2020-01-28 14:33:53
Abhishek Sharma1,2 & Ajay Kumar Mathur2 & Jawahar Ganpathy1 & Bhrugesh Joshi1 & Prittesh Patel1
Received: 4 September 2018 / Accepted: 27 January 2019 / Published online: 14 February 2019
# Institute of Molecular Biology, Slovak Academy of Sciences 2019
Introduction
The anti-neoplastic herb, Catharanthus roseus is a classified high-value low-volume medicinal herb which is in the global attention of scientific research for modulation of its terpenoid indole alkaloids (TIAs) pathway (Verma et al. 2015; Sharma et al. 2017). The plant belongs to the Family Apocynaceae and is known to harbor >130 different types of bioactive TIAs that make it an interesting ingredient in several traditional and modern therapies (Zhao and Verpoorte 2007; Sharma et al. 2018). In the modern pharmacopeia the plant is commercially valued for the strong anti-neoplastic efficacy of two of its leaf- derived dimeric TIAs namely vincristine and vinblastine. In addition, two of its root-derived monomeric alkaloids ajmalicine and serpentine are also in wide clinical usage to treat hypertension and other circulatory disorders (El-Sayed and Verpoorte 2007; Duarte and Cooper-DeHoff 2010). Vincristine and vinblastine are indispensable components of several chemotherapeutic regimens employed in the treatment for acute lymphoblastic leukemias, Hodgkin’s and non- Hodgkin’s lymphomas, Ewing’s sarcoma and breast cancer etc. (Neuss and Neuss 1990; Arora et al. 2010). The in-planta yields of these bioactive TIAs are extremely low (0.0001– 0.0005% leaf dry weight) that makes their extraction difficult and exorbitantly expensive (Sharma et al. 2018). At the same time, vincristine and vinblastine also represent a complex chi- rality that makes their chemical synthesis difficult and eco- nomically non-viable (Hughes and Shanks 2002). This makes Catharanthus roseus an exclusive bio-resource of these high value drugs. TIAs pathway, therefore, is in center-stage of several plant metabolic engineering pursuits because of the high commercial stakes attached to it (Wang et al. 2012; Li et al. 2013; Qu et al. 2015). The better insight of the pathway architecture, the spatial and temporal complexities associated with its expression and execution and different facets of its developmental, enzymatic and genetic regulations have pro- vided ample opportunity to strategize different pathway engi- neering options in this herb. Involvement of at least four dis- crete cell/tissue types (epidermis, internal phloem parenchy- ma, idioblasts) and five intra-cellular compartments namely cytosol, vacuole, thylakoid membrane, nucleus, and endoplas- mic reticulum has so far been implicated with TIAs metabo- lism in Catharanthus roseus plants (Sharma et al. 2018).
The detail description of TIAs biogenesis in Catharanthus roseus is provided in several research findings (Tatsis et al. 2017; Sharma et al. 2018; Caputi et al. 2018; Qu et al. 2018). Cell and tissue cultures of Catharanthus roseus have been investigated as an alternative source of TIA production. Major researches were contributed towards the improvement of alkaloid production in Catharanthus roseus cell suspension and hairy root cultures (Verpoorte et al. 1997; Verma et al. 2012). The obtained results were not as successful as expected as dimeric alkaloids are produced only in the green shoots of the plant and productivity of other alkaloids in these cultures is very low to be commercialized is limited by lack of that par- ticular precursor. The optimization of culture conditions such as medium composition, including the addition of biosynthet- ic precursor(s) and elicitors to the medium, may enhance al- kaloid production where the productivity is limited by lack of that particular precursor (Verpoorte et al. 1997, 2002). Precursor feeding and pathway up-regulation by biotic and abiotic elicitors are two standard strategies for improving the expression of an intended metabolic pathway in plants and their in vitro cultures (Karuppusamy 2009; Murthy et al. 2014; Giri and Zaheer 2016). Therefore, the elicitation and precursor feeding experiments in the present study were car- ried out with multiple shoots cultures of Catharanthus roseus that provide the required cellular complexity to complete TIAs pathway and leads to the synthesis of dimeric alkaloids vin- blastine and vincristine. A series of experiments with multiple shoots were therefore, laid down to monitor the influence of these exogenously applied TIAs precursors Tryptophan (TP) and Tryptamine (TM) and five abiotic elicitors salicylic acid (SA), succinic acid (SCA), potassium chloride (KCL), methyl jasmonate (MJ), mannitol (MN) on TIAs pathway biosynthe- sis. Whitmer et al. (1998) suggested the role of pathway pre- cursors like TP and TM as their availability may advances the TIAs production in Catharanthus roseus. Signaling molecules MJ and SA are frequently associated with up-regulation of secondary metabolites (Vázquez-Flota et al. 2009; Verma et al. 2014). Physiological stress also plays a crucial role in the secondary metabolites biosynthesis, therefore, KCL and MN are used to generate salt stress and osmotic stress, respec- tively (Zhao et al. 2000, 2001). Influence of these exogenous- ly applied factors was also chased in callus tissue to make a comparison of their efficacy at different levels of cellular or- ganization. Change in the transcripts of important pathway genes controlling the major steps of TIAs biosynthesis from tryptamine, strictosidine to vinblastine (Wang et al. 2016; Liu et al. 2017; Sharma et al. 2017, 2018) viz., CrTDC, CrSTR, CrSGD, CrT3O, CrT3R, CrD4H, CrDAT, and CrPRX1 along with the accumulation of monomeric TIAs vindoline and catharanthine and dimeric TIAs vinblastine and vincristine, were analyzed to understand the influence of these exoge- nously applied factors.
Materials and methods
Multiple shoot culture
The seeds of Catharanthus roseus BDhawal^ (National Gene Bank Accession Numbers: CIMAP-0859) were procured
from CSIR-CIMAP gene bank. Prior to culturing, the seeds were soaked overnight in running tap water. The surface ster- ilization was done by washing them in 70% ethanol for 30 s. and with HgCl2 (0.1% w/v) treatment for 2 min, followed by 2–3 times washing with sterilized distilled water and plated over basal MS medium (Murashige and Skoog 1962) under dark condition. The seeds germinated after 3–4 days and after 2 weeks, transferred over shoot multiplication medium (SMM) comprised of MS media supplemented with 1 mg/L 6-benzylaminopurine (BAP), 0.1 mg/L naphthalene acetic ac- id and 0.38 mg/L thymine HCL with 3% sucrose and 8 g/L agar (Sharma et al. 2018). The axillary shoot developed were separated and maintained/sub-cultured after every 8 weeks hiatus on SMM. Seedlings and multiple shoot cultures were maintained at 25 ± 2 °C, under 16 h photoperiod by cool day- light fluorescent incandescent tubes (48 μmol m−2 s−1 photo- synthetic flux).
Callus culture
Callus cultures were induced by using leaves from in vitro raised axillary shoot culture. Leaves were plated over MS medium (Murashige and Skoog 1962) supplemented with
1.0 mg/L 2,4-dichloro-phenoxy acetic acid (2,4-D) and
0.5 mg/L benzylaminopurine (BAP) and incubated under con- tinuous diffused light (15 μmol m−2 s−1 photosynthetic flux). After 2–3 weeks, callus induced from leaf and transferred to fresh callusing medium for growth and maintenance.
Precursor and elicitor preparation
Precursors and elicitors preparation were done by dissolving the required amount into the sterile distilled water; filter ster- ilized and added to 100 mL conical flask (for multiple shoot cultures) / petryplates (for callus cultures) containing 50 mL of MS medium specific to multiple shoot culture and callus cul- ture just before solidification under sterile conditions. The concentrations of the growth regulators with a final concen- tration in the medium were as follows: Salicylic acid (Sigma, USA) 1 mM, 5 mM and 10 mM; Succinic acid (Sigma, USA) 1 mM, 5 mM and 10 mM; Methyljasmonate solution (Sigma- Aldrich, Germany) 10 mM, 100 mM and 250 mM; KCL (SRL, India) 2 g/L, 3 g/L, 4 g/L and 5 g/L; Mannitol (HiMedia, India) 100 mM, 200 mM, 300 mM and 400 mM; Tryptophan (HiMedia, India) 300 mg/L, 500 mg/L and 1000 mg/L and Tryptamine (HiMedia, India) mg/L, 500 mg/ L and 1000 mg/L (Table 1). Culture medium of Control ex- plants were not fed with any of the above chemicals.
Method of feeding
All precursor and elicitor solutions were prepared by separate- ly dissolving the known amount of the component in sterile distilled water. They were filter-sterilized using a Millipore disposable syringe filter (0.2 μm). The required volume of such sterilized solutions was then aseptically added in 50 mL of callusing or shoots multiplication medium in 100 mL flask. Media with an equal amount of sterilized distilled water served as the control. Minimum of three replicates per treatment were taken and the experiment was repeated twice. The feeding and elicitation treatments were compared in terms of their influ- ence on growth indices, total alkaloid content, and quantifica- tion of four major TIAs namely vindoline, catharanthine, vin- blastine and vincristine in the alkaloid pool. Growth and me- tabolite production kinetics of Catharanthus roseus multiple shoot cultures and callus cultures were performed and based on the results the duration of all in vitro treatment was kept constant at 30 days. All treatments were initiated at 0 days of culture (30 days after seed germination for multiple shoot cul- tures and callus cultures development from leaves) and growth kinetics of callus and multiple shoot cultures were monitored in terms of their Growth Index (GI) over time. The GI was calculated by using the formula:
Alkaloid profiling
High performance liquid chromatography (HPLC) was per- formed to analyze the different TIAs in Catharanthus roseus. For alkaloids extraction, 100 mg of oven-dried (50–60°C) the harvested multiple shoots and callus tissue was taken and extracted thrice with HPLC grade methanol (3 × 30 mL) at room temperature. The methanolic extracts were pooled and dried in vacuum to 10 mL to which 10 mL of distilled water was then added. The mixture was acidified with 10 mL of 3% HCl and washed thrice with hexane (3 × 30 mL). The purified aqueous portion was basified with ammonia (to pH 8.0), ex- tracted with chloroform (3 × 30 mL), air dried over anhydrous sodium sulfate and weighed until constant reading. The HPLC analysis and quantification was carried out using a modular HPLC apparatus (Waters Corp., Milford, MA, USA) equipped with a 600E multi-solvent delivery system and a 2996 photo- diode array (PDA) detector and an RP-18e Reversed-Phase Chromolith Performance HPLC column (100-×4.6 mm). The mobile phase comprised of 21:79 (v/v) acetonitrile: 0.1 M phosphate buffer acidified with 0.5% glacial acetic acid (pH 3.5). The flow-rate was maintained at 1.2 mL/min during the entire analysis. The injector volume used for every sample was 10 μl. Peak detection was carried out at 254 nm. A stan- dard mixture of vindoline, catharanthine, vinblastine and vin- cristine (0.25 mg/ml methanol) was made using the stock solution of 1 mg/ml of each. The obtained data were processed using Empower Pro (Waters Corp.) chromatographic soft- ware. The entire run took 35 min.
Real-time PCR (RT-qPCR) analysis
To analyze the quantitative real-time PCR, the relative gene expression studies were performed using the Stepone real- time PCR system (Applied Biosystems, USA) with SYBR Green PCR Master Mix (Applied Biosystems, USA) as de- scribed in Sharma et al. (2018). Total RNA content was ex- tracted from elicited and control tissues as per the manufac- turer’s instructions (DSS Takara, Japan). The quality and quantity of extracted RNA were estimated using a NanoDrop spectrophotometer (Thermo Scientific, USA). The complementary DNA (cDNA) was generated from the isolated RNA using the random primers and MultiScribe Reverse Transcriptase kit (Applied Biosystems, USA). All real-time PCR quantifications were performed with endoge- nous control Actin and a non-template control (Pandey et al. 2016; Kumar and Bhatia 2016; Sharma et al. 2017, 2018). Real-time transcript analysis of five candidate genes viz., CrTDC, CrSTR, CrSGD, CrT3O, CrT3R, CrD4H, CrDAT, and CrPRX1 genes was calculated using the Livak’s method (RQ = 2 − ΔΔct). List of primers used in the present study is provided in Supplementary Material.
Statistical analysis
The data obtained from the experimental analysis is expressed as a mean ± standard deviation (SD) of three biological repli- cates. The statistical differences in elicited tissues for TIAs were analyzed by two-way analysis of variance (ANOVA) using the SPSS (Version 17.0). All the values at P < 0.05 were considered statistically significant.
Result
Seed germination, shoot multiplication and callus culture establishment
The seeds of Catharanthus roseus BDhawal^ were used to generate healthy growing multiple shoot cultures and callus
cultures. Overnight soaked seeds germinated with 100% seed germination rate within 3–4 days of culture over MS medium (Fig. 1a). Fifteen days old seedlings upon transfer to SMM, elongated to average length of 8–10 cm within 8 weeks of growth (Fig. 1c-e). The axillary bud explants from these 8 weeks old elongated shoots were used to feed with pathway precursors and chemical elicitors. Leaves from 6 to 8 weeks old invitro grown multiple shoot cultures of Catharanthus roseus were used to induce callus cultures. Callus initiation from leaf explants became evident after 16–20 days of plating and by 30th day the entire explant surface was covered with induced calli. The callus was subsequently maintained on the same callusing medium through regular sub-culturing every 4th–5th week (Fig. 1b). These 4–5 weeks old callus cultures were used for the further experimentation.
Growth kinetic of multiple shoot and callus cultures
The growth and metabolite production kinetics of shoot cul- tures through a 50 days culture cycle on MSB (MS + 1.0 mg/L BAP + 0.1 mg/L NAA + 0.4 mg/L THCL) was recorded (Fig. 2). The biomass production curve followed a steady exponential trend in biomass gain between 10th to 40th days of in vitro growth with a GI value of 75 to 800, respectively. Biomass built up on the 30th day was 3.5 fold more than on the 20th day of culture. The cultures maintained a near steady state of growth beyond 40th day with a GI of 820 on the 50th day of incubation. The alkaloid content in the regenerating shoots was low during the first 20 days of cultures (0.36– 0.51% dry wt). The alkaloid production was peaked on the 30th day with an alkaloid content of 1.56% dry wt. It gradu- ally declined to 1.32 and 0.47% dry wt on 40th and 50th day, respectively. The growth and metabolite production kinetics of callus culture thought a 40 days culture cycle on callus culture medium (MS + 1.0 mg/L 2,4 D + 0.1 mg/L BAP) was recorded (Fig. 3). The biomass production curve followed a steady exponential trend between 10th to 30th days of in vitro shoot growth with a GI value of 3.68 and 7.58, respec- tively. The cultures maintained a near steady state of growth beyond 30th day with a GI of 4.43 on the 40th day of incuba- tion. The alkaloid content in the growing callus was low dur- ing the first 20 days of cultures. The alkaloid production on peaked on the 30th day with an alkaloid content of 2.19% dry wt and was declined to 0.68% dry wt on the 40th day.
The callus and the multiple shoot cultures of Catharanthus roseus were fed to chase the feeding efficacy of the two TIAs biosynthesis precursors (tryptophan and tryptamine) and five chemicals (methyl jasmonate, salicylic acid, succinic acid, po- tassium chloride, and mannitol) (Table 1). All treatment doses were applied at the beginning of the culture cycle and the harvesting of the tissue was done on the 30th day of growth. Harvesting time was deduced on the basis of highest biomass and alkaloid productivity obtained on this day in the control cultures. Influence of various treatments was compared in terms of harvest biomass yield, crude alkaloid content and quantitative change in catharanthine, vindoline, vinblastine and vincristine levels in the tissue.
Modulation in TIAs biosynthesis in multiple shoot culture
In terms of shoot biomass production, methyl jasmonate elic- itation was found inhibitory and 1.5 to 2.0 fold depression over the control was evident in shoots treated with 10 mM and 100 mM methyl jasmonate. Salicylic acid, tryptophan and tryptamine treatments also failed to enhance biomass produc- tivity to any significant level when compared with non-treated controls. Most positive effect on shoot growth was evident in cultures treated with low levels of KCl (2 g/L) and higher dose (5–10 mM) of succinic acid. KCl at 2 g/L levels and succinic acid at 10 mM supplementation caused more than 2–4 fold increase in growth and biomass accumulation in shoot cul- tures. In comparison to 1.56%, dry wt content of total alka- loids in the control shoots, the alkaloid content in the treated shoots varies in a range from 1.67 to 6.42% dry wt (Fig. 4). Highest alkaloid levels of 6.2% dry weight were found in shoots that were fed with 1000 mg/L tryptamine followed by 4.22% dry wt at 500 mg/L tryptamine dose. In terms of total alkaloid yield, treatment of succinic acid at 10 mM was best as in addition to 3.15% dry wt alkaloid content it also supported a fourfold increase in biomass. Methyl jasmonate at higher doses (250 mM) and tryptophan at 1000 mg/L support mini- mum alkaloid production in comparison to control. The three elicitation treatments of KCl did not differ much in terms of alkaloid content 3.68, 3.43 and 3.51% dry wt and 2, 4, and 5 g/ L levels. Salicylic acid supplementation was found dose- dependent with a treatment comprising of 10 mM/L being best with TIAs content of 3.72% dry wt.
The highest accumulation (2 folds) of catharanthine (0.0729%) was observed in shoots exposed to the highest concentration of KCL (5 g/L). An organic acid, salicylic acid at its lower concentration of 1 mM also increased the production of catharanthine 1.25 folds, when compared to control. However, increase in the concentration of SA com- promises the cell growth. Alkaloid precursor, tryptamine at its low concentration of 300 mg/L also showed an increase in catharanthine production but no concurrent influences on growth. Treatments of methyl jasmonate and tryptamine were not found to affect production of catharanthine content. None of the precursor feeding and elicitation treatments were found to have any positive influence on vindoline content that varied from 0.0025 to 0.0763% dry wt in comparison to 0.1126% dry wt in the control shoots. Interestingly, while no vincristine peak could be seen in most of the alkaloids samples including that of control, methyl jasmonate (250 mM), salicylic acid (5 mM), succinic acid (1 mM) and tryptamine (500-100 mg/L) treatments showed the presence of trace amount of vincris- tine when the samples were analyzed for their alkaloid con- tent. Highest vinblastine production, on the other hand, was observed in the shoots fed with a lower concentration of trypt- amine (100 mg/L), wherein, nine-fold increase was evident (i.e. 0.0277% dry wt vis a vis 0.003% in the control). While the positive influence of tryptamine feeding remained only two folds at 500 mg/L, its accumulation was not seen in the sample treated with 1000 mg/L dose of tryptamine. Similar to tryptamine but to a lesser degree, tryptophan was also found favoring vinblastine accumulation in cultured shoots. The ef- fect was more prominent (>5 fold) at lower tryptophan sup- plementation doses of 300–500 mg/L. Salicylic acid at its lower concentration (1 mM) also increased the production of vinblastine by five folds with high cell growth increase. Increase in concentration of methyl jasmonate resulted in a marginal increase in vinblastine production but with a concur- rent decrease in the cell growth. The real time PCR gene expression analysis of the elicited multiple shoots cultures reported a very slight increase in the levels of CrSGD and CrPRX I genes (Fig. 5). CrSGD reported a marginal increase in the tissues treated with TP300 and TP500. SA1 treatment resulted in the 1.46 fold increased CrPRX11 followed by 1.32 fold increase with KCL5 treatment. Rest of the treatment failed to enhance the expression of other studied genes.
Effect of precursor feeding and elicitation over TIAs biosynthesis on callus cultures
In terms of callus biomass production, salicylic acid and meth- yl jasmonate elicitations were found inhibitory and showed
2.0 to 3.0 fold and 1.5 to 2.0 fold depressions over control. Tryptamine and tryptophan treatments also failed to enhance biomass productivity to any significant level when compared with non-treated controls. Most positive effect on callus growth was evident in cultures treated with KCl and mannitol. KCl at 3 g/L levels and mannitol at 100 mM supplementation induced 2 fold and 1.7 fold increase in growth and biomass accumulation in callus cultures. In comparison to 1.18% dry wt content of total alkaloids in the control callus culture, the alkaloid content in the treated callus cultures varies from a range of 0.46 to 2.38% dry wt (Fig. 6). Highest alkaloid levels of 2.38% and 2.28% dry weight were found in callus fed with 5 mM and 1 mM succinic acid dose and also recorded a 1.6 to 3 fold increase in biomass. Higher doses of Methyl jasmonate (100–250 mM) and mannitol (200-500 mM) supports the minimum alkaloid production in comparison to control. The elicitation treatments of KCl produced alkaloid content rang- ing from 1.53 to 2.07% dry wt at 2, 3, 4, and 5 g/L levels. The elicitation treatments with salicylic acid and feeding with tryp- tophan also did not differ much in terms of alkaloid content. Alkaloid production in these treatments ranges from 1.12 to 1.74% dry wt in tryptophan feeding and 1.04 to 1.59% dry wt in cultures elicited with salicylic acid. Increase in tryptamine doses at 300, 500 and 1000 mg/L doses caused a decrease in alkaloid content with 1.98, 1.68 and 1.55% dry wt.
Catharanthine and vindoline content were determined in the callus cultures treated with precursors and abiotic elicitors. In case of catharanthine production, callus cultures fed with 500 mg/L tryptophan recorded the trivial increase of 5% with a decrease in cell growth compared to non-treated control, otherwise, no other treatment enhanced catharanthine produc- tion that varied from 0.0007 to 0.0101% dry wt. Vindoline production in callus system is highly affected by salt and os- motic stress. KCl at its lower dose of 3 g/L recorded 12 folds increase (0.0451% dry wt) with an increase in cell growth (Fig. 6). However, the increase in KCl concentration gradually reduced vindoline production and limited to 2 fold increase. A lower dose of mannitol also affects the production of vindoline. Mannitol at 100 mM dose affects the vindoline production by 3 fold (0.0115% dry wt) with increased cell growth; however increase in mannitol concentrations (200 mM, 300 mM, 400 mM) reduced the vindoline produc- tion with variable increased in cell growth. Succinic acid with its higher dose of 10 mM resulted in 2fold (0.0065%) increase in vindoline content with similar growth compared to non-treated control. Meanwhile, the treatments of salicylic acid, methyl jasmonate, tryptophan, and tryptamine showed a var- iable decrease in vindoline production compared to non- treated control. The real time PCR gene expression analysis of the elicited callus cultures reported an increase in the levels of only CrDAT transcript levels (Fig. 7). KCL2 treatment in- creased the CrDAT transcript levels upto 1.34 fold increases followed by KCL4 treatment with a marginal increment of
1.09 fold. MN100 treatment also recorded 1.20 fold enhanced CrDAT transcript levels. Rest of the treatment failed to en- hance the expression of other studied genes whereas, the tran- script levels of CrPRX11 were not detected in all samples.
Discussion
Amenability and sensitivity of TIAs metabolism towards pre- cursor feeding, biotic and abiotic elicitations in cell and tissue cultures of Catharanthus roseus have been very well docu- mented in many previous studies. These attempts have been periodically reviewed in the literature (Verpoorte and Memelink 2002; Baenas et al. 2014; Verma et al. 2015). These approaches have resulted in higher accumulation of certain monomeric TIAs but most of the time the responses were found to be sporadic, inconsistent and tissue or cell line- specific. Long term stability of metabolite up-regulation in these lines was also a serious constraint in several of these studies. Ajmalicine, serpentine and tabersonine were the most preferred beneficiaries of these imposed elicitation treatments in these previous studies (Vázquez-Flota and Loyola-Vargas 1994; Rijhwani and Shanks 1998; Rodriguez et al. 2003; Thakore et al. 2015). Elicitors can regulate a large number of control points and trigger the expression of key genes with increased cellular activities at a biochemical and molecular level involving signal transduction molecules like jasmonate, salicylic acid, nitrous oxide etc. (Caarls et al. 2015; Zaheer and Giri 2015; Giri and Zaheer 2016). Similarly, the availability and abundance of a starting precursor or a limiting intermedi- ate in a given cellular environment may also advance the path- way in the desired direction but these responses are mostly governed by the metabolic status of the cell cultures (Whitmer et al. 1998; Verma et al. 2015). Results obtained in the pre- sented study in respect to abiotic elicitation of Catharanthus roseus callus and multiple shoot cultures also followed these general trends. In all, five abiotic elicitor molecules namely salicylic acid, succinic acid, methyl jasmonate, mannitol, po- tassium chloride and two TIAs precursors i.e., tryptophan and tryptamine were tested for eliciting the TIAs pathway in callus and multiple shoots.
Highest improvement in TIAs productivity (6.20% dry wt) was observed in shoots fed with 1000 mg/L of tryptamine at the beginning of the culture cycle, followed by 4.22% dry wt at 500 mg/L tryptamine dose in comparison to 1.56% dry wt. in the untreated control shoots. Catharanthine synthesis (0.0729% dry wt) was found to be specifically favored by elicitation with 5 g/L dose of KCl. None of the treatments showed a positive influence on vindoline synthesis that remained down-regulated (0.0025 to 0.763% dry wt) in all treated shoots when compared with its content (0.1126% dry wt) in the control tissue. Among individual alkaloids, none of the TIAs except vindoline recorded any significant change in the treated calli. In the case of vindoline, however, a three or twelve-fold increase was recorded in callus treated with 100 mM and 3 g/L dose of mannitol and KCl, respectively. While vincristine in all the analyzed samples was present in traces, vinblastine accumulation was found more responsive to feeding with 300 mg/L dose of tryptamine wherein a 9-fold increase was visible. Presence of lower concentration of path- way precursors like tryptophan and tryptamine was found effective for enhanced dimeric TIAs production as seen in our previous studies (Sharma et al. 2017; 2018). At the same time, this concentration has a direct effect over the expression of genes like CrTDC and CrSTR. Tryptophan is converted into tryptamine with the help of enzyme tryptophan decarbox- ylase synthesized by CrTDC. At the same time synthesized tryptamine proposed to have feedback inhibition towards en- zyme tryptophan decarboxylase (Noe et al. 1984). Studies made by Whitmer et al. (1998, 2002) and Morgan and Shanks (2000) also supports the fact that the utilization of tryptamine via its coupling with secologanin to produce strictosidine was a more limiting stem than its formation per se. Therefore, in the present study the lower tryptamine and tryptophan concentration availability supports the lower gene expression of CrTDC and CrSTR and other genes, except CrPRXI. Salicylic acid at 1 mM dose also increased the pro- duction of vinblastine by five-folds. With respect to elicitation through a salt stress imposed via KCl treatment, Smith et al. (1987) reported enhanced TIAs production in cell cultures treated with KCL. Zhao et al. (2000) reported the application of KCL and NaCl treatment for enhanced TIAs production in Catharanthus roseus. Compared to NaCl, KCL was found much more able to stimulate the increased catharanthine pro- duction with highest 15 mg/L concentration with 6 g/L KCL treatment. This yield was about 2.5 fold higher than the con- trol. A study carried out with compact callus cultures (CCC) by Zhao et al. (2001) demonstrated that the stress created by KCL and mannitol greatly enhances the TIAs production. Mannitol with 250 mM concentration produced highest level (4–5 folds) of catharanthine and ajmalicine whereas the in- crease in KCL concentration accumulated three to four folds catharanthine and serpentine production. The basic mecha- nism behind the stimulating effect of salt and osmotic stress on alkaloid accumulation in these cell cultures is still not clearly understood.
The overexpression of CrPRXI gene for lower tryptophan, tryptamine and salicylic acid, and high KCL treatments sup- ported the enhanced vinblastine production. To the less catharanthine and vindoline concentration estimated in the present study can be justified by assuming the rapid conver- sion of catharanthine and vindoline to vinblastine as discussed in several studies (Wang et al. 2016; Sharma et al. 2017, 2018). TIAs accumulation in case of callus cultures was most favored by succinic acid mediated abiotic elicitation. Both the tested doses of succinic acid treatment (1 and 5 mM) showed comparable influence resulting in total alkaloids concentration of 2.38 and 2.28% dry wt in comparison to 1.18% in control, however, not supported by the expression levels of all gene studied. Therefore, any concrete generalization cannot be made as was the case in a large number of earlier studies (Smith et al. 1987; Godoy–Hernandez and Loyola–Vargas 1991; Zhao et al. 2000). Methyl jasmonate, as signal mole- cules has been frequently associated with secondary metabolic pathway up-regulation in cell and tissue cultures of many plant species, including Catharanthus roseus cell lines. Jasmonic acid (JA) and its ester methyl jasmonate (MJ) are, otherwise, known to enhance the production of TIAs in cell suspension and hairy root cultures of Catharanthus roseus by activating several pathway genes and transcriptional factors. JA at 50 mg/L dose stimulated ORCA3 transcriptional regu- lator as evident by one hundred seventy-fold increase in ORCA3 transcripts in a Catharanthus roseus hairy root clone (Peebles et al. 2009). This was accompanied with a simulta- neous decrease in catharanthine accumulation. On the con- trary in another study, MJ (50 mg/L) treatment enhanced mRNA transcripts of pathway genes with a concurrent in- crease in catharanthine content (Zhou et al. 2010). In a sepa- rate study, Vázquez-Flota et al. (2009) observed that com- bined treatment of methyl jasmonate at 10 mM and ethylene at 5 ppm was effective in enhancing the vindoline concentra- tion in rootless shoot cultures of Catharanthus roseus that was about 6.5 times higher than in the untreated cultures. In our study, MJ treatment was not found effective with callus and multiple shoot cultures, however, only a marginal increase in vinblastine production was supported by 250 mg/L dose of methyl jasmonate. This too was occurred alongside the severe penalty on cell growth. This may again be a reflection of physiological and/or genotype-specificity of these elicitors as shown by Vázquez-Flota et al. (2009) and Verma et al. (2014).
2-Fluoro-pyridine-3-sulfonyl chlorideCatalog No.:AA01C4LV CAS No.:1089330-70-0 MDL No.:MFCD11227180 MF:C5H3ClFNO2S MW:195.5992 |
{3-[(acetyloxy)imino]-2,3-dihydro-1H-isoindol-1-ylidene}amino acetateCatalog No.:AA01BAA1 CAS No.:1089332-91-1 MDL No.:MFCD28397598 MF:C12H11N3O4 MW:261.2334 |
4-(4-(tert-Butyl)phenoxy)benzaldehydeCatalog No.:AA008ZCN CAS No.:108934-20-9 MDL No.:MFCD08446988 MF:C17H18O2 MW:254.3236 |
2'-Methyl-biphenyl-4-carbaldehydeCatalog No.:AA007BL8 CAS No.:108934-21-0 MDL No.:MFCD01631915 MF:C14H12O MW:196.2445 |
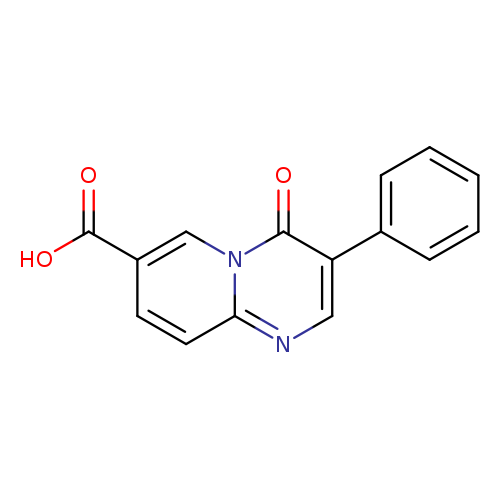
4-Oxo-3-phenyl-4H-pyrido[1,2-a]pyrimidine-7-carboxylic acidCatalog No.:AA00JD90 CAS No.:1089342-74-4 MDL No.:MFCD11853610 MF:C15H10N2O3 MW:266.2515 |
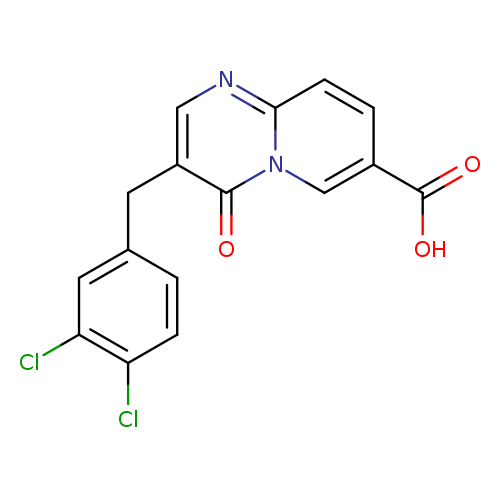
3-(3,4-Dichlorobenzyl)-4-oxo-4H-pyrido[1,2-a]pyrimidine-7-carboxylic acidCatalog No.:AA0090JE CAS No.:1089342-78-8 MDL No.:MFCD11853611 MF:C16H10Cl2N2O3 MW:349.1682 |
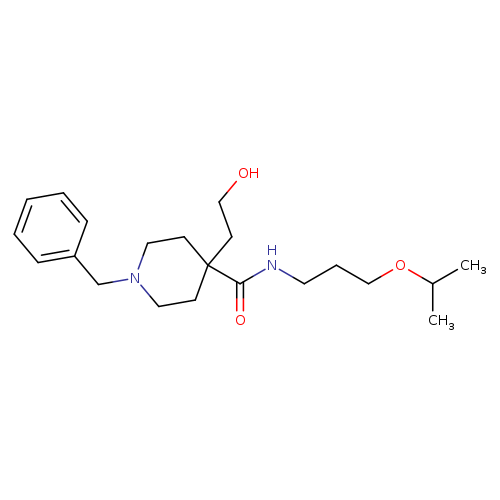
1-BENZYL-4-(2-HYDROXYETHYL)-N-[3-(PROPAN-2-YLOXY)PROPYL]PIPERIDINE-4-CARBOXAMIDECatalog No.:AA01FOH3 CAS No.:1089344-33-1 MDL No.:MFCD18533464 MF:C21H34N2O3 MW:362.5063 |
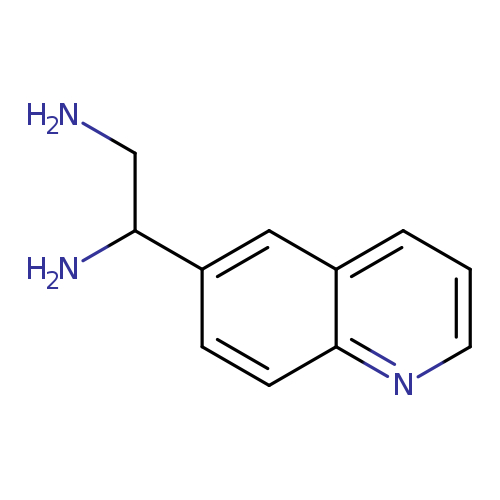
1-(quinolin-6-yl)ethane-1,2-diamineCatalog No.:AA01A2Q0 CAS No.:1089346-08-6 MDL No.:MFCD11503693 MF:C11H13N3 MW:187.2410 |
1-(5-Methylfuran-2-yl)ethane-1,2-diamineCatalog No.:AA01A2X1 CAS No.:1089347-06-7 MDL No.:MFCD11503437 MF:C7H12N2O MW:140.1830 |
1-cyclopentylethane-1,2-diamineCatalog No.:AA01BEFC CAS No.:1089358-93-9 MDL No.:MFCD11503939 MF:C7H16N2 MW:128.2153 |
4-Methoxyindoline-2,3-dioneCatalog No.:AA0082V7 CAS No.:108937-87-7 MDL No.:MFCD10000746 MF:C9H7NO3 MW:177.1568 |
7-Bromo-5-methylindoline-2,3-dioneCatalog No.:AA007TQB CAS No.:108938-16-5 MDL No.:MFCD00462954 MF:C9H6BrNO2 MW:240.0534 |
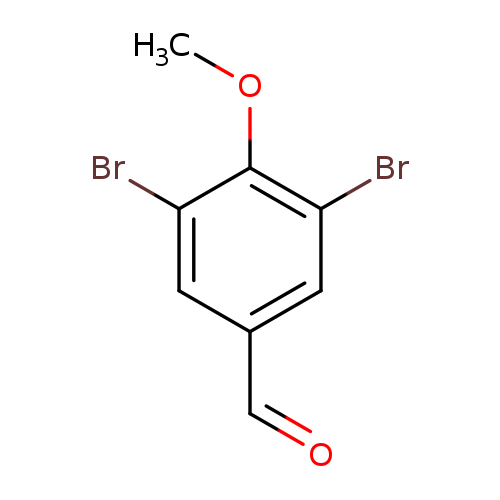
3,5-dibromo-4-methoxybenzaldehydeCatalog No.:AA0082V3 CAS No.:108940-96-1 MDL No.:MFCD00858984 MF:C8H6Br2O2 MW:293.9400 |

D-ribo-Hexopyranose, 2,6-dideoxy-, 1,3,4-triacetateCatalog No.:AA01CBP7 CAS No.:108942-62-7 MDL No.: MF:C12H18O7 MW:274.2671 |
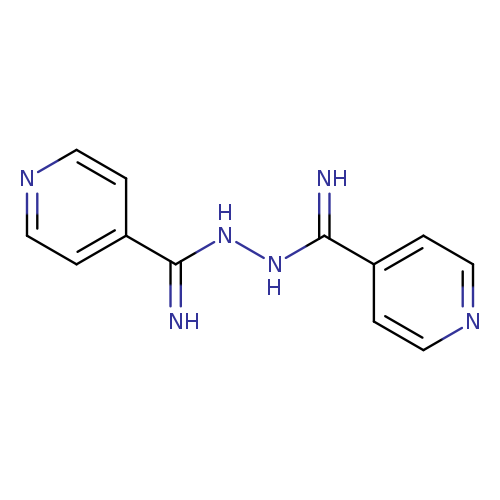
N-(Pyridin-4-ylmethanimidamido)pyridine-4-carboximidamideCatalog No.:AA01F3PZ CAS No.:108952-34-7 MDL No.:MFCD06637722 MF:C12H12N6 MW:240.2639 |
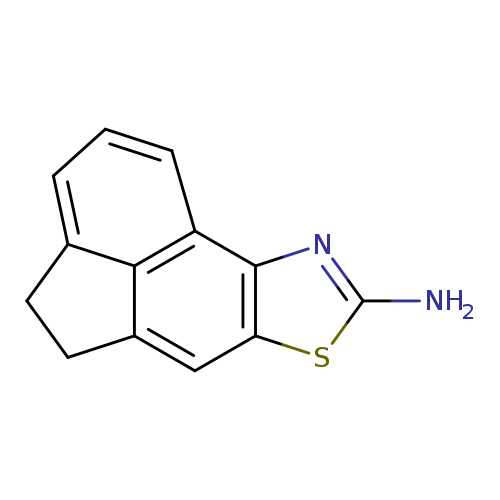
5-thia-3-azatetracyclo[6.6.1.0^{2,6}.0^{11,15}]pentadeca-1(14),2(6),3,7,11(15),12-hexaen-4-amineCatalog No.:AA01ARYR CAS No.:108954-84-3 MDL No.:MFCD03030160 MF:C13H10N2S MW:226.2969 |
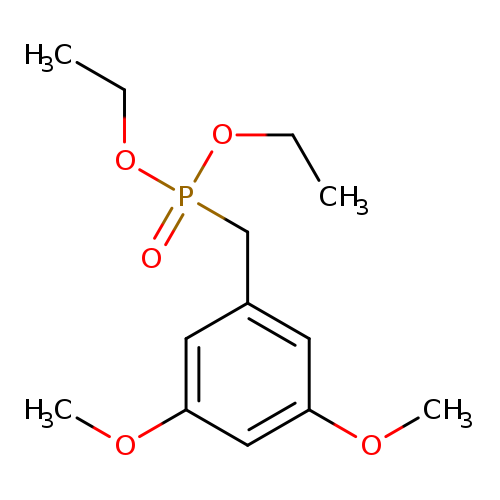
Diethyl 3,5-dimethoxybenzylphosphonateCatalog No.:AA0082UZ CAS No.:108957-75-1 MDL No.:MFCD18252337 MF:C13H21O5P MW:288.2766 |
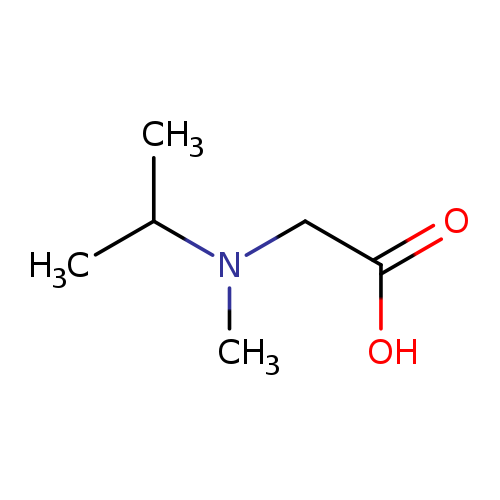
N-Isopropyl-n-methylglycineCatalog No.:AA008V79 CAS No.:108957-96-6 MDL No.:MFCD09864552 MF:C6H13NO2 MW:131.1729 |
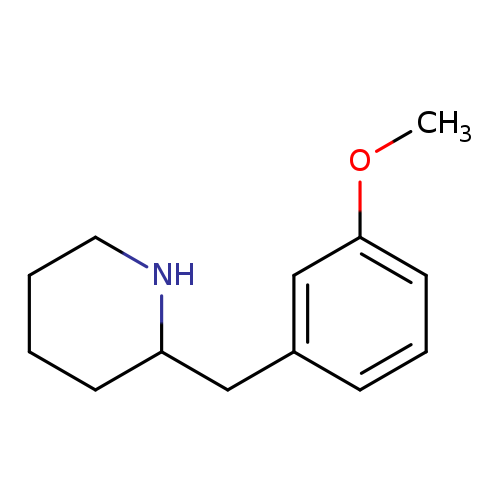
2-(3-Methoxy-benzyl)-piperidineCatalog No.:AA007BL0 CAS No.:108958-36-7 MDL No.:MFCD02663630 MF:C13H19NO MW:205.2961 |
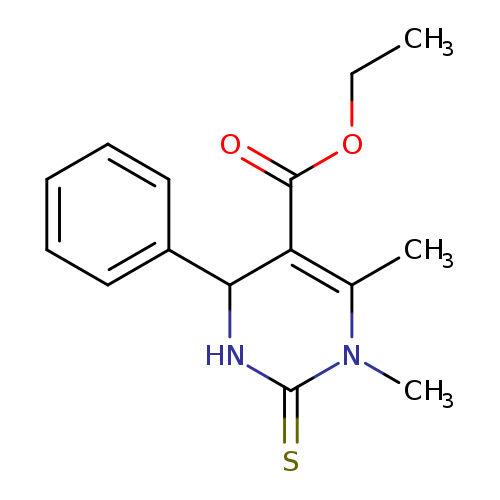
Ethyl 1,6-dimethyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylateCatalog No.:AA0082UY CAS No.:108958-81-2 MDL No.:MFCD01112452 MF:C15H18N2O2S MW:290.3806 |
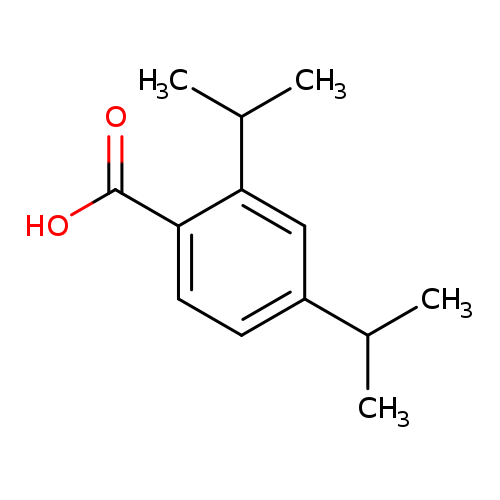
Benzoic acid,2,4-bis(1-methylethyl)-Catalog No.:AA0082UX CAS No.:108961-55-3 MDL No.:MFCD06655349 MF:C13H18O2 MW:206.2808 |
N-(2,2-Dimethoxyethyl)-2-(3-methoxyphenyl)acetamideCatalog No.:AA00HB6W CAS No.:108962-85-2 MDL No.:MFCD17294877 MF:C13H19NO4 MW:253.2943 |
1,2,4-TRIS(METHANESULFONYLOXY)BUTANECatalog No.:AA003D8M CAS No.:108963-16-2 MDL No.:MFCD00191688 MF:C7H16O9S3 MW:340.3915 |
3-PyrrolidinaMine, N,1-diethyl-Catalog No.:AA009TND CAS No.:108963-17-3 MDL No.:MFCD00059062 MF:C8H18N2 MW:142.2419 |
(3S)-(+)-1-Benzyl-3-(methylamino)pyrrolidineCatalog No.:AA008RYF CAS No.:108963-18-4 MDL No.:MFCD02184351 MF:C8H20Cl2N2 MW:215.1638 |
N,N'-Dibenzyl-3-aminopyrrolidineCatalog No.:AA003SFO CAS No.:108963-20-8 MDL No.:MFCD00059060 MF:C18H22N2 MW:266.3807 |
D-Lys(Z)-Pro-Arg-pNA (diacetate)Catalog No.:AA008TJ1 CAS No.:108963-70-8 MDL No.:MFCD29472230 MF:C35H51N9O11 MW:773.8331 |
Boc-L-pyroglutamic acid methyl esterCatalog No.:AA0034HD CAS No.:108963-96-8 MDL No.:MFCD06809720 MF:C11H17NO5 MW:243.2564 |
Bis(acetoxymethyl) 2,2'-((2-(5-((acetoxymethoxy)carbonyl)oxazol-2-yl)-5-(2-(2-(bis(2-(acetoxymethoxy)-2-oxoethyl)amino)-5-methylphenoxy)ethoxy)benzofuran-6-yl)azanediyl)diacetateCatalog No.:AA007TQ2 CAS No.:108964-32-5 MDL No.:MFCD00036976 MF:C44H47N3O24 MW:1001.8497 |
GlcNAcβ(1-4)[Fucα(1-6)]GlcNAcCatalog No.:AA00IMF4 CAS No.:108964-40-5 MDL No.: MF:C22H38N2O15 MW:570.5415 |
3,4-DifluorobenzenesulfonamideCatalog No.:AA003IE5 CAS No.:108966-71-8 MDL No.:MFCD00728800 MF:C6H5F2NO2S MW:193.1712 |
(6-Bromobenzofuran-2-yl)methanolCatalog No.:AA01DEDM CAS No.:1089682-06-3 MDL No.:MFCD22370368 MF:C9H7BrO2 MW:227.0547 |
Dichlorobis(2-ethylhexyl)silaneCatalog No.:AA007TPW CAS No.:1089687-03-5 MDL No.:MFCD22370170 MF:C16H34Cl2Si MW:325.4327 |
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4h-silolo[3,2-b:4,5-b']dithiopheneCatalog No.:AA0091WJ CAS No.:1089687-05-7 MDL No.:MFCD16621138 MF:C24H36Br2S2Si MW:576.5661 |
2-(difluoromethoxy)acetic acidCatalog No.:AA01BDTB CAS No.:1089695-61-3 MDL No.:MFCD28118272 MF:C3H4F2O3 MW:126.0589 |
1-(5-bromo-3-fluoro-2-hydroxyphenyl)ethanoneCatalog No.:AA0091D9 CAS No.:1089706-28-4 MDL No.:MFCD16742826 MF:C8H6BrFO2 MW:233.0344 |
3-Hydroxycyclobutyl pivalateCatalog No.:AA00HB70 CAS No.:1089709-03-4 MDL No.:MFCD27955486 MF:C9H16O3 MW:172.2215 |
3-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]cyclobutanolCatalog No.:AA00999D CAS No.:1089709-08-9 MDL No.:MFCD23105929 MF:C10H22O2Si MW:202.3660 |
benzyl 2-(difluoromethoxy)acetateCatalog No.:AA01BHMC CAS No.:1089709-30-7 MDL No.:MFCD28246278 MF:C10H10F2O3 MW:216.1814 |
benzyl[1-(2-methoxyphenyl)propan-2-yl]amineCatalog No.:AA01A7UP CAS No.:108971-51-3 MDL No.:MFCD12509323 MF:C17H21NO MW:255.3547 |
1-cyclopropyl-2-methanesulfonylethan-1-oneCatalog No.:AA01BF34 CAS No.:1089722-36-0 MDL No.:MFCD19606969 MF:C6H10O3S MW:162.2068 |
4-(ethylamino)-2-(methylthio)-5-Pyrimidinecarboxylic acidCatalog No.:AA019EL6 CAS No.:1089724-08-2 MDL No.:MFCD12911522 MF:C8H11N3O2S MW:213.2568 |
(3-Methoxypropyl)boronic acidCatalog No.:AA01ADCH CAS No.:1089725-75-6 MDL No.:MFCD06212375 MF:C4H11BO3 MW:117.9393 |
Valsartan-d8Catalog No.:AA00923C CAS No.:1089736-72-0 MDL No.:MFCD12022350 MF:C24H21D8N5O3 MW:443.5681 |
L-Valine, N-(1-oxopentyl-d9)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]Methyl]-Catalog No.:AA008TKL CAS No.:1089736-73-1 MDL No.:MFCD08063540 MF:C24H20D9N5O3 MW:444.5742 |
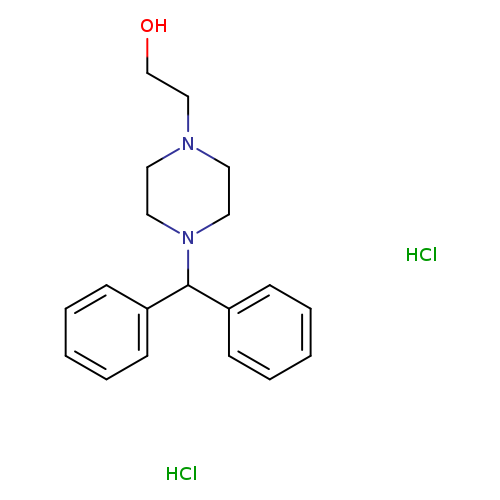
4-(Diphenylmethyl)-1-piperazineethanol dihydrochlorideCatalog No.:AA0082UN CAS No.:108983-83-1 MDL No.:MFCD03844661 MF:C19H26Cl2N2O MW:369.3285 |
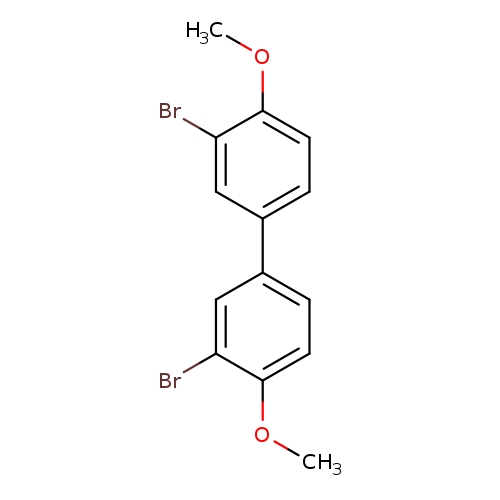
3,3'-Dibromo-4,4'-dimethoxybiphenylCatalog No.:AA003I9X CAS No.:108989-36-2 MDL No.:MFCD10000957 MF:C14H12Br2O2 MW:372.0519 |
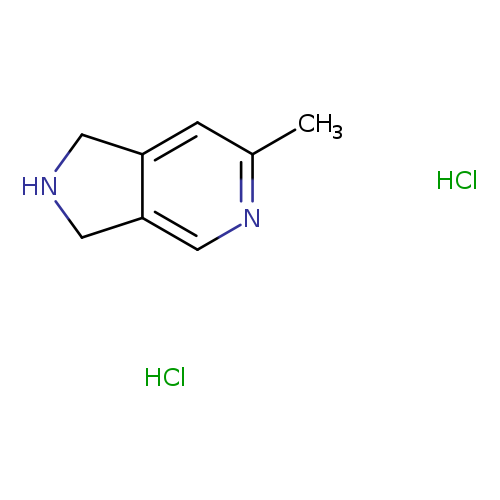
6-methyl-2,3-dihydro-1H-pyrrolo[3,4-c]pyridine hydrochlorideCatalog No.:AA008ZQ8 CAS No.:108989-52-2 MDL No.:MFCD30479746 MF:C8H12Cl2N2 MW:207.1003 |
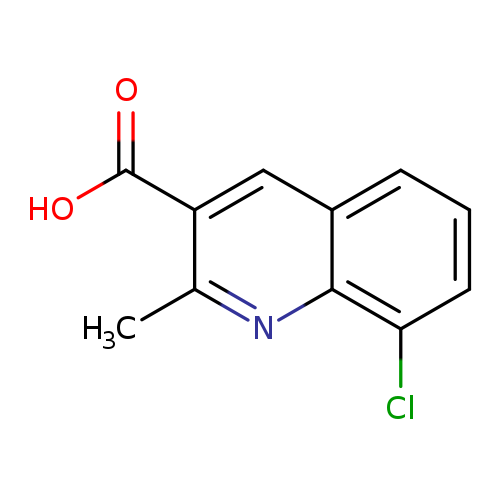
8-chloro-2-methylquinoline-3-carboxylic acidCatalog No.:AA01BXIE CAS No.:1089898-90-7 MDL No.:MFCD20036314 MF:C11H8ClNO2 MW:221.6397 |
5-methyl-1-phenyl-1H-1,2,4-triazol-3-amineCatalog No.:AA01AN0S CAS No.:1089899-70-6 MDL No.:MFCD20693895 MF:C9H10N4 MW:174.2025 |
6,7-Dihydro-5H-cyclopenta[d]pyrimidin-2-amineCatalog No.:AA007BGU CAS No.:108990-72-3 MDL No.:MFCD09864349 MF:C7H9N3 MW:135.1665 |
N,N-Bis(propan-2-yl)piperidine-4-carboxamide hydrochlorideCatalog No.:AA01A8G5 CAS No.:108992-66-1 MDL No.:MFCD16040073 MF:C12H25ClN2O MW:248.7927 |
Ethyl 3-methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-2-carboxylateCatalog No.:AA00IXIK CAS No.:108993-84-6 MDL No.:MFCD01044397 MF:C12H14O4 MW:222.2372 |
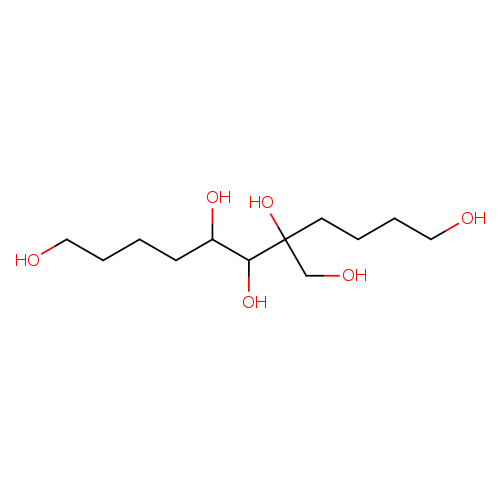
5-(Hydroxymethyl)undecane-1,5,6,7,11-pentaolCatalog No.:AA01FOOQ CAS No.:108993-87-9 MDL No.:MFCD00085565 MF:C12H26O6 MW:266.3312 |
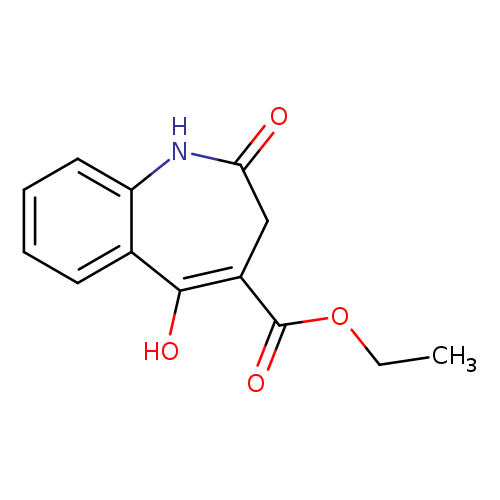
5-HYDROXY-2-OXO-2,3-DIHYDRO-1H-[1]BENZAZEPHE-4-CARBOXYLIC ACID ETHYL ESTERCatalog No.:AA008WGR CAS No.:108993-98-2 MDL No.:MFCD00666164 MF:C13H13NO4 MW:247.2466 |
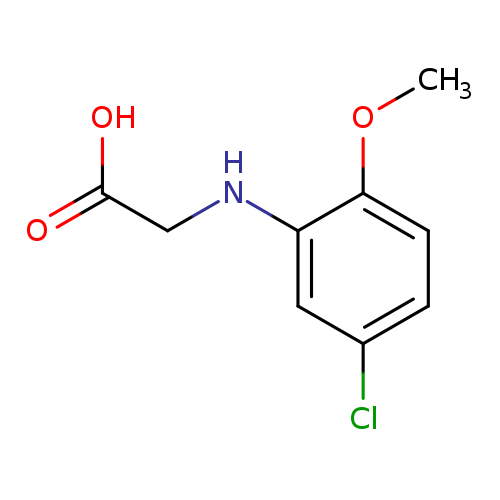
2-[(5-Chloro-2-methoxyphenyl)amino]acetic acidCatalog No.:AA019WWM CAS No.:108994-41-8 MDL No.:MFCD06849813 MF:C9H10ClNO3 MW:215.6336 |
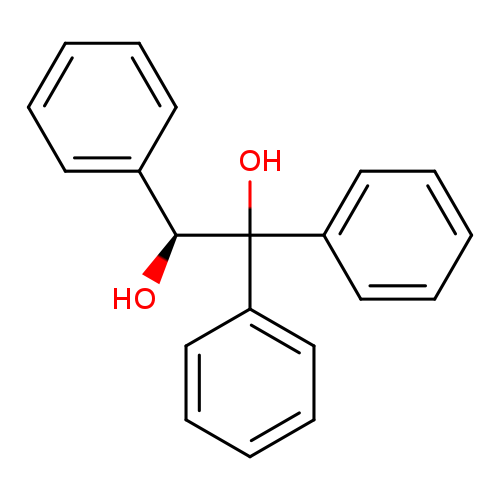
(S)-1,1,2-Triphenyl-1,2-ethanediolCatalog No.:AA007BGR CAS No.:108998-83-0 MDL No.:MFCD19232237 MF:C20H18O2 MW:290.3557 |
3-HydroxypyridineCatalog No.:AA003JI6 CAS No.:109-00-2 MDL No.:MFCD00006378 MF:C5H5NO MW:95.0993 |
1-MethylpiperazineCatalog No.:AA0032QJ CAS No.:109-01-3 MDL No.:MFCD00005966 MF:C5H12N2 MW:100.1622 |
4-MethylmorpholineCatalog No.:AA003416 CAS No.:109-02-4 MDL No.:MFCD00006175 MF:C5H11NO MW:101.1469 |
2-BromopyridineCatalog No.:AA003346 CAS No.:109-04-6 MDL No.:MFCD00006219 MF:C5H4BrN MW:157.9960 |
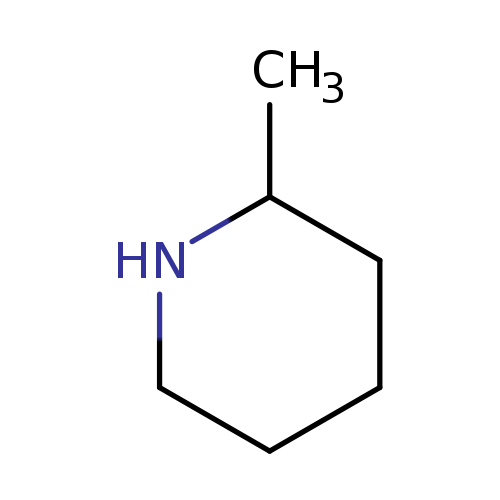
2-MethylpiperidineCatalog No.:AA0033BL CAS No.:109-05-7 MDL No.:MFCD00005982 MF:C6H13N MW:99.1741 |
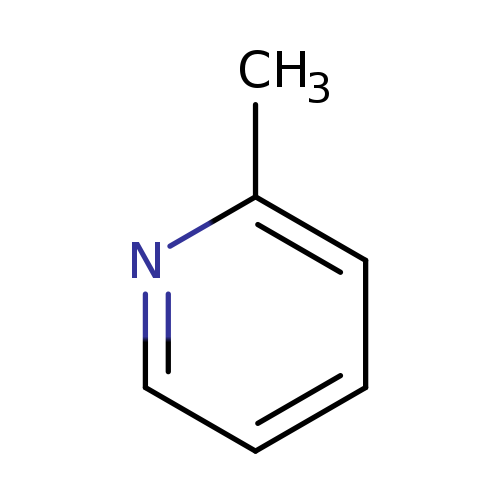
2-MethylpyridineCatalog No.:AA00HB73 CAS No.:109-06-8 MDL No.:MFCD00006332 MF:C6H7N MW:93.1265 |
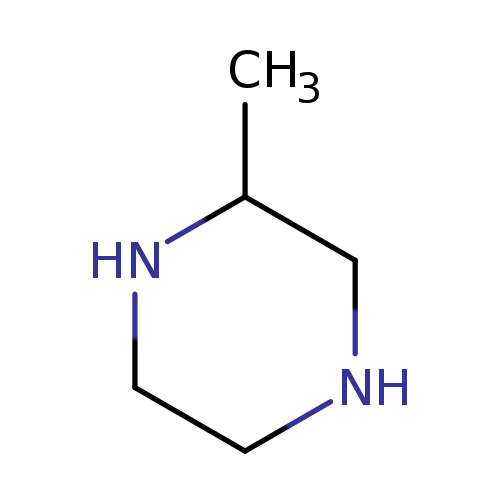
2-MethylpiperazineCatalog No.:AA0033BK CAS No.:109-07-9 MDL No.:MFCD00005954 MF:C5H12N2 MW:100.1622 |
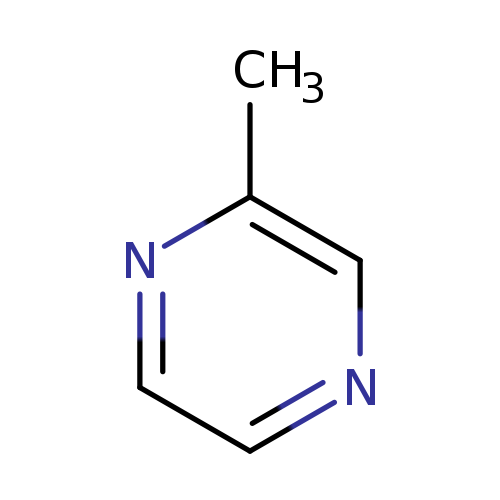
2-MethylpyrazineCatalog No.:AA003HNI CAS No.:109-08-0 MDL No.:MFCD00006142 MF:C5H6N2 MW:94.1145 |
2-ChloropyridineCatalog No.:AA00IKAQ CAS No.:109-09-1 MDL No.:MFCD00006228 MF:C5H4ClN MW:113.5450 |
Morpholin-3-oneCatalog No.:AA003JJF CAS No.:109-11-5 MDL No.:MFCD00631009 MF:C4H7NO2 MW:101.1039 |
2-AminopyrimidineCatalog No.:AA00331O CAS No.:109-12-6 MDL No.:MFCD00006089 MF:C4H5N3 MW:95.1026 |
Triethylene glycol dimethacrylateCatalog No.:AA003UZ3 CAS No.:109-16-0 MDL No.:MFCD00008591 MF:C14H22O6 MW:286.3209 |
Tetraethylene glycol dimethacrylateCatalog No.:AA003ULF CAS No.:109-17-1 MDL No.:MFCD00014932 MF:C16H26O7 MW:330.3734 |
Butyric Acid Butyl EsterCatalog No.:AA003OJR CAS No.:109-21-7 MDL No.:MFCD00009450 MF:C8H16O2 MW:144.2114 |
16-HexadecanolideCatalog No.:AA003DST CAS No.:109-29-5 MDL No.:MFCD00039668 MF:C16H30O2 MW:254.4082 |
Dibutyl decanedioateCatalog No.:AA003P9L CAS No.:109-43-3 MDL No.:MFCD00027218 MF:C18H34O4 MW:314.4602 |
Bis(2-ethoxyethyl) adipateCatalog No.:AA003O5M CAS No.:109-44-4 MDL No.:MFCD00041926 MF:C14H26O6 MW:290.3526 |

1,3-Dibutyl-2-thioureaCatalog No.:AA003DGE CAS No.:109-46-6 MDL No.:MFCD00004926 MF:C9H20N2S MW:188.3335 |
5-Hexen-2-oneCatalog No.:AA00347B CAS No.:109-49-9 MDL No.:MFCD00008793 MF:C6H10O MW:98.1430 |
Valeric AcidCatalog No.:AA0035PQ CAS No.:109-52-4 MDL No.:MFCD00004413 MF:C5H10O2 MW:102.1317 |
Isobutyl vinyl etherCatalog No.:AA003R07 CAS No.:109-53-5 MDL No.:MFCD00008934 MF:C6H12O MW:100.1589 |
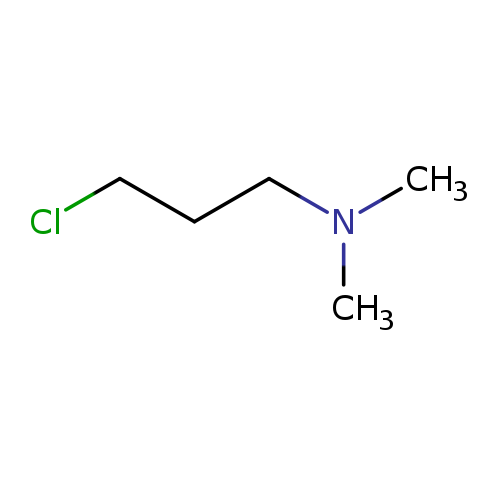
3-Chloro-1-(N,N-dimethyl)propylamineCatalog No.:AA008R9P CAS No.:109-54-6 MDL No.:MFCD00044496 MF:C5H12ClN MW:121.6085 |
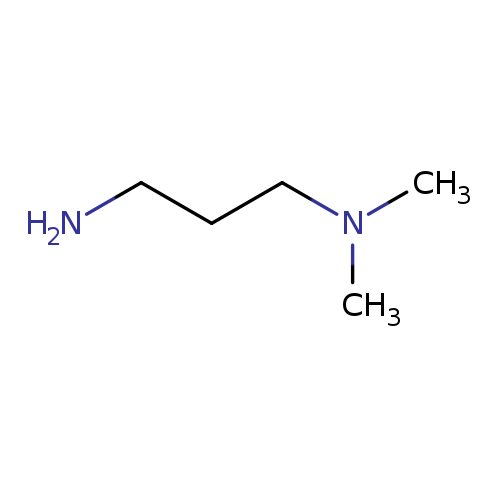
N,N-Dimethyl-1,3-propanediamineCatalog No.:AA0033M2 CAS No.:109-55-7 MDL No.:MFCD00008216 MF:C5H14N2 MW:102.1781 |
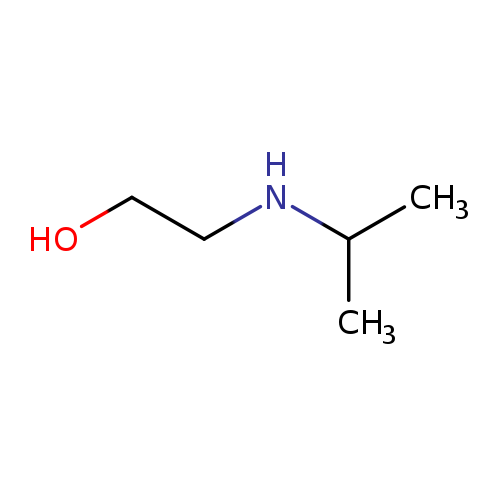
2-(Isopropylamino)ethanolCatalog No.:AA00IKDT CAS No.:109-56-8 MDL No.:MFCD00041755 MF:C5H13NO MW:103.1628 |
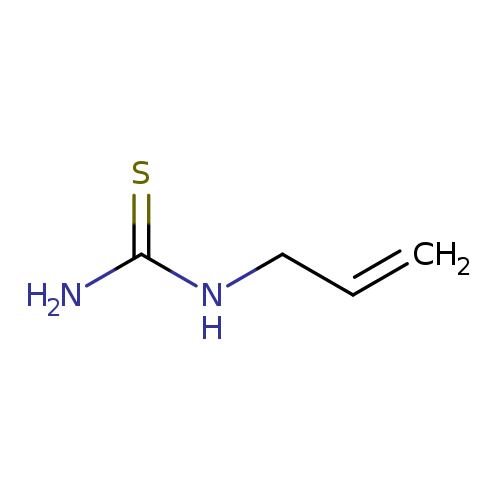
N-AllylthioureaCatalog No.:AA003NNT CAS No.:109-57-9 MDL No.:MFCD00004940 MF:C4H8N2S MW:116.1847 |
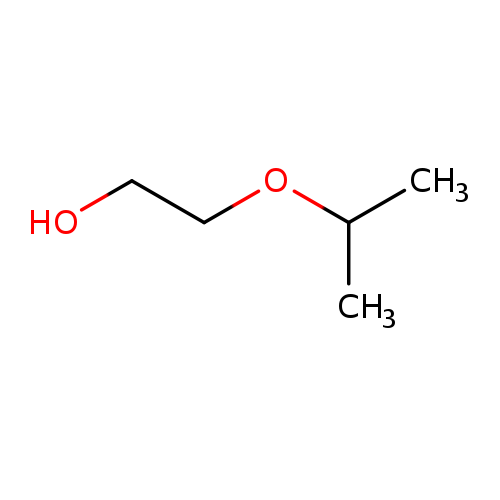
2-IsopropoxyethanolCatalog No.:AA003HEM CAS No.:109-59-1 MDL No.:MFCD00002866 MF:C5H12O2 MW:104.1476 |
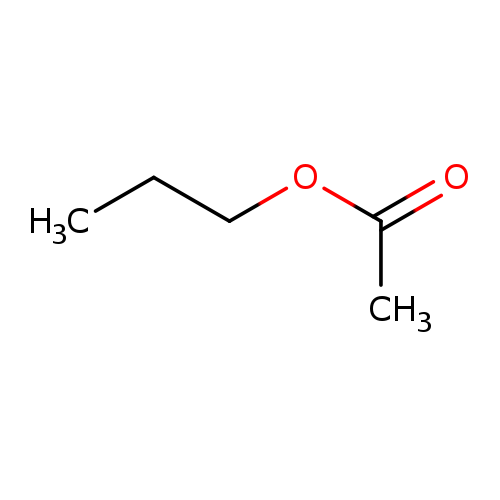
Propyl acetateCatalog No.:AA003TX0 CAS No.:109-60-4 MDL No.:MFCD00009372 MF:C5H10O2 MW:102.1317 |
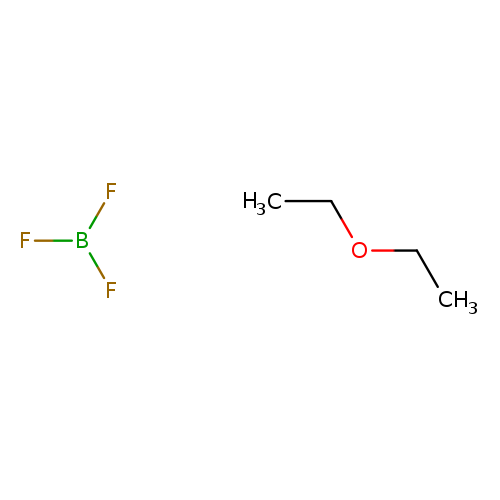
boron trifluoride ethyl etherCatalog No.:AA0035SP CAS No.:109-63-7 MDL No.:MFCD00013194 MF:C4H10BF3O MW:141.9278 |
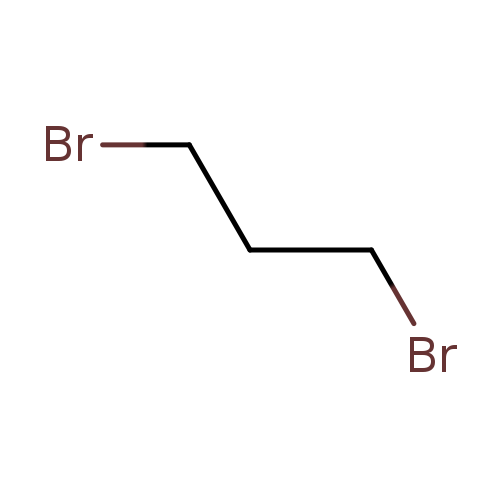
1,3-DibromopropaneCatalog No.:AA0032IS CAS No.:109-64-8 MDL No.:MFCD00000255 MF:C3H6Br2 MW:201.8877 |
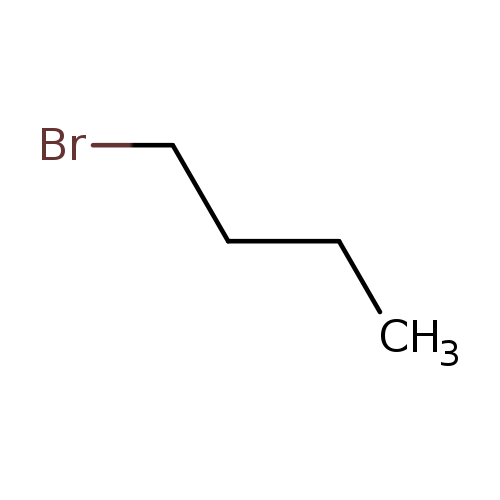
1-BromobutaneCatalog No.:AA0034HU CAS No.:109-65-9 MDL No.:MFCD00000260 MF:C4H9Br MW:137.0183 |
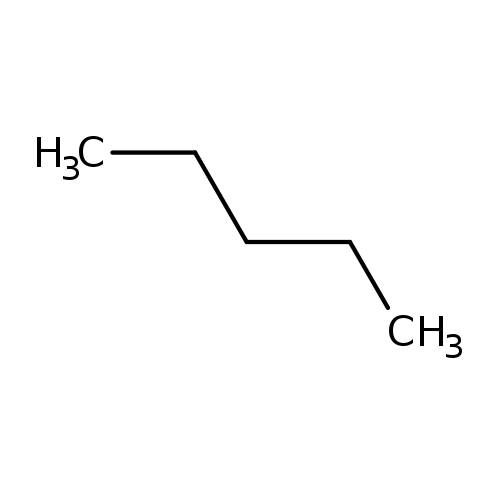
n-PentaneCatalog No.:AA009QSB CAS No.:109-66-0 MDL No.:MFCD00009498 MF:C5H12 MW:72.1488 |
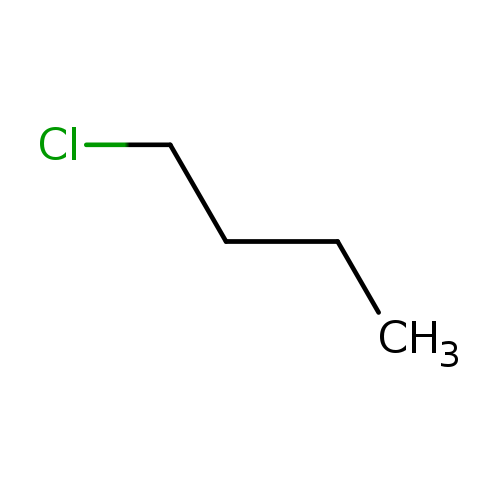
1-ChlorobutaneCatalog No.:AA0032O3 CAS No.:109-69-3 MDL No.:MFCD00001009 MF:C4H9Cl MW:92.5673 |
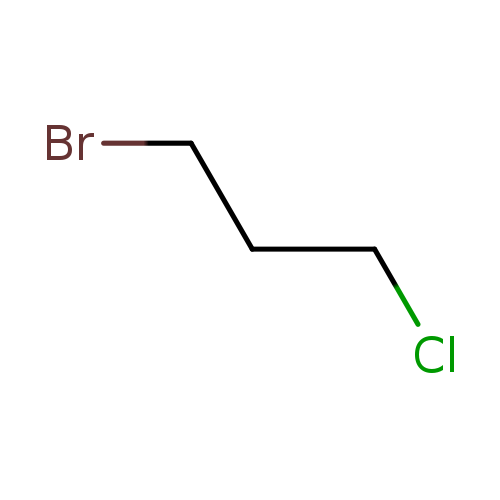
1-Bromo-3-chloropropaneCatalog No.:AA003E1B CAS No.:109-70-6 MDL No.:MFCD00000998 MF:C3H6BrCl MW:157.4367 |
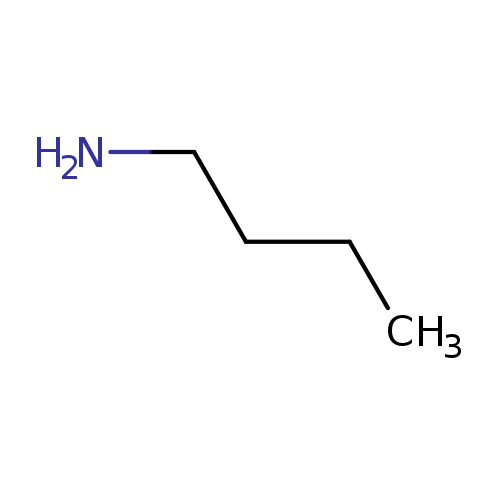
ButylamineCatalog No.:AA0035AH CAS No.:109-73-9 MDL No.:MFCD00011690 MF:C4H11N MW:73.1368 |
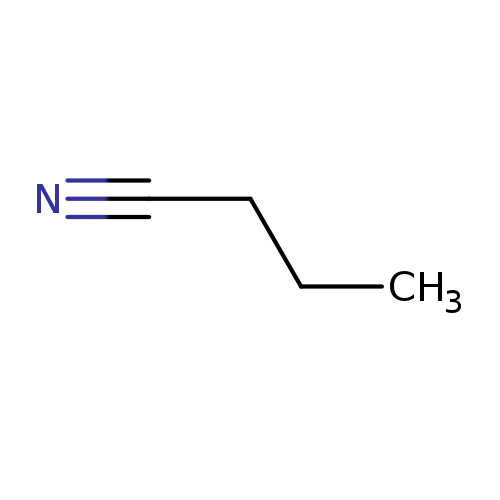
ButanenitrileCatalog No.:AA007SEG CAS No.:109-74-0 MDL No.:MFCD00001968 MF:C4H7N MW:69.1051 |
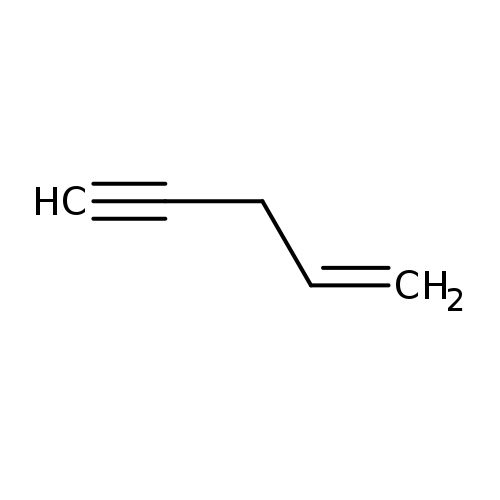
3-ButenenitrileCatalog No.:AA003J2U CAS No.:109-75-1 MDL No.:MFCD00001962 MF:C5H6 MW:66.1011 |
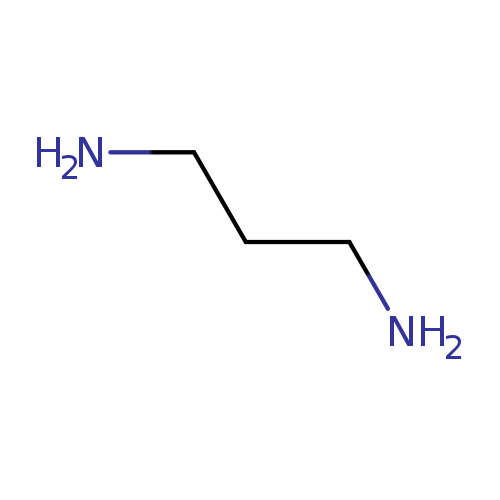
1,3-DiaminopropaneCatalog No.:AA0032IP CAS No.:109-76-2 MDL No.:MFCD00008228 MF:C3H10N2 MW:74.1249 |
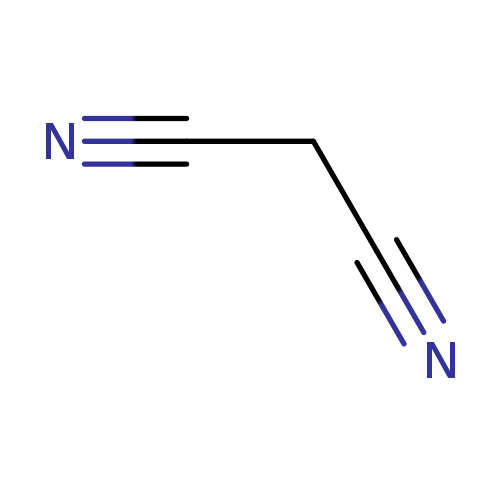
MalononitrileCatalog No.:AA003RCS CAS No.:109-77-3 MDL No.:MFCD00001883 MF:C3H2N2 MW:66.0614 |
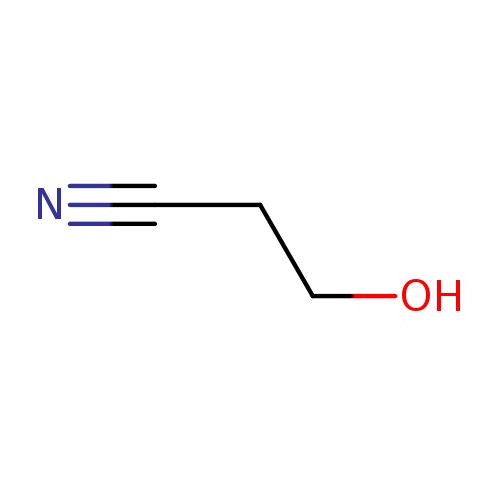
3-HydroxypropionitrileCatalog No.:AA0033NH CAS No.:109-78-4 MDL No.:MFCD00002826 MF:C3H5NO MW:71.0779 |
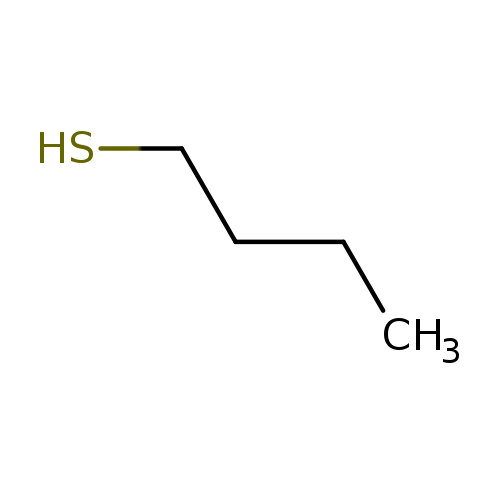
1-ButanethiolCatalog No.:AA003E34 CAS No.:109-79-5 MDL No.:MFCD00004905 MF:C4H10S MW:90.1872 |
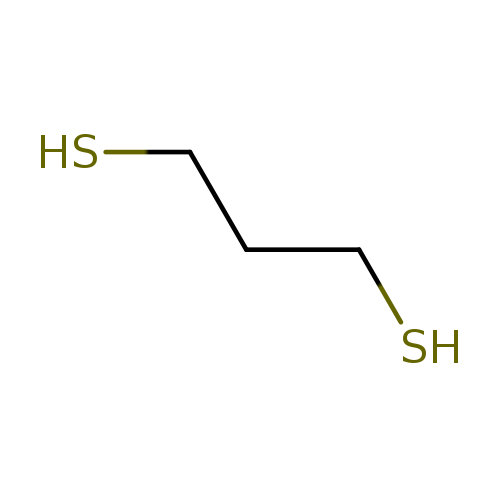
1,3-DimercaptopropaneCatalog No.:AA0032J0 CAS No.:109-80-8 MDL No.:MFCD00004904 MF:C3H8S2 MW:108.2256 |
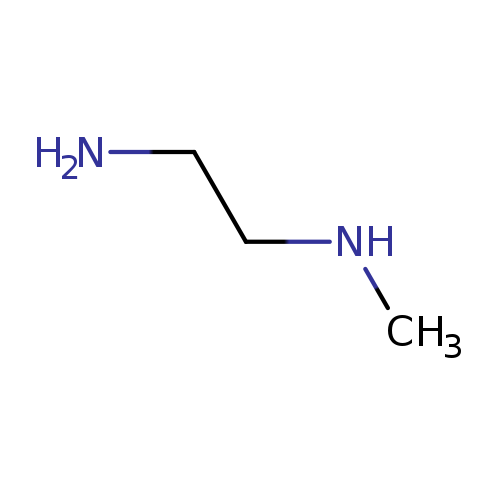
N-MethylethylenediamineCatalog No.:AA0035BM CAS No.:109-81-9 MDL No.:MFCD00008165 MF:C3H10N2 MW:74.1249 |
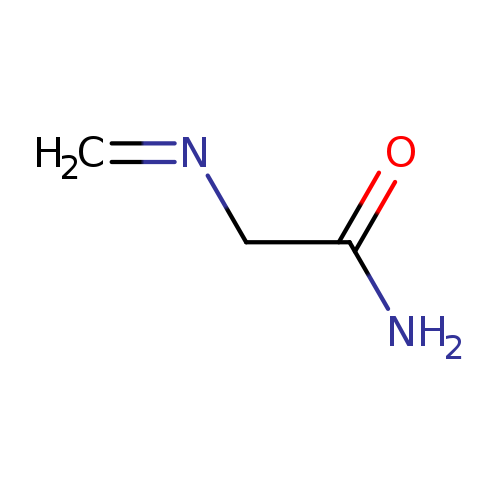
MethylenaminoacetonitrileCatalog No.:AA003S0Y CAS No.:109-82-0 MDL No.:MFCD00001889 MF:C3H6N2O MW:86.0925 |
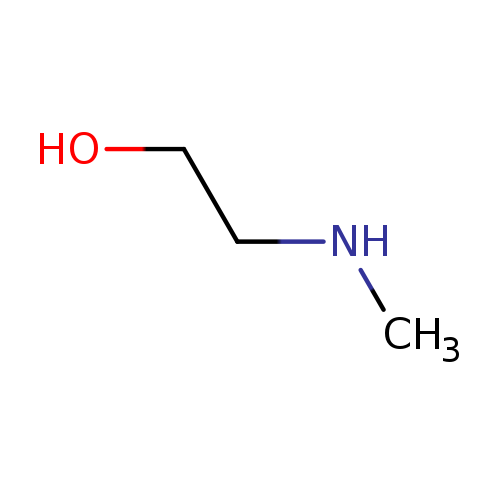
2-(Methylamino)ethanolCatalog No.:AA0032SW CAS No.:109-83-1 MDL No.:MFCD00002839 MF:C3H9NO MW:75.1097 |
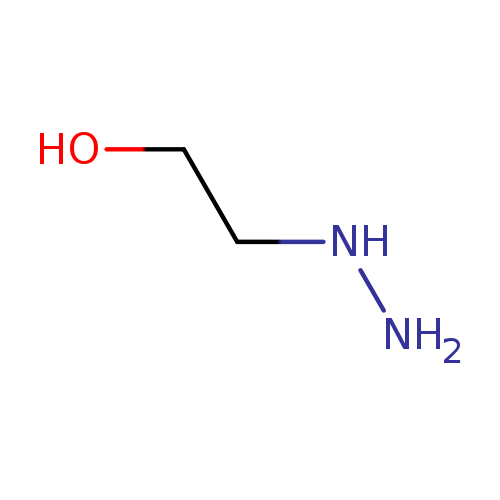
2-HydroxyethylhydrazineCatalog No.:AA00339B CAS No.:109-84-2 MDL No.:MFCD00007623 MF:C2H8N2O MW:76.0977 |
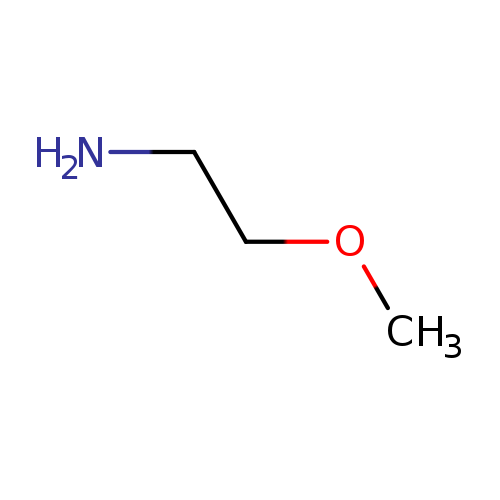
2-MethoxyethylamineCatalog No.:AA0033AX CAS No.:109-85-3 MDL No.:MFCD00008180 MF:C3H9NO MW:75.1097 |
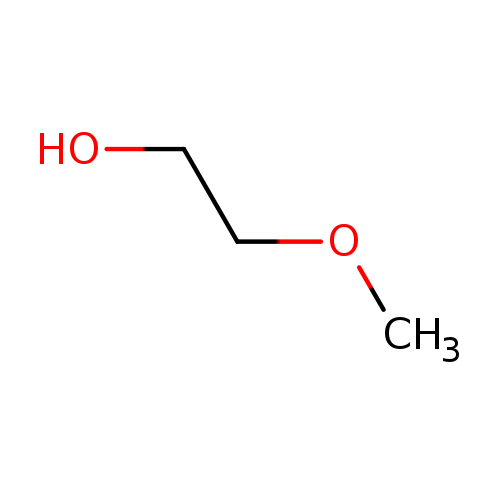
2-MethoxyethanolCatalog No.:AA0033AU CAS No.:109-86-4 MDL No.:MFCD00002867 MF:C3H8O2 MW:76.0944 |