Title: Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells.
Journal: Chemico-biological interactions 20150605
Title: Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues.
Journal: Chemico-biological interactions 20130225
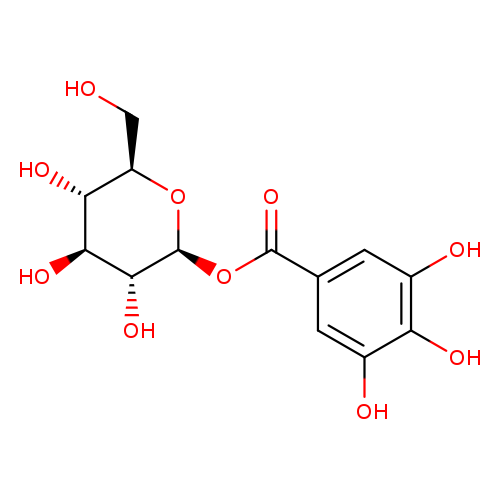
Title: The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis.
Journal: PloS one 20120101
Title: Method development for β-glucogallin and gallic acid analysis: application to urinary pharmacokinetic studies.
Journal: Journal of pharmaceutical and biomedical analysis 20110325
Title: Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo.
Journal: Biological & pharmaceutical bulletin 20110101
Title: Characterization of gallotannins from Astronium species by flow injection analysis- electrospray ionization-ion trap-tandem mass spectrometry and matrix-assisted laser desorption/ionization time-of- flight mass spectrometry.
Journal: European journal of mass spectrometry (Chichester, England) 20110101
Title: Diterpene esters and phenolic compounds from Sapium insigne (ROYLE) BENTH. ex HOOK. fil.
Journal: Chemical & pharmaceutical bulletin 20091101
Title: [Studies on chemical constituents of leaves of Psidium guajava].
Journal: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20090301
Title: Identification of multiple constituents in the traditional Chinese medicine formula GuiZhiFuLing-Wan by HPLC-DAD-MS/MS.
Journal: Journal of pharmaceutical and biomedical analysis 20090220
Title: Chemical profiling of Radix Paeoniae evaluated by ultra-performance liquid chromatography/photo-diode-array/quadrupole time-of-flight mass spectrometry.
Journal: Journal of pharmaceutical and biomedical analysis 20090220
Title: High-speed separation and characterization of major constituents in Radix Paeoniae Rubra by fast high-performance liquid chromatography coupled with diode-array detection and time-of-flight mass spectrometry.
Journal: Rapid communications in mass spectrometry : RCM 20090101
Title: [Study on hydrolysable tannin constituents of seed of Juglans regia II].
Journal: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20080701
Title: Comparative study of chemical constituents of rhubarb from different origins.
Journal: Chemical & pharmaceutical bulletin 20061101
Title: [The chemical constituents of Breynia rostrata].
Journal: Yao xue xue bao = Acta pharmaceutica Sinica 20060201
Title: Anti-babesial and anti-plasmodial compounds from Phyllanthus niruri.
Journal: Journal of natural products 20050401
Title: Novel lipid-peroxidation- and cyclooxygenase-inhibitory tannins from Picrorhiza kurroa seeds.
Journal: Chemistry & biodiversity 20040301
Title: [Studies on chemical constituents in fruits of Tibetan medicine Phyllanthus emblica].
Journal: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20031001
Title: Gallotannin biosynthesis: two new galloyltransferases from Rhus typhina leaves preferentially acylating hexa- and heptagalloylglucoses.
Journal: Planta 20021101
Title: Gallic esters of sucrose as efficient radical scavengers in lipid peroxidation.
Journal: Journal of agricultural and food chemistry 20020605
Title: Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis.
Journal: Planta medica 20020501
Title: Ethyl m-digallate from red maple, Acer rubrum L., as the major resistance factor to forest tent caterpillar, Malacosoma disstria Hbn.
Journal: Journal of chemical ecology 20011201
Title: Gallotannin biosynthesis: beta-glucogallin: hexagalloyl 3-O-galloyltransferase from Rhus typhina leaves.
Journal: Phytochemistry 20011101
Title: A new galloylglucoside from Cleyera ochnacea DC.
Journal: Chemical & pharmaceutical bulletin 20011101
Title: Synthesis of gallotannins.
Journal: Carbohydrate research 20011015
Title: Ellagic acid formation from galloylglucoses by a crude enzyme of Cornus capitata adventitious roots.
Journal: Bioscience, biotechnology, and biochemistry 20010801
Title: Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens.
Journal: Phytochemistry 20010501
Title: Inhibitory effects of ellagi- and gallotannins on rat intestinal alpha-glucosidase complexes.
Journal: Bioscience, biotechnology, and biochemistry 20010301
Title: Inhibitory effects of Egyptian folk medicines on human immunodeficiency virus (HIV) reverse transcriptase.
Journal: Chemical & pharmaceutical bulletin 19950401