig. 3 only displays one molecule within the unit cell for simplification due to their similar nature. The single-crystal X-ray structure of compound 2 has been previously reported as a co-crystalized product of a heterobimetallic coordination polymer, [(L)2Cu] [{Cu(L)2}-{n-Bu2Sn(H2O)2}]n [6] and its bis(3,5-dimethylpyrazol-1-yl)acetate congener has also been reported [5]. To our knowledge, this report is the first structurallycharacterized discrete, well-defined, bis[bis(pyrazol-1-yl) acetate] copper (II) compound. The single-crystal X-ray structure provides details on the structure and bonding of 2. The two unique copper atoms in 2 sit on special positions and have a second symmetry-related ligand (L) per copper formulated as the bis-ligated copper(II) complex.
Four waters of hydration are found in the complex. It is noteworthy that the crystal has to be coated in order to prohibit further deterioration, presumably because of its significant deliquescent behavior. The copper center resides in a six-coordinate pseudo-octahedral environment with the bond angles significantly constrained arising from a single ligand (L). The N1–Cu1–O1 and N3–Cu1–N1 bond angles are 85.58 (9)° and 88.64 (11)°, respectively, while the N3–Cu1–O1 bond angle is constrained to 79.76 (9)° in the molecule containing the Cu1 atom. Similarly, these features are consistent in the discrete complex containing the Cu2 atom with the N7–Cu2–N5, N7–Cu2–O3 and N5–Cu2–O3 bond angles being 88.07 (10)°, 84.52 (10)° and 81.13 (10)°, respectively. The copper-pyrazole Cu–N bond distances (Cu1–N1=2.035 (3) Å; Cu1–N3=1.972 (2) Å; Cu2–N5i=2.011 (3) Å, Cu2–N7=2.006 (3) Å) were found to be significantly shorter (~0.4 Å) in both molecules than the copper–acetate C–O bond distances (Cu1–O1=2.430 (3) Å; Cu2–O3 = 2.405 (3) Å). This follows expected Jahn–Teller distortion for octahedral CuN4O2 complexes, wherein the C–O bonds elongate the octahedron [5]. The water molecules in 2 are configured in a hydrogen bonding network between themselves and the carbonyl unit of the bis(pyrazolyl)acetate ligands (L) (Fig. 4). A list of hydrogenbonding geometries for 2 are shown in Table 3. A comparison of the bond distances and angles of 2 with those in the water free complex [(L)2Cu][{Cu(L)2}-{n-Bu2Sn(H2O)2}]n(2·[Sn]) reveals the C–O distances in 2 are longer than that of its previously reported analogue (2.381 Å), which may be the result of the carbonyl oxygen engaging in hydrogen bonding with water. The C–N bonds in 2 are statistically equivalent to those in 2·[Sn], albeit on the longer side. Both 2 and 2·[Sn] share Jahn–Teller distortions, with elongation along the C-O bonds and widened O–Cu–N bond angles (97.162 (6)° for 2·[Sn], 94.42 (9)° for 2) as compared to ideal octahedral. Both complexes have O–C–O bond angles that are essentially linear.
Conclusion and Summary
Two new copper complexes supported by bis(pyrazol-1-yl) acetic acid have been disclosed. Both compounds represent the first, well-defined, independent copper(II) compounds supported by the bis(pyrazolyl)acetic acid ligand. Compound 1, to our knowledge, is the first CuBr2 complex supported by any of the ligands included in the bis(pyrazolyl) acetic acid genre of ligands. These complexes add to the collection of copper complexes reported in the literature. Acknowledgements BQ would like to acknowledge the American Chemical Society Petroleum Research Fund for support or partial support of this research (PRF# 53848-UNI3). Compliance with Ethical Standards Conflict of interest The authors declare no competing financial interest.
N-(3,5-Dibromophenyl)pivalamideCatalog No.:AA0006A2 CAS No.:1020252-74-7 MDL No.:MFCD09972089 MF:C11H13Br2NO MW:335.0350 |
Benzyl 3-bromo-5-nitrophenylcarbamateCatalog No.:AA0006A1 CAS No.:1020252-75-8 MDL No.:MFCD01013391 MF:C14H11BrN2O4 MW:351.1521 |
N-Benzyl 4-bromo-3-methylbenzamideCatalog No.:AA0006A0 CAS No.:1020252-76-9 MDL No.:MFCD09972121 MF:C15H14BrNO MW:304.1818 |
N-Isopropyl 4-bromo-3-methylbenzamideCatalog No.:AA00069Z CAS No.:1020252-77-0 MDL No.:MFCD09972122 MF:C11H14BrNO MW:256.1390 |
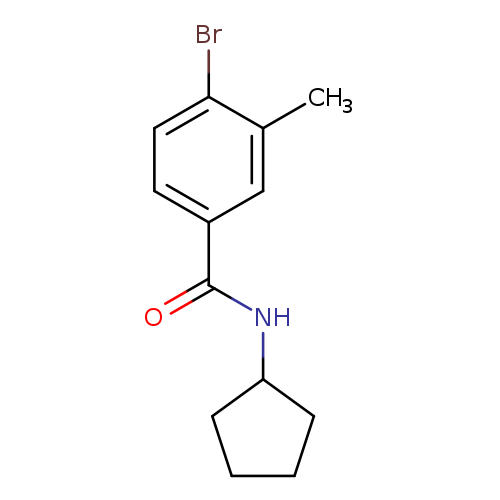
N-Cyclopentyl 4-bromo-3-methylbenzamideCatalog No.:AA00069Y CAS No.:1020252-78-1 MDL No.:MFCD09972124 MF:C13H16BrNO MW:282.1762 |
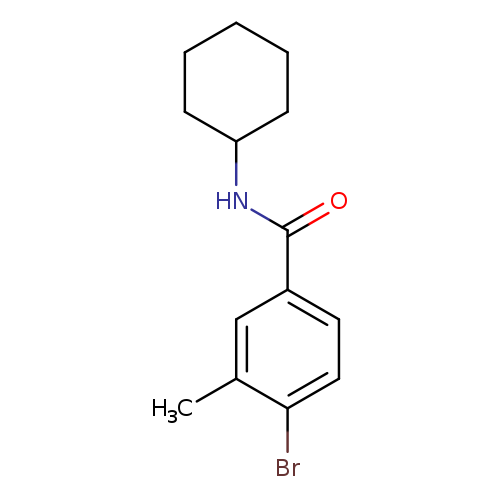
N-Cyclohexyl-4-bromo-3-methylbenzamideCatalog No.:AA00069W CAS No.:1020252-80-5 MDL No.:MFCD09972128 MF:C14H18BrNO MW:296.2028 |
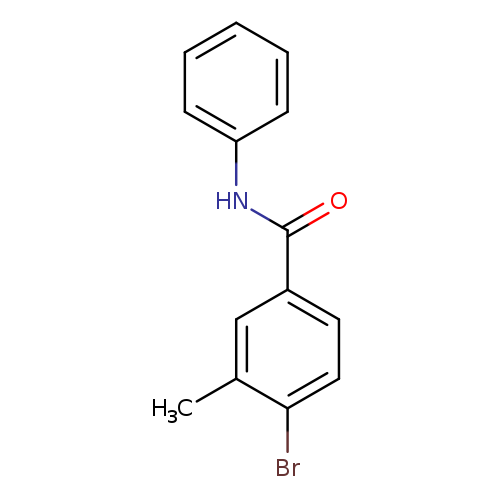
N-Phenyl 4-bromo-3-methylbenzamideCatalog No.:AA00069V CAS No.:1020252-81-6 MDL No.:MFCD09972129 MF:C14H12BrNO MW:290.1552 |
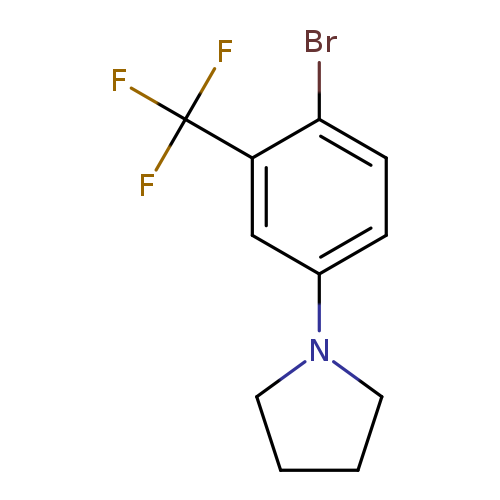
1-(4-Bromo-3-trifluoromethylphenyl)pyrrolidineCatalog No.:AA00069Q CAS No.:1020252-86-1 MDL No.:MFCD09972140 MF:C11H11BrF3N MW:294.1109 |
(5-(3-Chlorophenyl)oxazol-4-yl)methanolCatalog No.:AA00069O CAS No.:1020252-88-3 MDL No.:MFCD09972143 MF:C10H8ClNO2 MW:209.6290 |
N-(Furan-2-ylmethyl) 3-bromo-5-methylbenzenesulfonamideCatalog No.:AA00069M CAS No.:1020252-90-7 MDL No.:MFCD09972146 MF:C12H12BrNO3S MW:330.1976 |
N,N-Dimethyl 3-bromo-5-methylbenzenesulfonamideCatalog No.:AA0006AY CAS No.:1020252-92-9 MDL No.:MFCD09972148 MF:C9H12BrNO2S MW:278.1661 |
N-Butyl 3-bromo-5-methylbenzenesulfonamideCatalog No.:AA0006AX CAS No.:1020252-93-0 MDL No.:MFCD09972149 MF:C11H16BrNO2S MW:306.2192 |
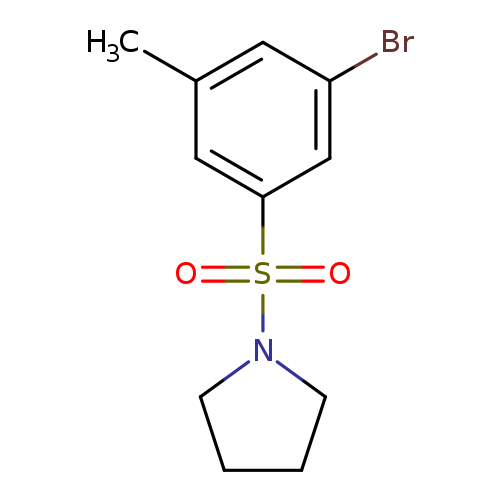
1-(3-Bromo-5-methylphenylsulfonyl)pyrrolidineCatalog No.:AA0006AU CAS No.:1020252-96-3 MDL No.:MFCD09972152 MF:C11H14BrNO2S MW:304.2034 |
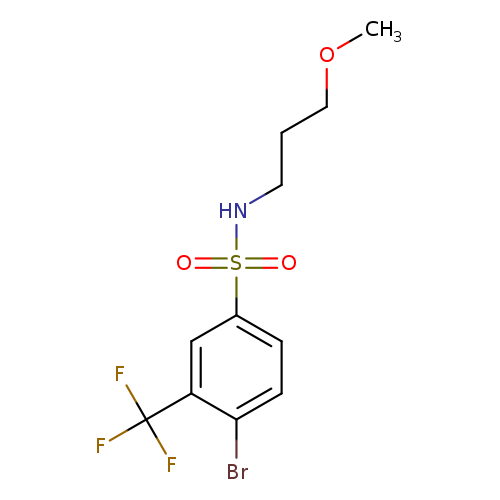
N-(3-Methoxypropyl) 4-bromo-3-trifluoromethylbenzenesulfonamideCatalog No.:AA0006AS CAS No.:1020252-98-5 MDL No.:MFCD09972154 MF:C11H13BrF3NO3S MW:376.1900 |
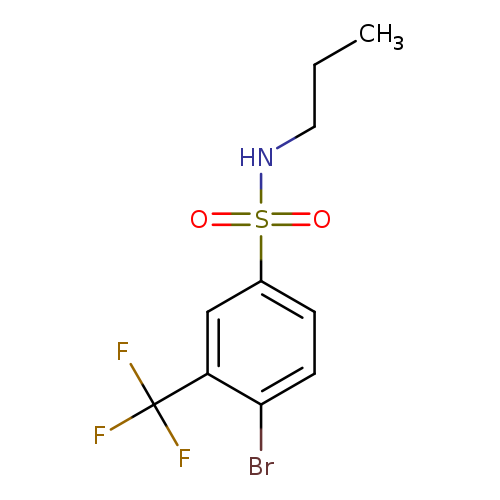
N-Propyl 4-Bromo-3-trifluoromethylbenzenesulfonamideCatalog No.:AA0006AQ CAS No.:1020253-00-2 MDL No.:MFCD09972156 MF:C10H11BrF3NO2S MW:346.1640 |
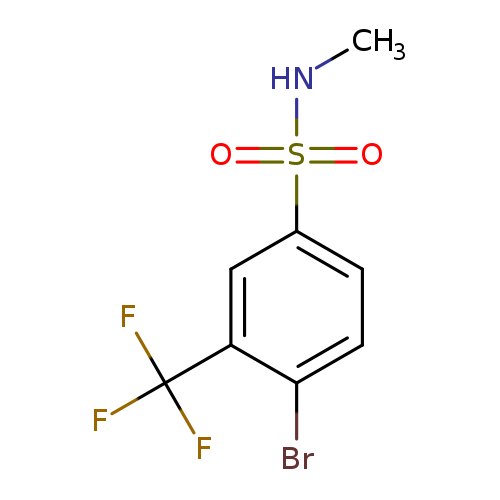
N-Methyl 4-bromo-3-trifluoromethylbenzenesulfonamideCatalog No.:AA0006AP CAS No.:1020253-01-3 MDL No.:MFCD09972157 MF:C8H7BrF3NO2S MW:318.1109 |
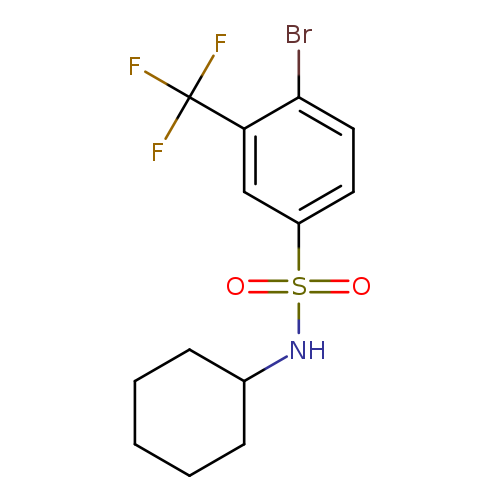
4-Bromo-N-cyclohexyl-3-(trifluoromethyl)benzenesulfonamideCatalog No.:AA0006AO CAS No.:1020253-02-4 MDL No.:MFCD09972158 MF:C13H15BrF3NO2S MW:386.2279 |
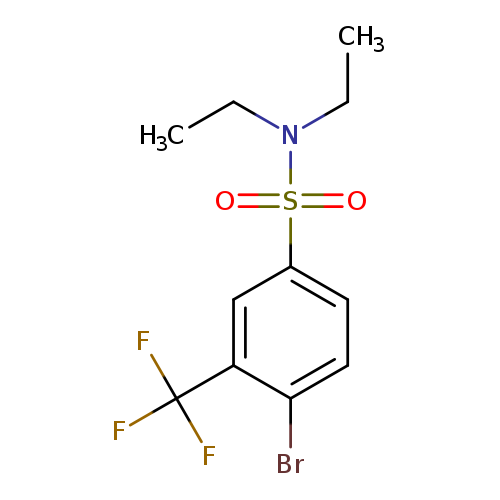
N,N-Diethyl 4-bromo-3-trifluoromethylbenzenesulfonamideCatalog No.:AA0006AN CAS No.:1020253-03-5 MDL No.:MFCD09972159 MF:C11H13BrF3NO2S MW:360.1906 |
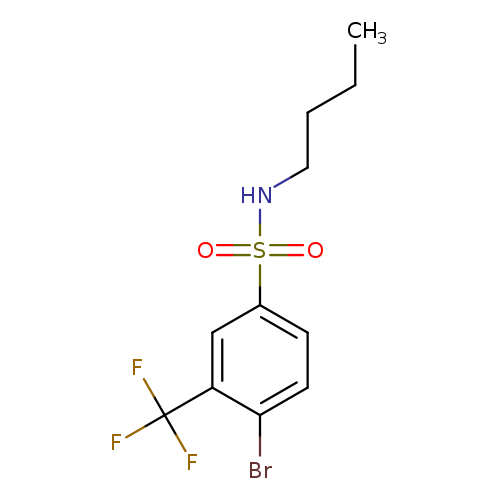
4-Bromo-N-butyl-3-(trifluoromethyl)benzenesulfonamideCatalog No.:AA0006AL CAS No.:1020253-05-7 MDL No.:MFCD09972161 MF:C11H13BrF3NO2S MW:360.1906 |
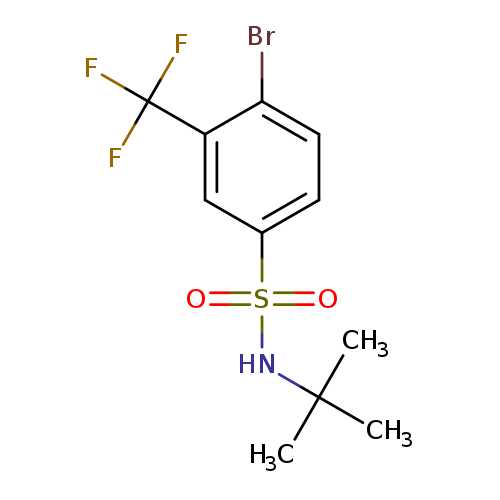
4-Bromo-N-tert-butyl-3-(trifluoromethyl)benzenesulfonamideCatalog No.:AA0006AK CAS No.:1020253-06-8 MDL No.:MFCD09972162 MF:C11H13BrF3NO2S MW:360.1906 |
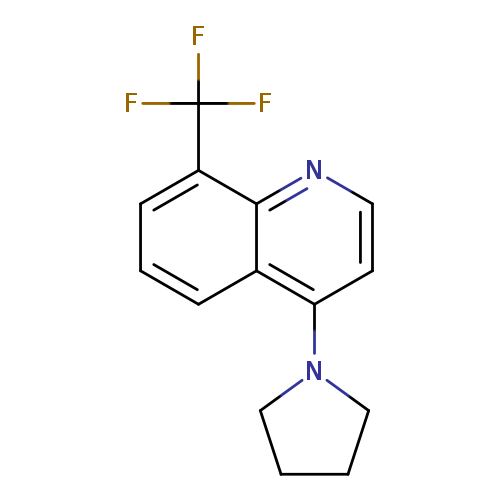
4-(Pyrrolidin-1-yl)-8-(trifluoromethyl)quinolineCatalog No.:AA0006AJ CAS No.:1020253-07-9 MDL No.:MFCD09972163 MF:C14H13F3N2 MW:266.2616 |
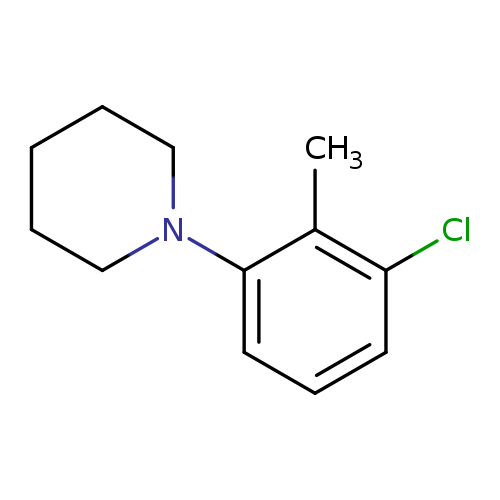
1-(3-Chloro-2-methylphenyl)piperidineCatalog No.:AA0006AI CAS No.:1020253-08-0 MDL No.:MFCD09972164 MF:C12H16ClN MW:209.7151 |
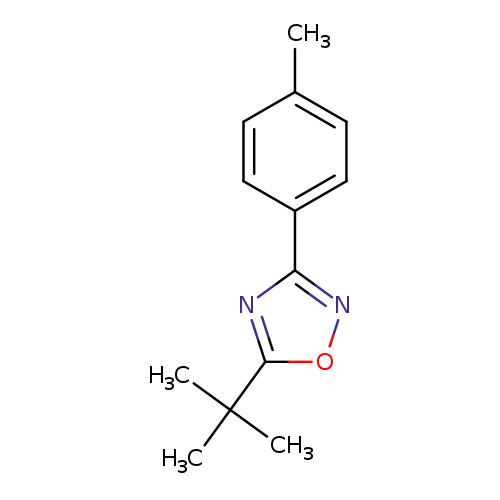
5-tert-Butyl-3-p-tolyl-1,2,4-oxadiazoleCatalog No.:AA0006AG CAS No.:1020253-10-4 MDL No.:MFCD02310122 MF:C13H16N2O MW:216.2789 |
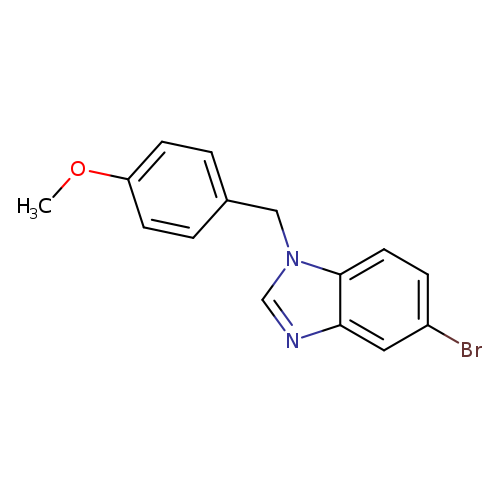
5-Bromo-1-(4-methoxybenzyl)-1H-benzo[d]imidazoleCatalog No.:AA0006AF CAS No.:1020253-11-5 MDL No.:MFCD09972169 MF:C15H13BrN2O MW:317.1805 |
(4-Bromo-2-fluorophenyl)(4-methoxybenzyl)sulfaneCatalog No.:AA0006AE CAS No.:1020253-12-6 MDL No.:MFCD09972171 MF:C14H12BrFOS MW:327.2119 |
1-Bromo-4,4-dimethylcyclohex-1-eneCatalog No.:AA0006AD CAS No.:1020253-13-7 MDL No.:MFCD09972172 MF:C8H13Br MW:189.0928 |
6-Chloro-5-fluoronicotinonitrileCatalog No.:AA0006AC CAS No.:1020253-14-8 MDL No.:MFCD09972184 MF:C6H2ClFN2 MW:156.5449 |
4-Bromo-2-chloro-5-methoxypyridineCatalog No.:AA0006AB CAS No.:1020253-15-9 MDL No.:MFCD09972186 MF:C6H5BrClNO MW:222.4670 |
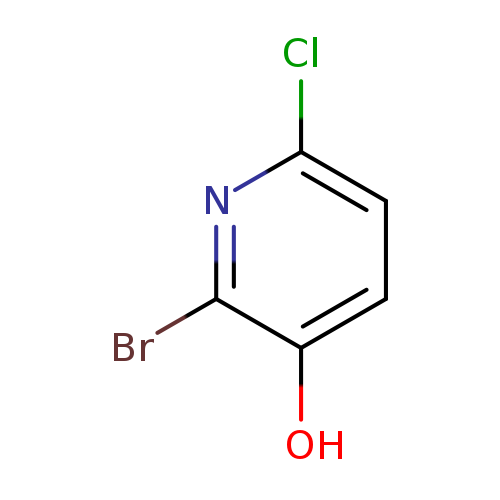
2-Bromo-6-chloro-3-hydroxypyridineCatalog No.:AA0006BH CAS No.:1020253-16-0 MDL No.:MFCD09972190 MF:C5H3BrClNO MW:208.4404 |
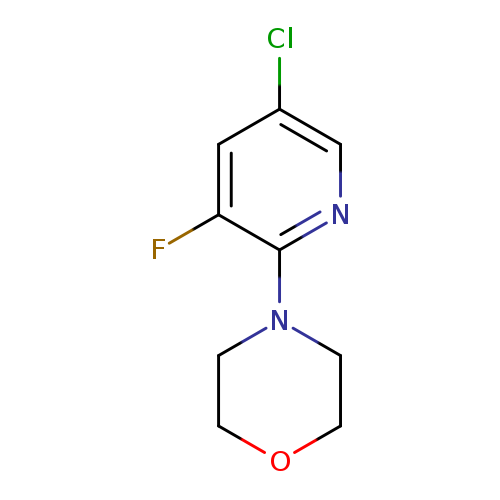
4-(5-Chloro-3-fluoro-2-pyridinyl)morpholineCatalog No.:AA0006BG CAS No.:1020253-17-1 MDL No.:MFCD09972191 MF:C9H10ClFN2O MW:216.6399 |
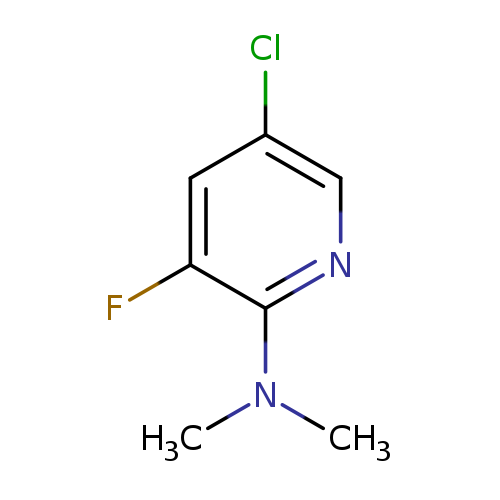
5-Chloro-2-(N,N-dimethylamino)-3-fluoropyridineCatalog No.:AA0006BE CAS No.:1020253-19-3 MDL No.:MFCD09972193 MF:C7H8ClFN2 MW:174.6032 |
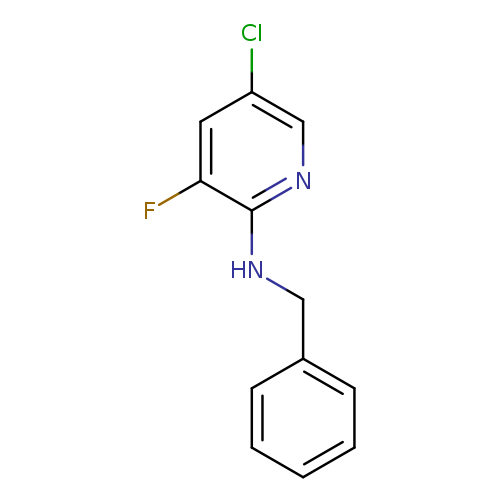
2-(N-Benzylamino)-5-chloro-3-fluoropyridineCatalog No.:AA0006BD CAS No.:1020253-20-6 MDL No.:MFCD09972194 MF:C12H10ClFN2 MW:236.6726 |
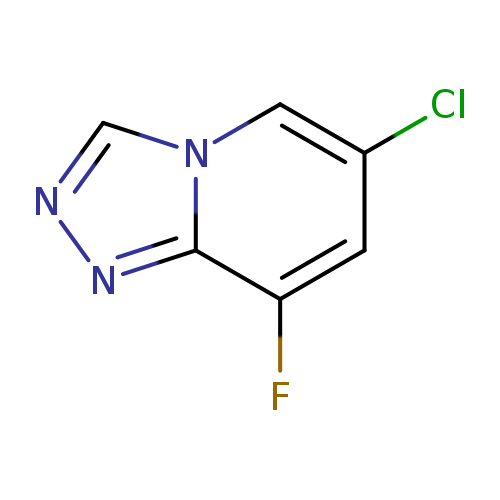
6-Chloro-8-fluoro-[1,2,4]triazolo[4,3-a]pyridineCatalog No.:AA0006BC CAS No.:1020253-21-7 MDL No.:MFCD09972195 MF:C6H3ClFN3 MW:171.5595 |
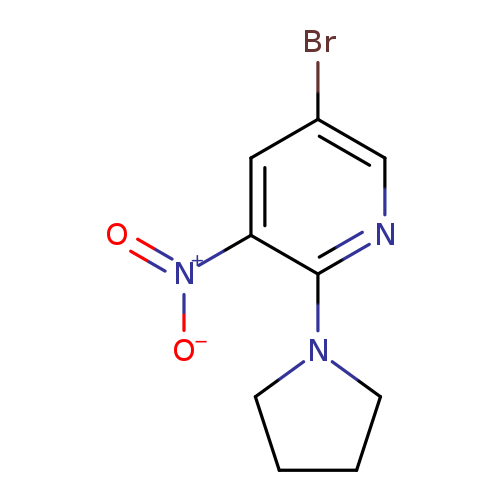
4-(5-Bromo-3-nitropyridin-2-yl)pyrrolidineCatalog No.:AA0006BB CAS No.:1020253-22-8 MDL No.:MFCD22123606 MF:C9H10BrN3O2 MW:272.0986 |
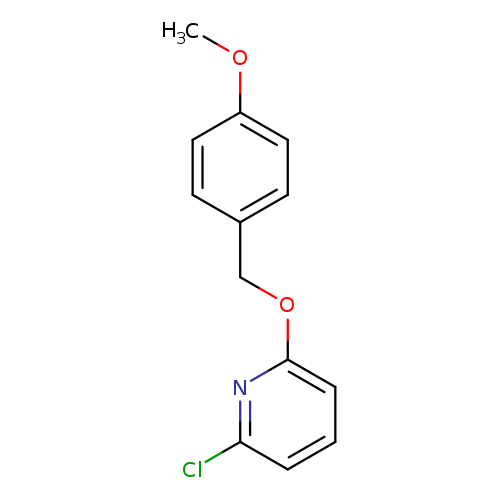
2-Chloro-6-(4-methoxybenzyloxy)pyridineCatalog No.:AA0006BA CAS No.:1020253-23-9 MDL No.:MFCD09972200 MF:C13H12ClNO2 MW:249.6929 |
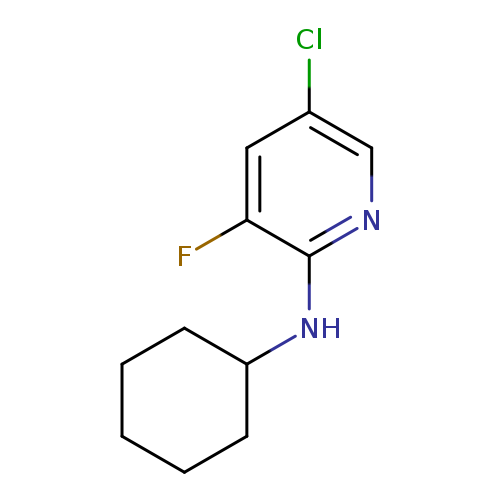
5-Chloro-2-cyclohexylamino-3-fluoropyridineCatalog No.:AA0006B9 CAS No.:1020253-24-0 MDL No.:MFCD09972201 MF:C11H14ClFN2 MW:228.6937 |
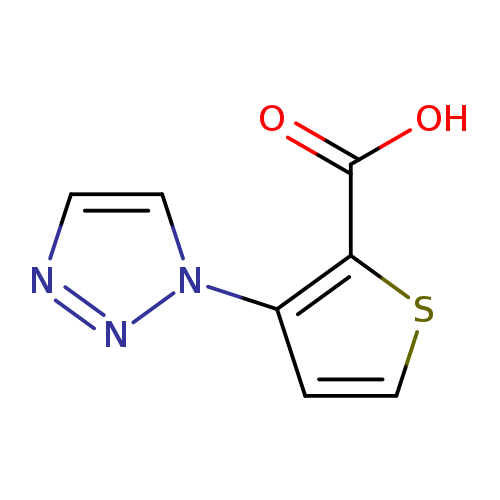
3-(1H-1,2,3-triazol-1-yl)thiophene-2-carboxylic acidCatalog No.:AA01ACA7 CAS No.:1020253-47-7 MDL No.:MFCD09260337 MF:C7H5N3O2S MW:195.1985 |
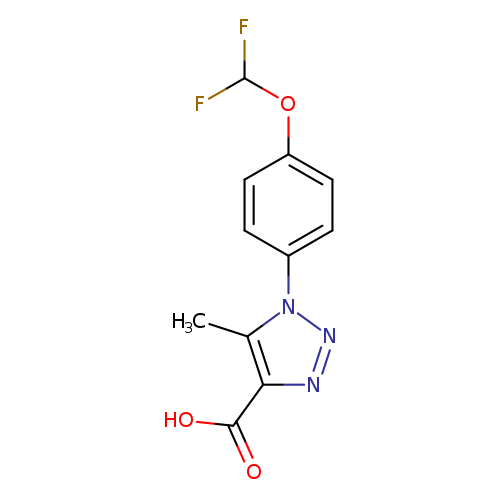
1-[4-(difluoromethoxy)phenyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acidCatalog No.:AA01AGTL CAS No.:1020253-48-8 MDL No.:MFCD09260343 MF:C11H9F2N3O3 MW:269.2043 |
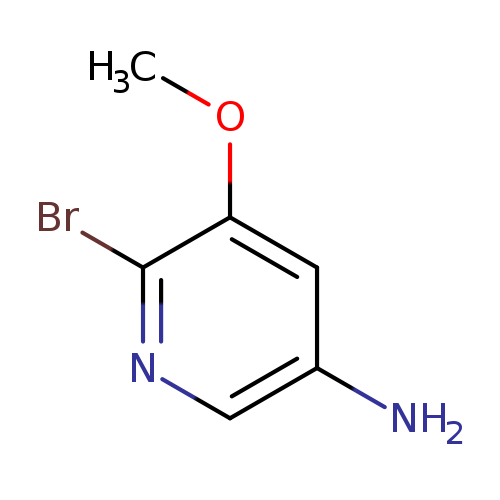
6-Bromo-5-methoxypyridin-3-amineCatalog No.:AA0006B5 CAS No.:1020253-85-3 MDL No.:MFCD21603641 MF:C6H7BrN2O MW:203.0366 |
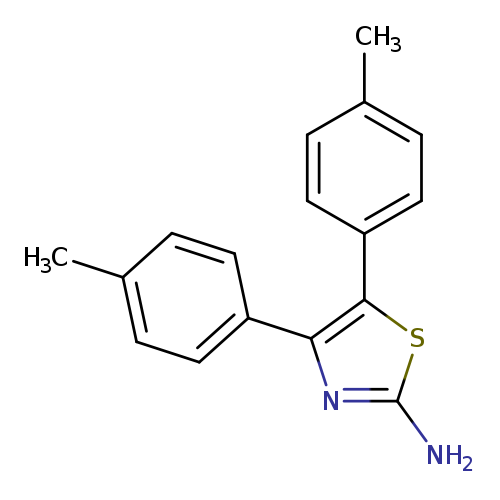
2-Thiazolamine, 4,5-bis(4-methylphenyl)-Catalog No.:AA0006BO CAS No.:102026-45-9 MDL No.:MFCD03655002 MF:C17H16N2S MW:280.3873 |
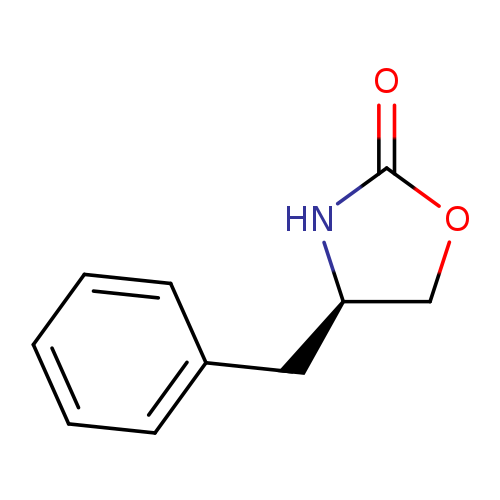
(R)-4-Benzyl-2-oxazolidinoneCatalog No.:AA0006BM CAS No.:102029-44-7 MDL No.:MFCD00010846 MF:C10H11NO2 MW:177.1998 |
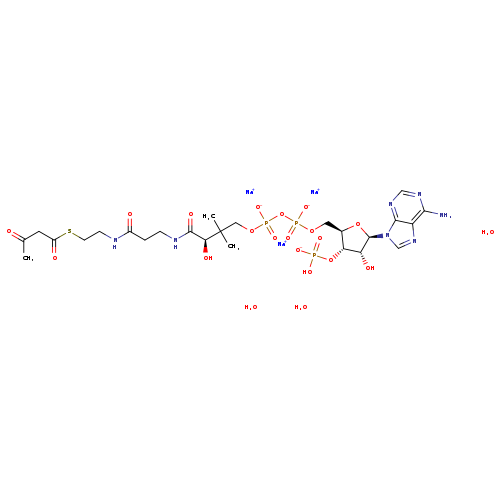
ACETOACETYL COENZYME A SODIUM SALTCatalog No.:AA008YFY CAS No.:102029-52-7 MDL No.:MFCD00084766 MF:C25H43N7Na3O21P3S MW:971.5988 |
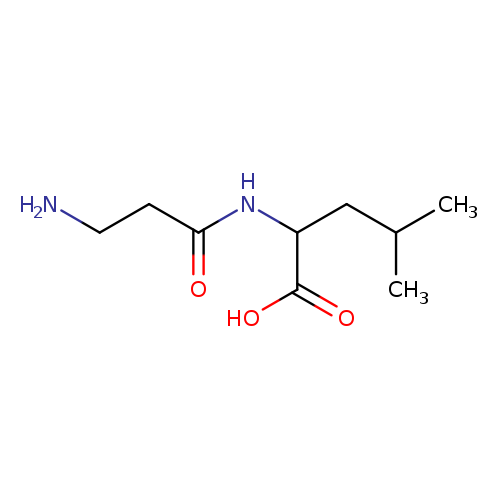
H-BETA-ALA-DL-LEU-OHCatalog No.:AA008XLF CAS No.:102029-56-1 MDL No.:MFCD00025609 MF:C9H18N2O3 MW:202.2508 |
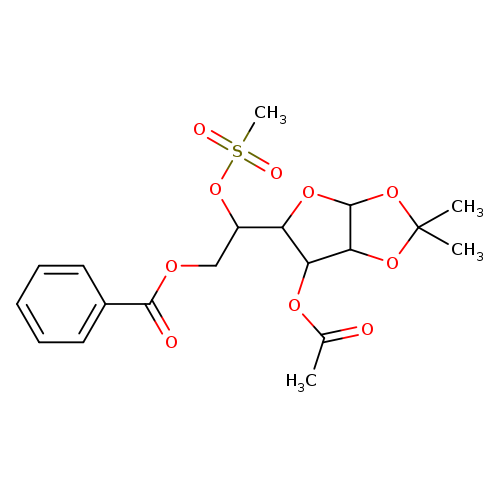
3-O-ACETYL-6-O -BENZOYL-5-O-(METHYLSULFONYL)-1,2-O-ISOPROPYLIDENE-ALPHA-D-GLUCOFURANOSECatalog No.:AA008VED CAS No.:102029-58-3 MDL No.:MFCD00074967 MF:C19H24O10S MW:444.4529 |
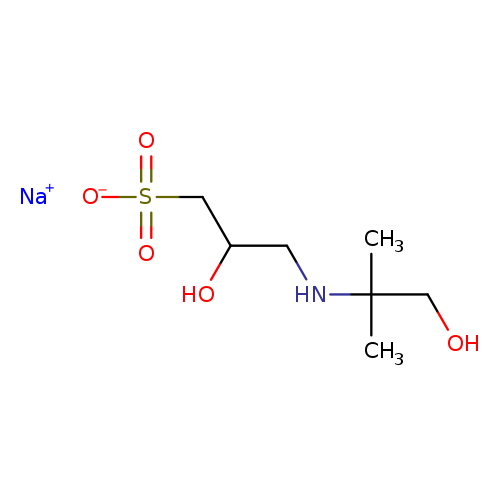
1-Propanesulfonic acid, 2-hydroxy-3-[(2-hydroxy-1,1-dimethylethyl)amino]-, sodium salt (1:1)Catalog No.:AA0006BL CAS No.:102029-60-7 MDL No.:MFCD00067500 MF:C7H16NNaO5S MW:249.2604 |
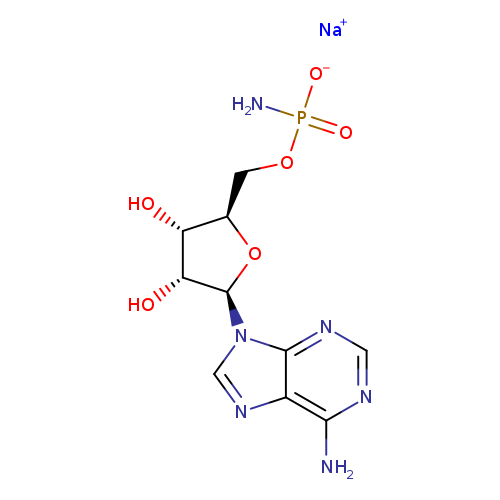
ADENOSINE 5'-MONOPHOSPHORAMIDATE SODIUM SALTCatalog No.:AA008UC3 CAS No.:102029-68-5 MDL No.:MFCD00042776 MF:C10H14N6NaO6P MW:368.2183 |
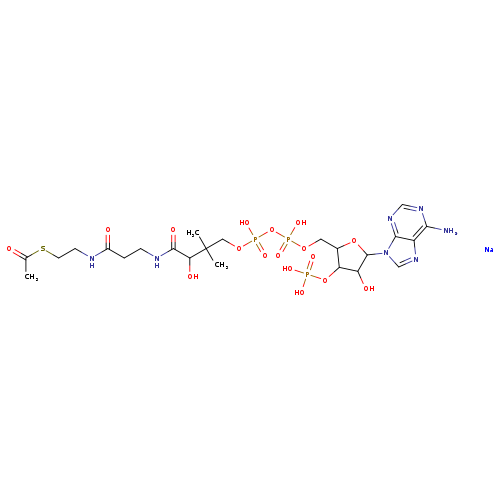
Coenzyme A, S-acetate, trisodium salt (9CI)Catalog No.:AA0006BI CAS No.:102029-73-2 MDL No.:MFCD00078858 MF:C23H38N7NaO17P3S MW:832.5606 |
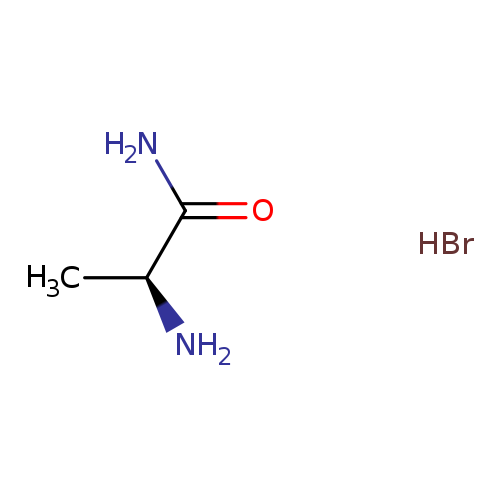
H-Ala-nh2 HBrCatalog No.:AA0006BZ CAS No.:102029-80-1 MDL No.:MFCD00050707 MF:C3H9BrN2O MW:169.0204 |
Adp di(monocyclohexylammonium) saltCatalog No.:AA0006BY CAS No.:102029-87-8 MDL No.:MFCD00058338 MF:C22H41N7O10P2 MW:625.5494 |
N-Acetyl-d-glucosamine-6-phosphate disodium saltCatalog No.:AA0006BX CAS No.:102029-88-9 MDL No.:MFCD11114536 MF:C8H16NNaO9P MW:324.1775 |
5'-Adenylic acid, anhydride with sulfuric acid, sodium salt (1:1:2)Catalog No.:AA0006BV CAS No.:102029-95-8 MDL No.:MFCD00057011 MF:C10H14N5NaO10PS MW:450.2742 |
Bzl-his-ome 2hclCatalog No.:AA009135 CAS No.:102029-99-2 MDL No.:MFCD00167636 MF:C14H19Cl2N3O2 MW:332.2256 |
3,5-DichlorobenzaldehydeCatalog No.:AA0006CA CAS No.:10203-08-4 MDL No.:MFCD00003352 MF:C7H4Cl2O MW:175.0121 |
2-DodecanolCatalog No.:AA0006C9 CAS No.:10203-28-8 MDL No.:MFCD00004551 MF:C12H26O MW:186.3342 |
Diethyl isobutylmalonateCatalog No.:AA0006C3 CAS No.:10203-58-4 MDL No.:MFCD00026869 MF:C11H20O4 MW:216.2741 |
1-Pentanol, 4-methyl-2-(methylamino)-, (2S)-Catalog No.:AA0006C0 CAS No.:10203-89-1 MDL No.:MFCD19217154 MF:C7H17NO MW:131.2160 |
1-Piperidinecarboxamide, N-3-pyridazinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methylene]-Catalog No.:AA0006BU CAS No.:1020315-31-4 MDL No.:MFCD18782721 MF:C23H20F3N5O2 MW:455.4324 |
tert-Butyl 4-(bromomethylene)piperidine-1-carboxylateCatalog No.:AA0006BP CAS No.:1020329-80-9 MDL No.:MFCD01632525 MF:C11H18BrNO2 MW:276.1701 |
Tyrosine, 2-fluoro-5-hydroxy-Catalog No.:AA0006CZ CAS No.:102034-49-1 MDL No.:MFCD08669846 MF:C9H10FNO4 MW:215.1784 |
Tetrahydro-1h-oxazolo[3,4-a]pyrazin-3(5h)-one hydrochlorideCatalog No.:AA0006CF CAS No.:1020349-31-8 MDL No.:MFCD09878828 MF:C6H11ClN2O2 MW:178.6167 |
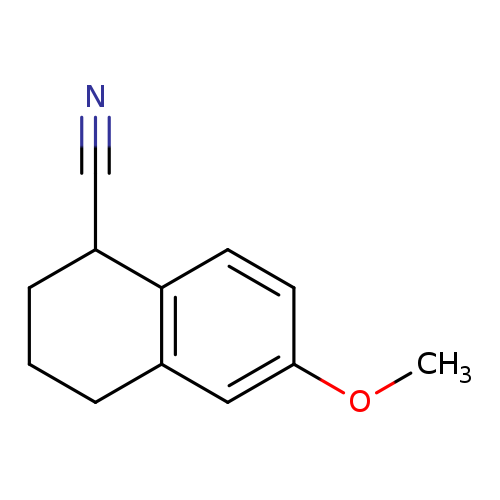
6-methoxy-1,2,3,4-tetrahydronaphthalene-1-carbonitrileCatalog No.:AA01A629 CAS No.:102035-35-8 MDL No.:MFCD11537295 MF:C12H13NO MW:187.2377 |
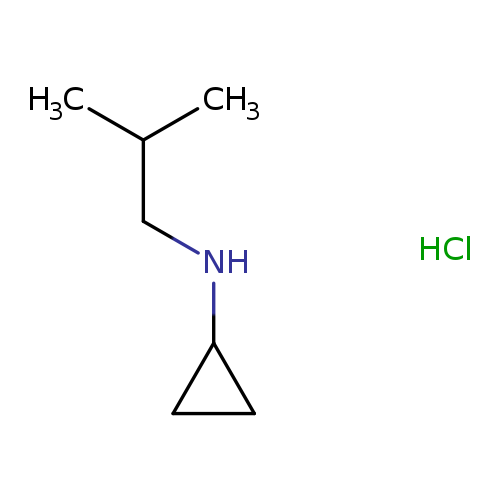
N-Cyclopropyl-n-isobutylamine hydrochlorideCatalog No.:AA0006CE CAS No.:1020353-46-1 MDL No.:MFCD09971419 MF:C7H16ClN MW:149.6616 |
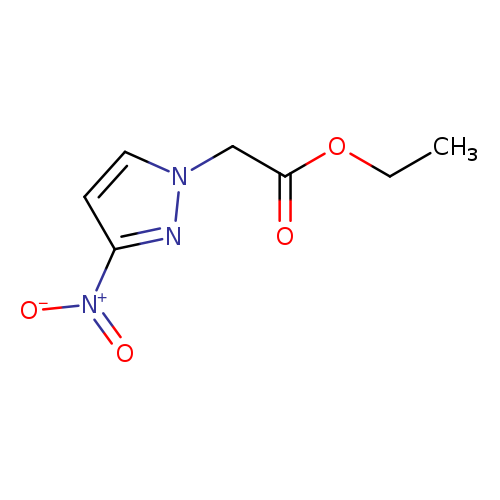
Ethyl (3-nitro-1h-pyrazol-1-yl)acetateCatalog No.:AA008RVT CAS No.:102039-43-0 MDL No.:MFCD01622828 MF:C7H9N3O4 MW:199.1641 |
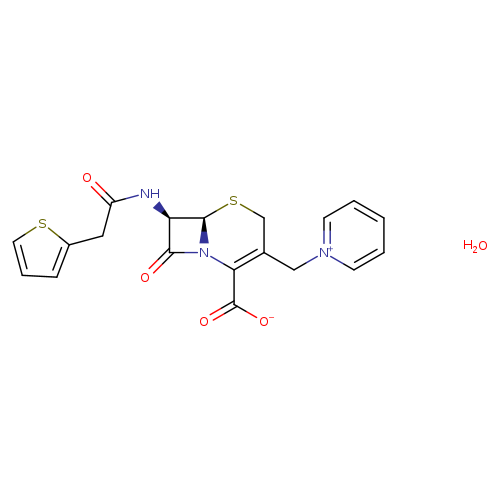
CephaloridineMonohydrateCatalog No.:AA01DZ9W CAS No.:102039-86-1 MDL No.: MF:C19H19N3O5S2 MW:433.5013 |
Carm1-in-1Catalog No.:AA008TIU CAS No.:1020399-49-8 MDL No.:MFCD23115307 MF:C26H21Br2NO3 MW:555.2578 |
Tubeimoside iCatalog No.:AA0006DF CAS No.:102040-03-9 MDL No.:MFCD03427680 MF:C63H98O29 MW:1319.4348 |
GardiquiModCatalog No.:AA008XZ9 CAS No.:1020412-43-4 MDL No.:MFCD11041176 MF:C17H23N5O MW:313.3974 |
(S)-5-(4-(((2-((1-Cyclohexylethyl)amino)-2-oxoethyl)((p-tolyloxy)carbonyl)amino)methyl)phenyl)nicotinic acidCatalog No.:AA0006D2 CAS No.:1020412-97-8 MDL No.:MFCD00216556 MF:C31H35N3O5 MW:529.6267 |
Benzyl 4-(hydroxymethyl)benzylcarbamateCatalog No.:AA0096SP CAS No.:1020415-08-0 MDL No.:MFCD11616716 MF:C16H17NO3 MW:271.3111 |
21-hydroxyoligomycin ACatalog No.:AA008SLV CAS No.:102042-09-1 MDL No.:MFCD09752734 MF:C45H74O12 MW:807.0619 |
Benzenamine, 4-(2-benzothiazolyl)-N,N-dimethyl-Catalog No.:AA0006DN CAS No.:10205-56-8 MDL No.:MFCD00937700 MF:C15H14N2S MW:254.3501 |
1-(2,3-Dichlorophenyl)propan-2-oneCatalog No.:AA0006D6 CAS No.:102052-39-1 MDL No.:MFCD03412402 MF:C9H8Cl2O MW:203.0652 |
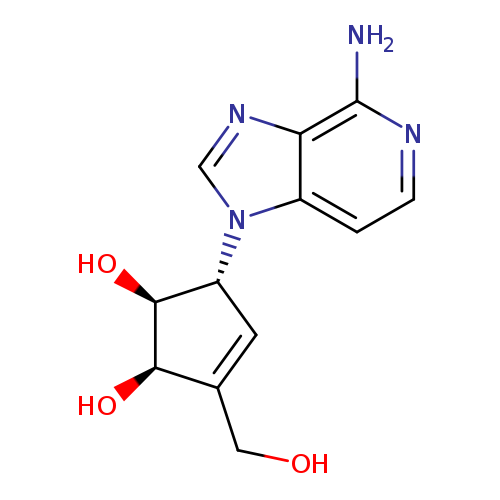
3-Deazaneplanocin ACatalog No.:AA0006ED CAS No.:102052-95-9 MDL No.:MFCD12545899 MF:C12H14N4O3 MW:262.2646 |
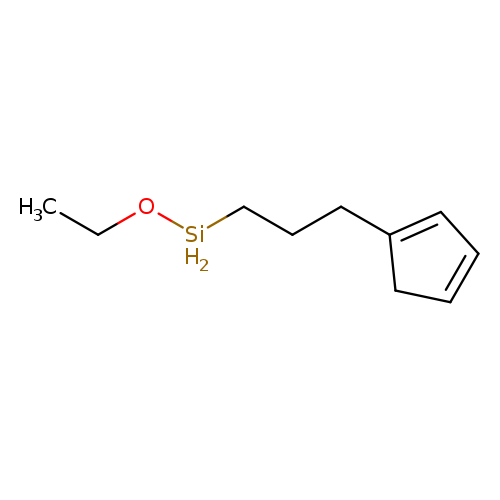
1,3-Cyclopentadiene, [3-(ethoxysilyl)propyl]-Catalog No.:AA0006E6 CAS No.:102056-64-4 MDL No.:MFCD00054919 MF:C10H18OSi MW:182.3348 |
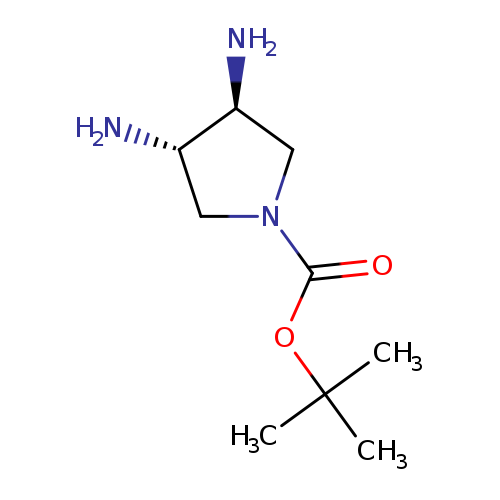
(3S,4S)-tert-Butyl 3,4-diaminopyrrolidine-1-carboxylateCatalog No.:AA0006DV CAS No.:1020571-45-2 MDL No.:MFCD14635703 MF:C9H19N3O2 MW:201.2661 |
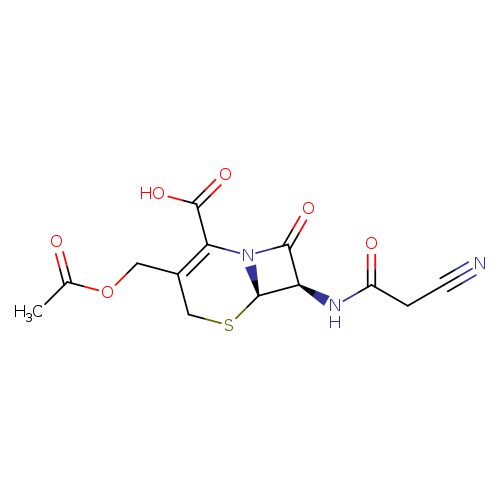
(6R,7R)-3-(Acetoxymethyl)-7-(2-cyanoacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acidCatalog No.:AA0085Q0 CAS No.:10206-21-0 MDL No.:MFCD00865099 MF:C13H13N3O6S MW:339.3238 |
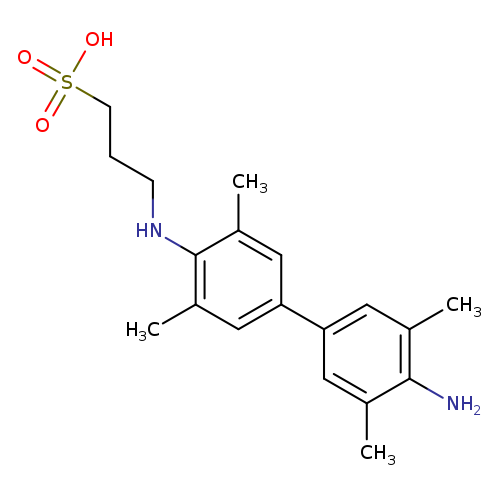
N-(3-Sulfopropyl)-3,3',5,5'-tetramethylbenzidine sodium saltCatalog No.:AA0006EZ CAS No.:102062-36-2 MDL No.:MFCD00077876 MF:C19H26N2O3S MW:362.4863 |
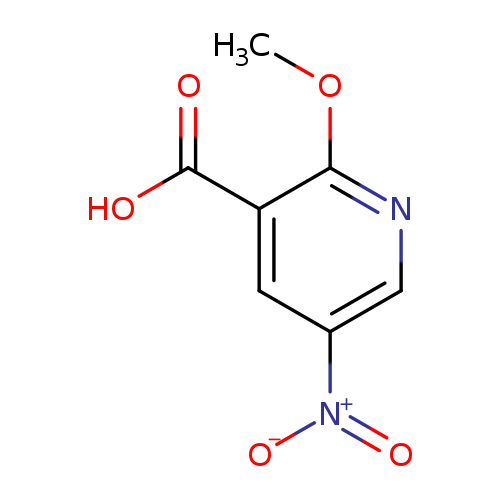
2-Methoxy-5-nitronicotinic acidCatalog No.:AA0006EJ CAS No.:1020635-54-4 MDL No.:MFCD11052413 MF:C7H6N2O5 MW:198.1329 |
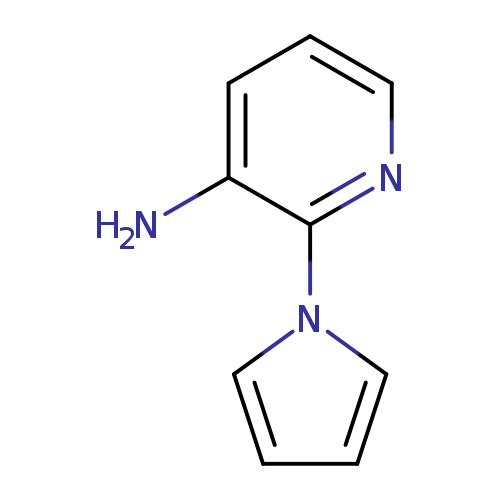
2-(1H-PYRROL-1-YL)PYRIDIN-3-AMINECatalog No.:AA01C5TB CAS No.:102064-32-4 MDL No.:MFCD20694581 MF:C9H9N3 MW:159.1879 |
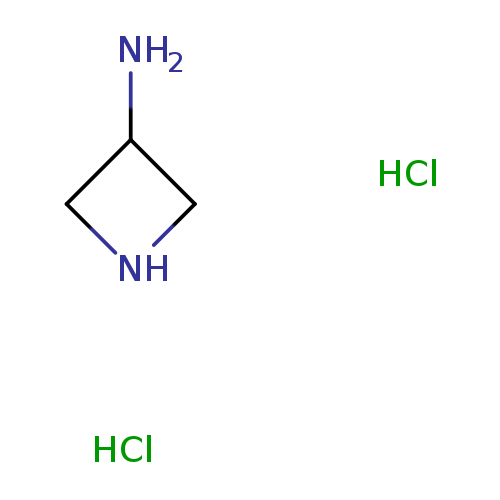
Azetidin-3-amine DiHClCatalog No.:AA0006ER CAS No.:102065-89-4 MDL No.:MFCD09910173 MF:C3H10Cl2N2 MW:145.0309 |
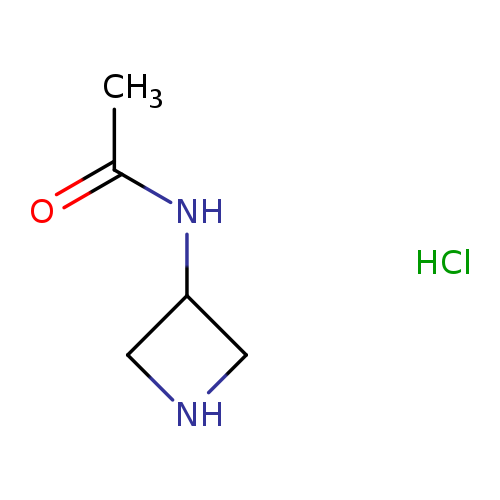
N-(Azetidin-3-yl)acetamide hydrochlorideCatalog No.:AA0006EQ CAS No.:102065-92-9 MDL No.:MFCD08059477 MF:C5H11ClN2O MW:150.6066 |
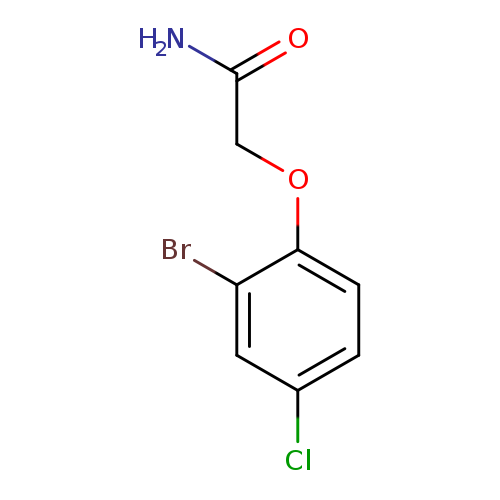
2-(2-Bromo-4-chlorophenoxy)acetamideCatalog No.:AA019WVD CAS No.:102065-98-5 MDL No.:MFCD01625285 MF:C8H7BrClNO2 MW:264.5037 |
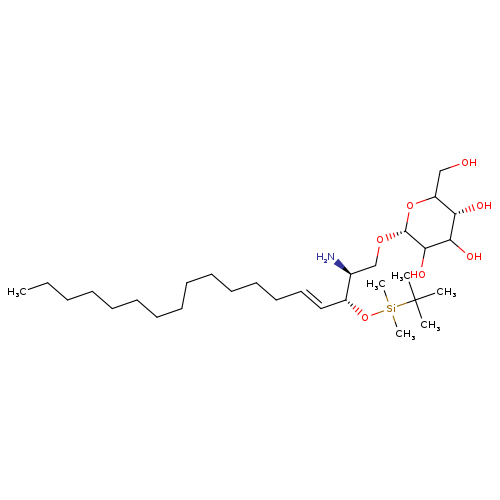
N,O3'-dibenzoylgeMcitabineCatalog No.:AA0091VV CAS No.:1020657-43-5 MDL No.: MF:C30H61NO7Si MW:575.8933 |
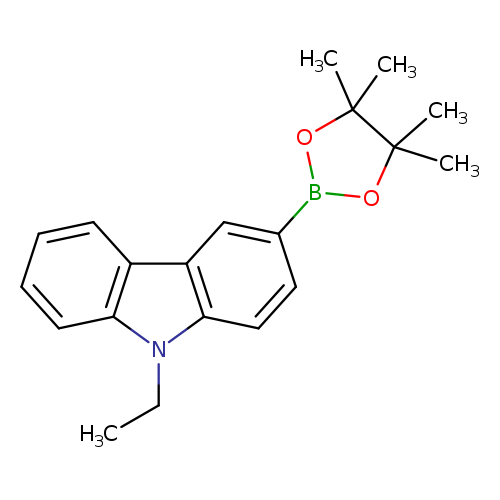
9-ethyl-3-(tetramethyl-1,3,2-dioxaborolan-2-yl)carbazoleCatalog No.:AA0093P6 CAS No.:1020657-86-6 MDL No.:MFCD19053172 MF:C20H24BNO2 MW:321.2211 |
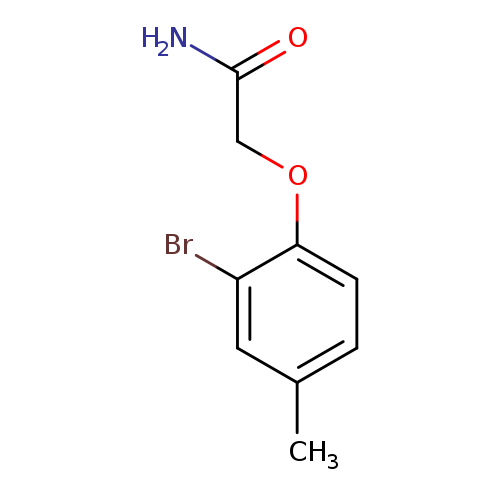
2-(2-Bromo-4-methylphenoxy)acetamideCatalog No.:AA0006EO CAS No.:102066-01-3 MDL No.:MFCD02012260 MF:C9H10BrNO2 MW:244.0852 |
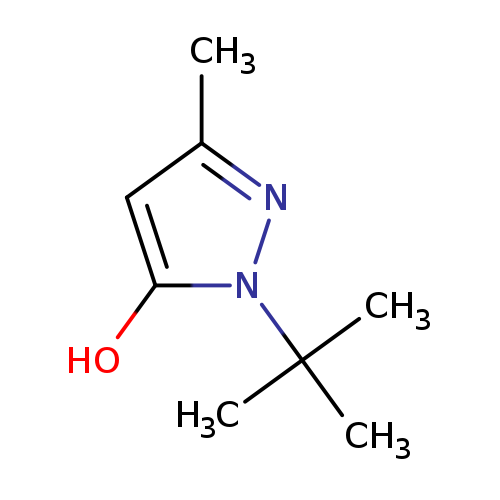
1-tert-butyl-3-methyl-1H-pyrazol-5-olCatalog No.:AA01AA2D CAS No.:1020661-22-6 MDL No.:MFCD00106918 MF:C8H14N2O MW:154.2096 |
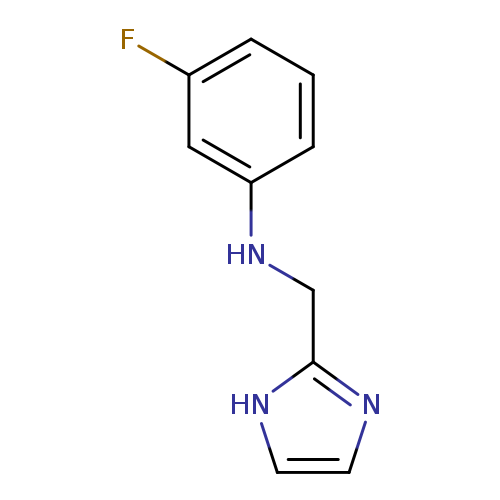
3-fluoro-N-(1H-imidazol-2-ylmethyl)anilineCatalog No.:AA019TF7 CAS No.:1020670-43-2 MDL No.:MFCD14693417 MF:C10H10FN3 MW:191.2049 |
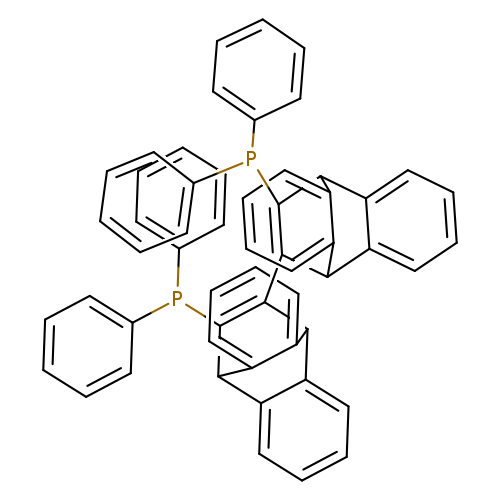
12,12'-Bis(diphenylphosphino)-9,9',10,10'-tetrahydro-11,11'-bi-9,10-ethenoanthracene, min. 98% CATPHOSCatalog No.:AA008WF1 CAS No.:1020670-88-5 MDL No.:MFCD11045085 MF:C112H46P4 MW:1549.7286 |
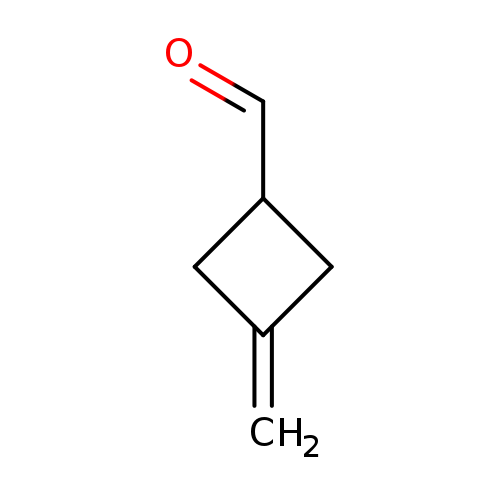
3-methylenecyclobutanecarboxaldehydeCatalog No.:AA0091MC CAS No.:1020675-51-7 MDL No.:MFCD22208481 MF:C6H8O MW:96.1271 |
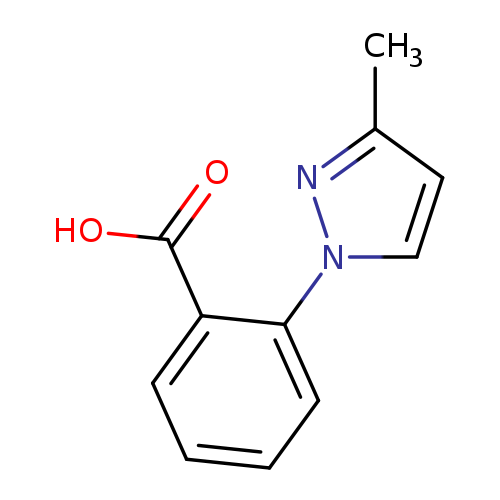
2-(3-methyl-1H-pyrazol-1-yl)benzoic acidCatalog No.:AA01AND6 CAS No.:1020703-44-9 MDL No.:MFCD06254508 MF:C11H10N2O2 MW:202.2093 |
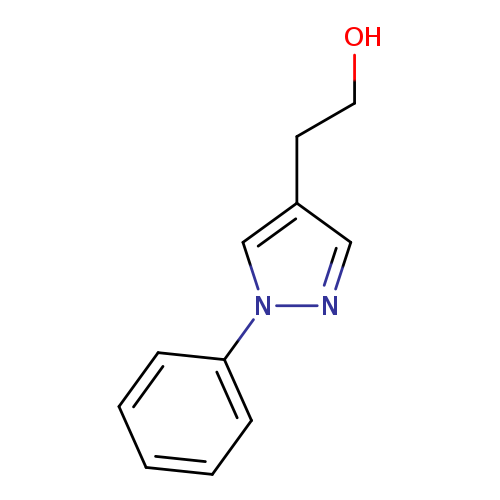
2-(1-Phenyl-1h-pyrazol-4-yl)ethan-1-olCatalog No.:AA01BH50 CAS No.:1020703-52-9 MDL No.:MFCD07440132 MF:C11H12N2O MW:188.2258 |
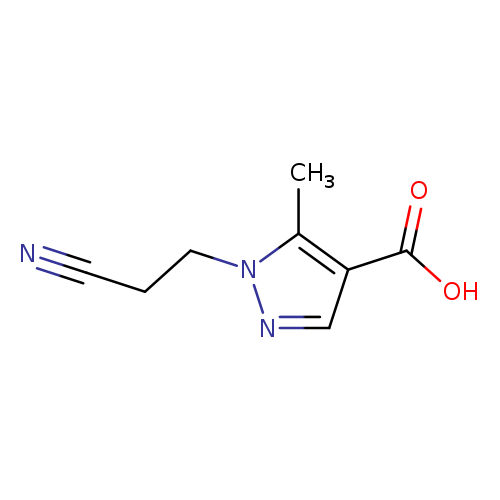
1-(2-Cyanoethyl)-5-methyl-1h-pyrazole-4-carboxylic acidCatalog No.:AA019XJP CAS No.:1020703-75-6 MDL No.:MFCD09051313 MF:C8H9N3O2 MW:179.1760 |
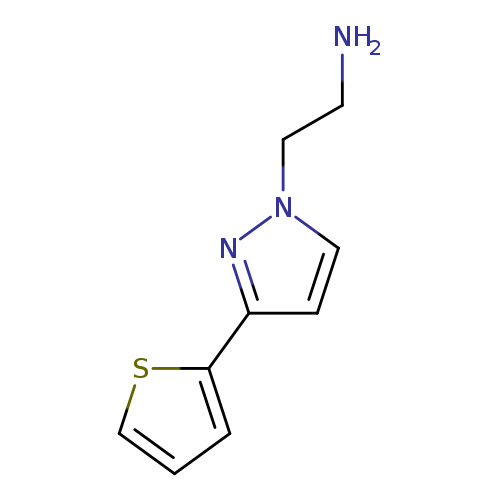
2-(3-(thiophen-2-yl)-1H-pyrazol-1-yl)ethan-1-amineCatalog No.:AA01FLSH CAS No.:1020706-50-6 MDL No.:MFCD06804815 MF:C9H11N3S MW:193.2687 |
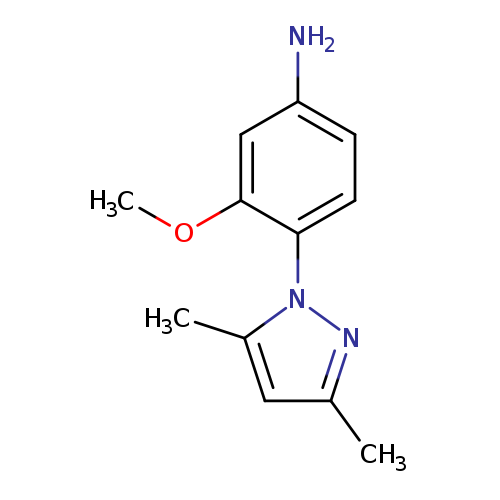
4-(3,5-dimethyl-1H-pyrazol-1-yl)-3-methoxyanilineCatalog No.:AA01EJEE CAS No.:1020713-23-8 MDL No.:MFCD07432785 MF:C12H15N3O MW:217.2670 |
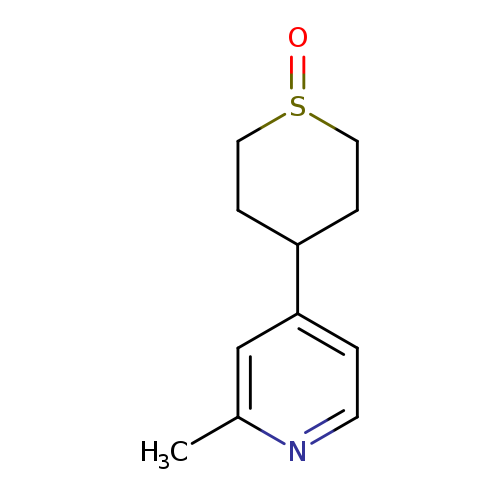
2-Methyl-4-(1-oxo-hexahydro-thiopyran-4-yl)-pyridineCatalog No.:AA01F8B8 CAS No.:1020717-37-6 MDL No.:MFCD06637785 MF:C11H15NOS MW:209.3079 |
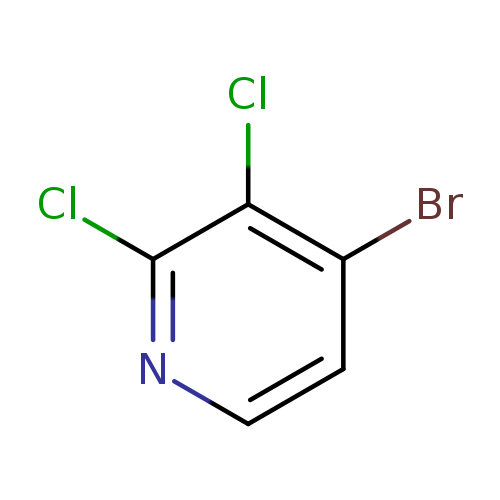
4-Bromo-2,3-dichloropyridineCatalog No.:AA0006F5 CAS No.:1020717-98-9 MDL No.:MFCD09743493 MF:C5H2BrCl2N MW:226.8861 |
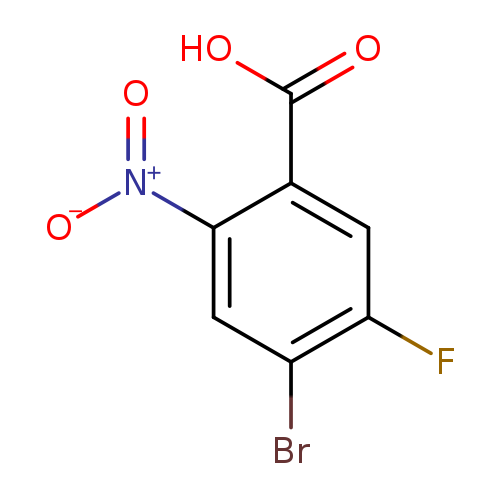
4-Bromo-5-fluoro-2-nitrobenzoic acidCatalog No.:AA0006F4 CAS No.:1020717-99-0 MDL No.:MFCD08702773 MF:C7H3BrFNO4 MW:264.0054 |
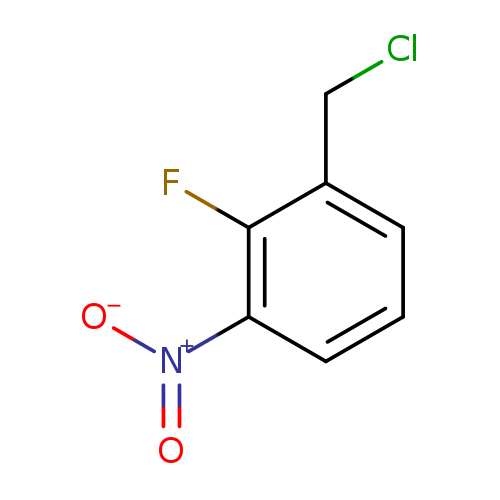
2-Fluoro-3-nitrobenzyl chlorideCatalog No.:AA0006F3 CAS No.:1020718-00-6 MDL No.:MFCD09955448 MF:C7H5ClFNO2 MW:189.5715 |
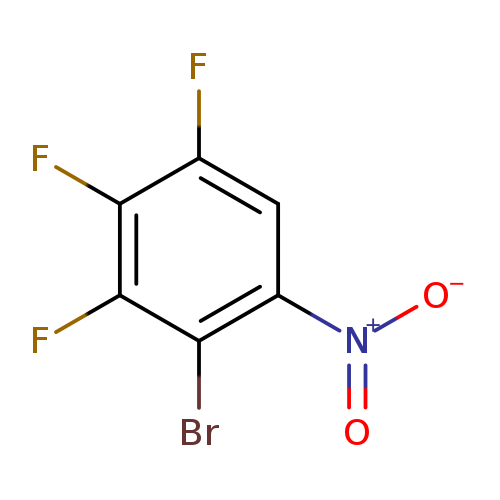
2-Bromo-3,4,5-trifluoro-1-nitrobenzeneCatalog No.:AA0006GG CAS No.:1020718-01-7 MDL No.:MFCD09955450 MF:C6HBrF3NO2 MW:255.9768 |
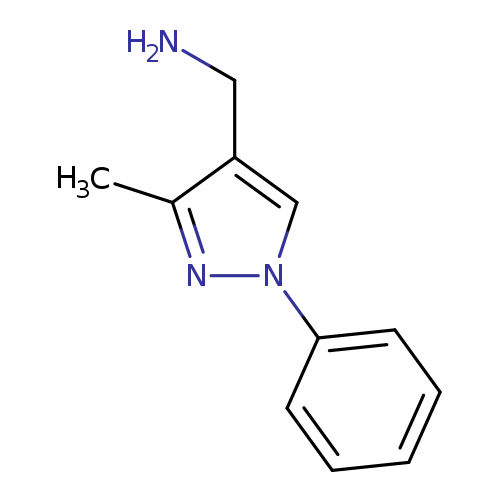
(3-Methyl-1-phenyl-1h-pyrazol-4-yl)methanamineCatalog No.:AA01A5DD CAS No.:1020718-05-1 MDL No.:MFCD06660880 MF:C11H13N3 MW:187.2410 |
2-Bromo-6-fluorobenzoyl chlorideCatalog No.:AA0006GF CAS No.:1020718-20-0 MDL No.:MFCD09038470 MF:C7H3BrClFO MW:237.4535 |
2-Pyrrolidinone, 4-(2-methoxyphenyl)Catalog No.:AA0006GD CAS No.:1020718-50-6 MDL No.:MFCD09955050 MF:C11H13NO2 MW:191.2264 |
MINOXIDIL-D10Catalog No.:AA008SYA CAS No.:1020718-66-4 MDL No.:MFCD07369552 MF:C9H5D10N5O MW:219.3099 |
Benzaldehyde-2,3,4,5-d4, 6-nitro-Catalog No.:AA0006G7 CAS No.:1020718-69-7 MDL No.:MFCD04039636 MF:C7HD4NO3 MW:155.1441 |
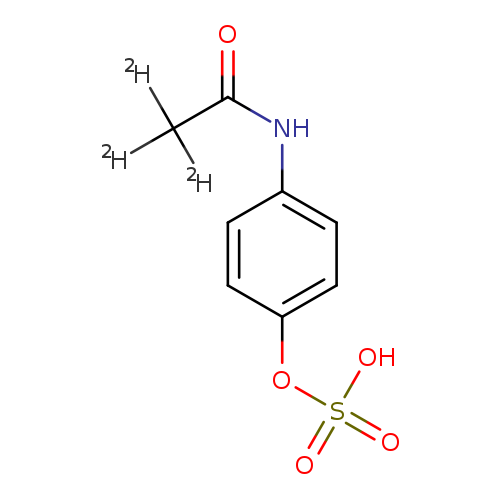
Acetamide-2,2,2-d3, N-[4-(sulfooxy)phenyl]-Catalog No.:AA0006G2 CAS No.:1020718-78-8 MDL No.:MFCD04116085 MF:C8H6D3NO5S MW:234.2442 |
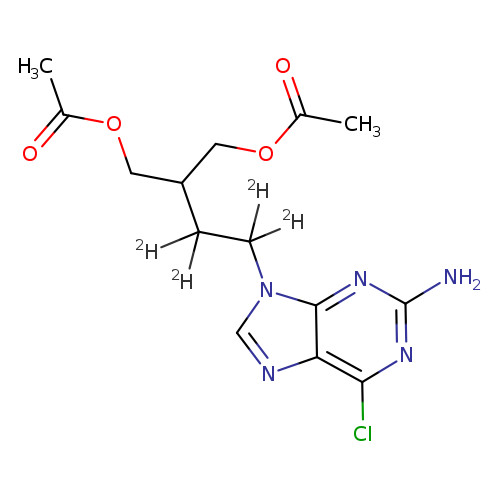
1,3-Propanediol, 2-[2-(2-amino-6-chloro-9H-purin-9-yl)ethyl-1,1,2,2-d4]-, 1,3-diacetateCatalog No.:AA0006G1 CAS No.:1020718-81-3 MDL No.:MFCD07367586 MF:C14H14ClD4N5O4 MW:359.8015 |
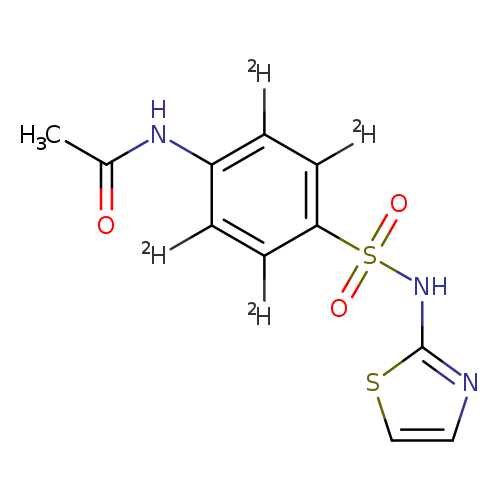
Acetamide, N-[4-[(2-thiazolylamino)sulfonyl]phenyl-2,3,5,6-d4]-Catalog No.:AA0006FZ CAS No.:1020718-91-5 MDL No.:MFCD03425466 MF:C11H7D4N3O3S2 MW:301.3780 |
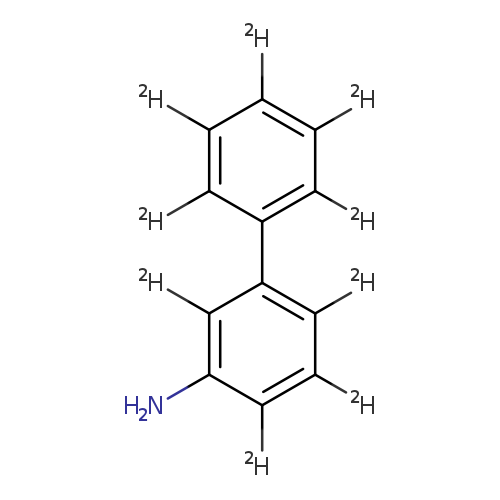
[1,1'-Biphenyl-2,2',3',4,4',5,5',6,6'-d9]-3-amineCatalog No.:AA0006FY CAS No.:1020718-93-7 MDL No.:MFCD03425468 MF:C12H2D9N MW:178.2779 |
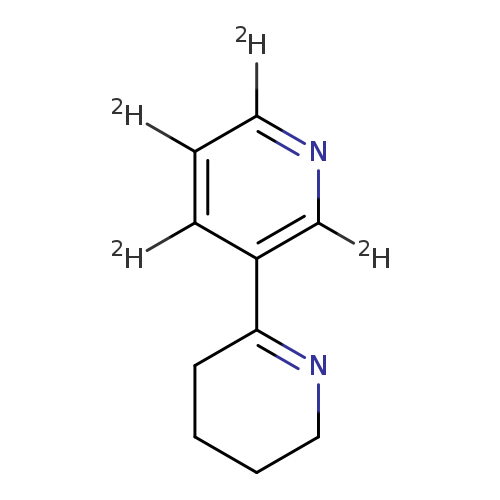
2,3'-Bipyridine-2',4',5',6'-d4, 3,4,5,6-tetrahydro-Catalog No.:AA0006FT CAS No.:1020719-05-4 MDL No.:MFCD07367637 MF:C10H8D4N2 MW:164.2403 |
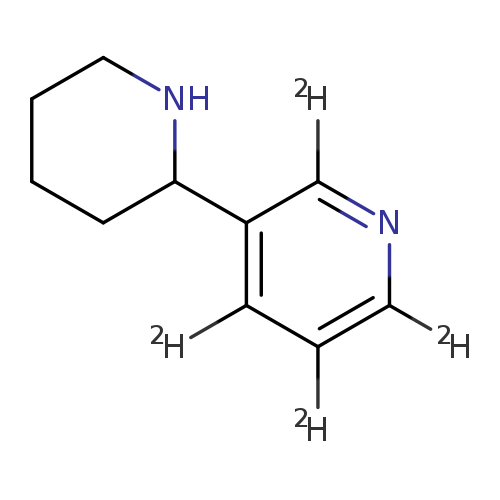
(R,S)-ANABASINE-2,4,5,6-D4Catalog No.:AA008S17 CAS No.:1020719-08-7 MDL No.:MFCD04039400 MF:C10H10D4N2 MW:166.2562 |
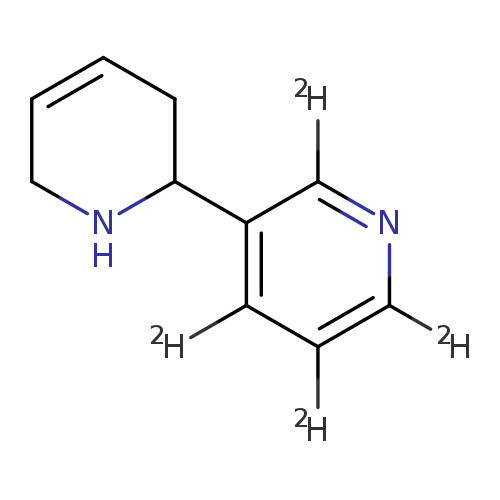
2,3'-Bipyridine-2',4',5',6'-d4, 1,2,3,6-tetrahydro-Catalog No.:AA0006FS CAS No.:1020719-11-2 MDL No.:MFCD04039401 MF:C10H8D4N2 MW:164.2403 |
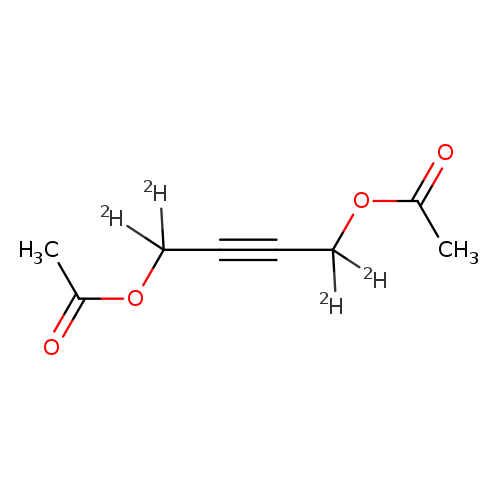
2-Butyne-1,1,4,4-d4-1,4-diol, 1,4-diacetateCatalog No.:AA0006H1 CAS No.:1020719-23-6 MDL No.:MFCD07369213 MF:C8H6D4O4 MW:174.1872 |
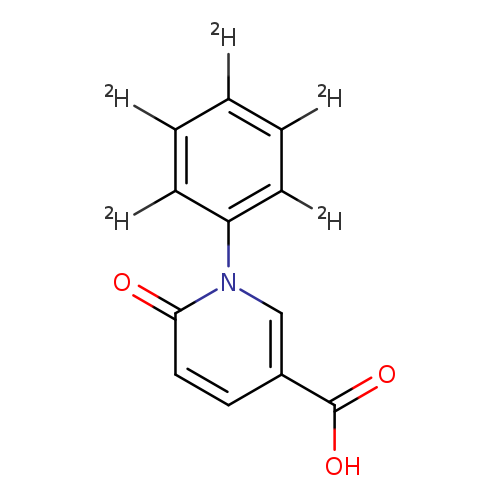
3-Pyridinecarboxylic acid, 1,6-dihydro-6-oxo-1-(phenyl-2,3,4,5,6-d5)-Catalog No.:AA0006H0 CAS No.:1020719-24-7 MDL No.:MFCD07369219 MF:C12H4D5NO3 MW:220.2356 |
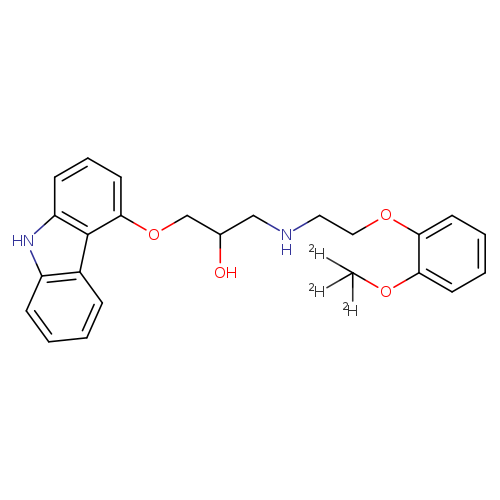
2-Propanol, 1-(9H-carbazol-4-yloxy)-3-[[2-[2-(methoxy-d3)phenoxy]ethyl]amino]-Catalog No.:AA0006GZ CAS No.:1020719-25-8 MDL No.:MFCD07369225 MF:C24H23D3N2O4 MW:409.4927 |
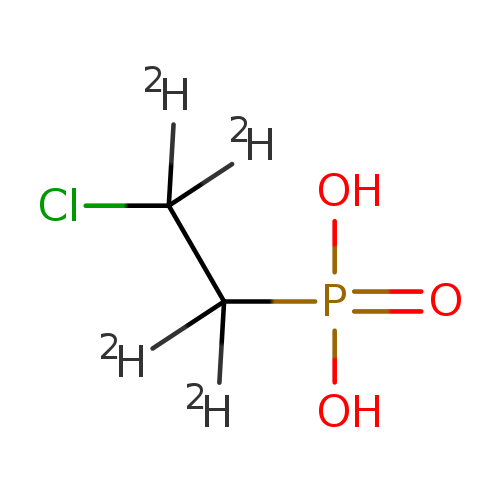
2-(CHLOROETHYL) PHOSPHONIC ACID-D4Catalog No.:AA008Y0N CAS No.:1020719-29-2 MDL No.:MFCD04039474 MF:C2H2ClD4O3P MW:148.5186 |
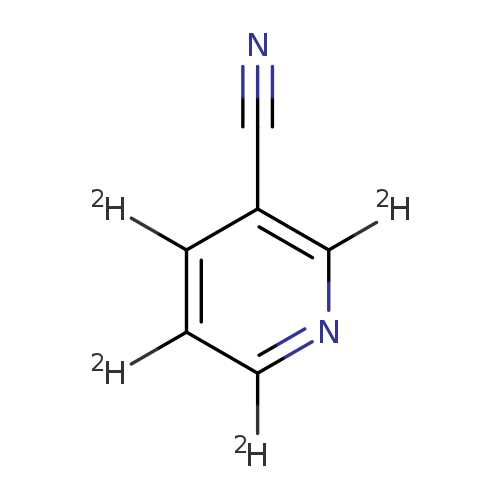
3-Pyridine-2,4,5,6-d4-carbonitrileCatalog No.:AA0006GU CAS No.:1020719-32-7 MDL No.:MFCD07369271 MF:C6D4N2 MW:108.1340 |
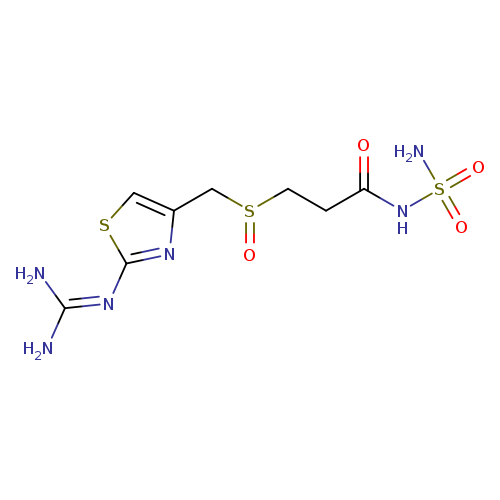
3-[2-(Diaminomethyleneamino)-1,3-thiazol-4-ylmethylsulphinyl]-N-sulphamoyl propanamideCatalog No.:AA008WHJ CAS No.:1020719-36-1 MDL No.:MFCD03788836 MF:C8H14N6O4S3 MW:354.4296 |
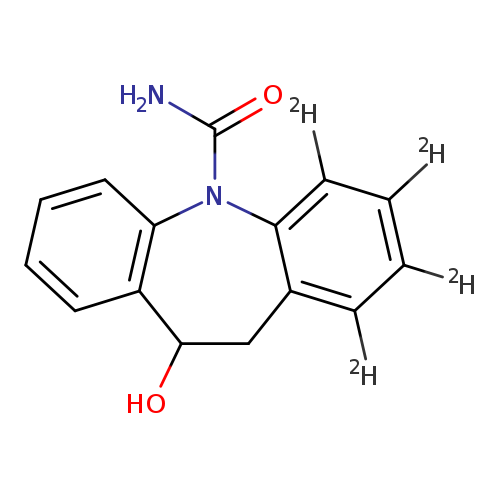
5H-Dibenz[b,f]azepine-1,2,3,4-d4-5-carboxamide, 10,11-dihydro-10-hydroxy-Catalog No.:AA0006GT CAS No.:1020719-39-4 MDL No.:MFCD07369334 MF:C15H10D4N2O2 MW:258.3085 |
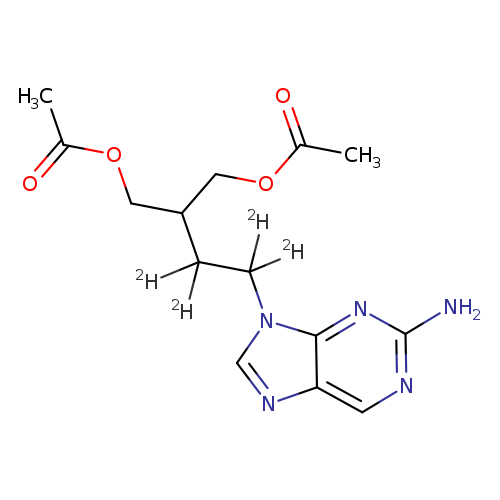
FAMCICLOVIR-D4Catalog No.:AA008S1Z CAS No.:1020719-42-9 MDL No.:MFCD07369397 MF:C14H15D4N5O4 MW:325.3564 |
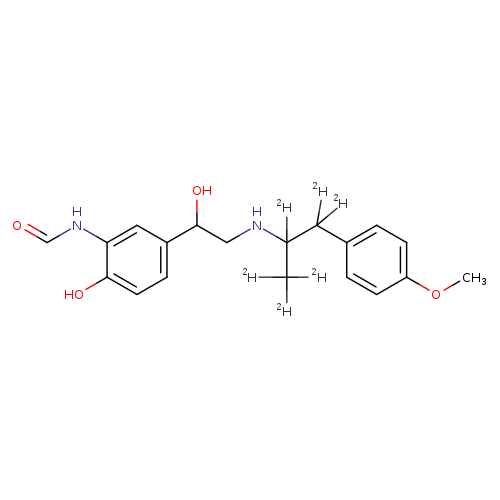
ForMoterol-D6 (Major) (Mixture of DiastereoMers)Catalog No.:AA0093LD CAS No.:1020719-45-2 MDL No.: MF:C19H18D6N2O4 MW:350.4418 |
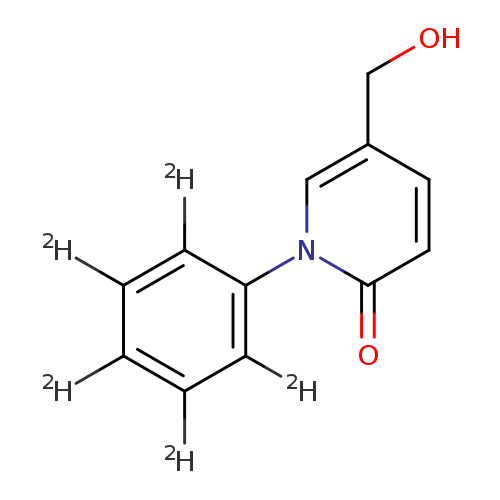
2(1H)-Pyridinone, 5-(hydroxymethyl)-1-(phenyl-2,3,4,5,6-d5)-Catalog No.:AA0006GJ CAS No.:1020719-52-1 MDL No.:MFCD03701685 MF:C12H6D5NO2 MW:206.2520 |
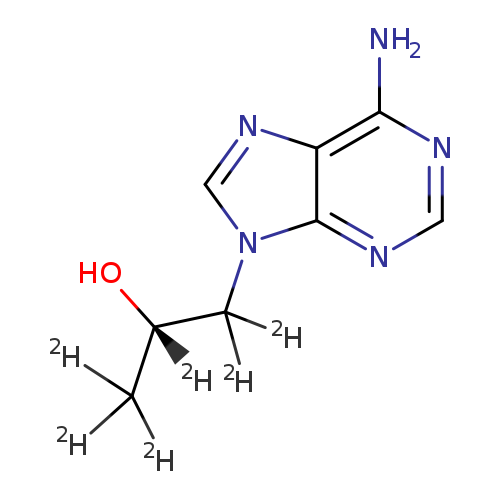
9H-Purine-9-ethan-α,β,β-d3-ol, 6-amino-α-(methyl-d3)-Catalog No.:AA0006GI CAS No.:1020719-54-3 MDL No.:MFCD07369452 MF:C8H5D6N5O MW:199.2428 |
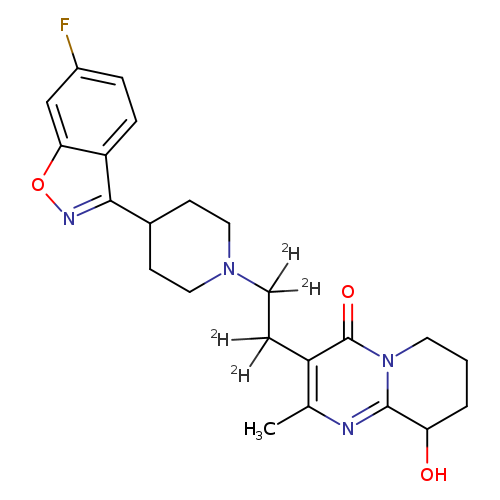
4H-Pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-1,1,2,2-d4]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-Catalog No.:AA0006GH CAS No.:1020719-55-4 MDL No.:MFCD07369454 MF:C23H23D4FN4O3 MW:430.5085 |
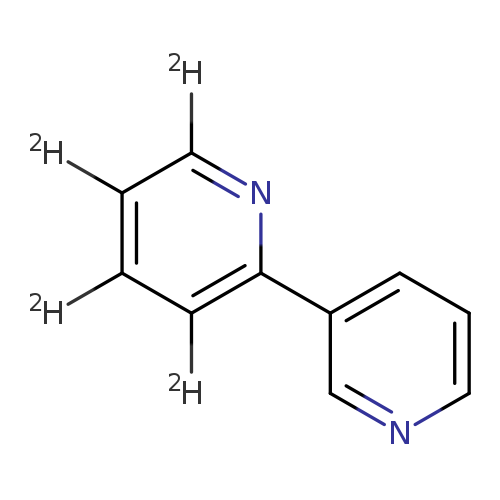
Isonicoteine-3,4,5,6-d4Catalog No.:AA01CBVW CAS No.:1020719-56-5 MDL No.: MF:C10H4D4N2 MW:160.2086 |
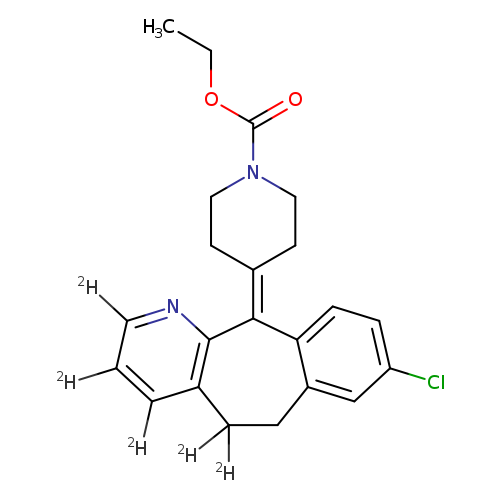
Loratadine-D5Catalog No.:AA008WXN CAS No.:1020719-57-6 MDL No.: MF:C22H18ClD5N2O2 MW:387.9140 |

3-Pyridinemethanol, α-[3-(methyl-d3-nitrosoamino)propyl]-Catalog No.:AA0006HO CAS No.:1020719-61-2 MDL No.:MFCD03425608 MF:C10H12D3N3O2 MW:212.2635 |
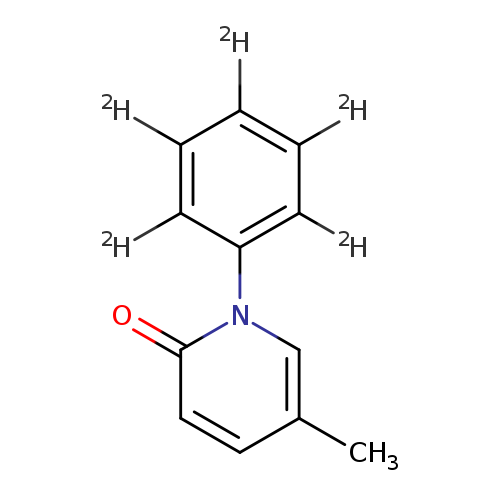
2(1H)-Pyridinone, 5-methyl-1-(phenyl-2,3,4,5,6-d5)-Catalog No.:AA0006HN CAS No.:1020719-62-3 MDL No.:MFCD07369537 MF:C12H6D5NO MW:190.2526 |
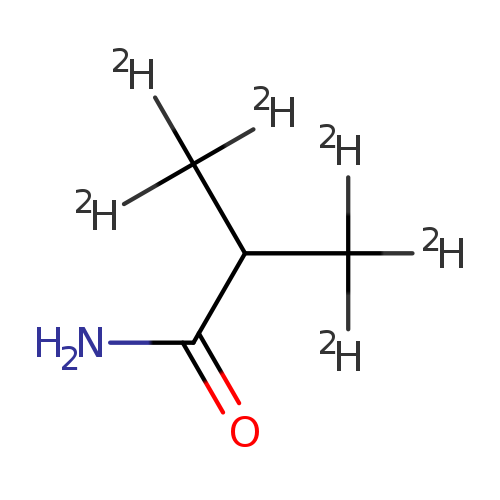
Propanamide-3,3,3-d3, 2-(methyl-d3)-Catalog No.:AA0006HL CAS No.:1020719-64-5 MDL No.:MFCD07369540 MF:C4H3D6NO MW:93.1573 |
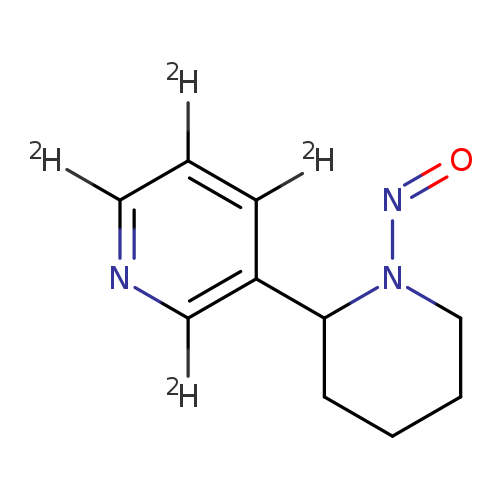
Pyridine-2,3,4,6-d4, 5-(1-nitroso-2-piperidinyl)-Catalog No.:AA0006HK CAS No.:1020719-68-9 MDL No.:MFCD07369613 MF:C10H9D4N3O MW:195.2544 |
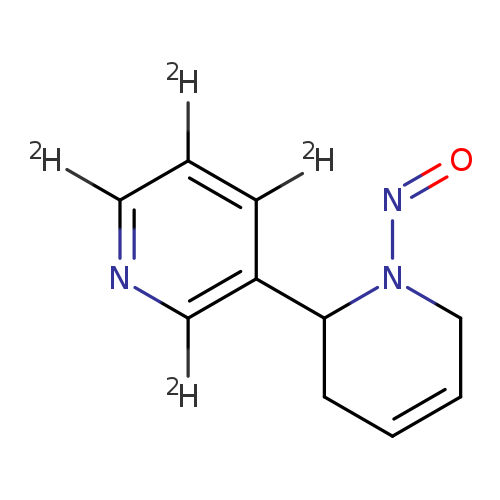
2,3'-Bipyridine-2',4',5',6'-d4, 1,2,3,6-tetrahydro-1-nitroso-Catalog No.:AA0006HJ CAS No.:1020719-69-0 MDL No.:MFCD07369614 MF:C10H7D4N3O MW:193.2385 |