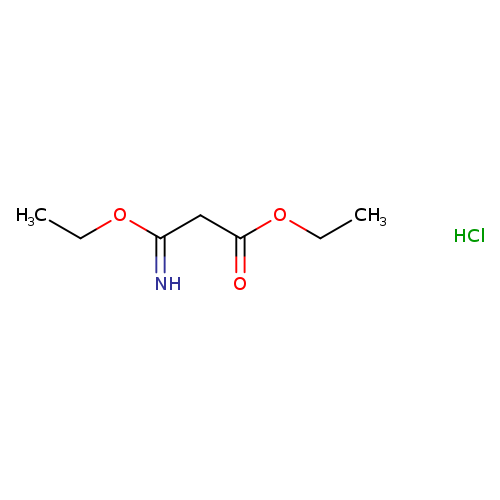
[1]JournalofMedicinalChemistry,2016,vol.59,#13,p.6070-6085
[2]JournalofMedicinalChemistry,2012,vol.55,#7,p.3398-3413
[3]RussianJournalofGeneralChemistry,2001,vol.71,#7,p.1076-1087
[1]RussianJournalofGeneralChemistry,2001,vol.71,#7,p.1076-1087
[2]Patent:WO2013/101974,2013,A1,.Locationinpatent:Paragraph00574;00575;00576;00577
[3]JournalofMedicinalChemistry,2016,vol.59,#13,p.6070-6085
[4]Patent:US9617268,2017,B2,.Locationinpatent:Page/Pagecolumn319
[1]BioorganicandMedicinalChemistryLetters,2012,vol.22,#23,p.7207-7213
[2]ChemicalandPharmaceuticalBulletin,1985,vol.33,#7,p.3046-3049
[3]Heterocycles,1996,vol.43,#9,p.1981-1989
[4]Tetrahedron,2009,vol.65,#4,p.757-764
[5]Patent:WO2006/7700,2006,A1,.Locationinpatent:Page/Pagecolumn66-67
[6]Patent:US2006/19905,2006,A1,.Locationinpatent:Page/Pagecolumn27
[7]Patent:WO2006/85,2006,A1,.Locationinpatent:Page/Pagecolumn80-81
[8]Patent:WO2007/9227,2007,A1,.Locationinpatent:Page/Pagecolumn36
[9]RussianJournalofGeneralChemistry,2001,vol.71,#7,p.1076-1087
[10]JournalofMedicinalChemistry,2012,vol.55,#7,p.3398-3413
[11]Synthesis(Germany),2016,vol.48,#17,p.2851-2862
[12]Patent:WO2008/6540,2008,A1,.Locationinpatent:Page/Pagecolumn34,53
[13]JournaloftheAmericanChemicalSociety,1945,vol.67,p.1012,1015
[14]JournaloftheChemicalSociety,PerkinTransactions1:OrganicandBio-OrganicChemistry(1972-1999),1987,p.1845-1852
[15]JournaloftheAmericanChemicalSociety,1983,vol.105,#12,p.3824-3832
[16]Farmaco,1993,vol.48,#3,p.357-374
[17]ChemicalandPharmaceuticalBulletin,1995,vol.43,#5,p.788-796
[18]ChemicalandPharmaceuticalBulletin,1995,vol.43,#5,p.797-817
[19]Patent:WO2008/33745,2008,A2,.Locationinpatent:Page/Pagecolumn70
[20]Patent:WO2008/51514,2008,A2,.Locationinpatent:Page/Pagecolumn44
[21]Patent:WO2008/57208,2008,A2,.Locationinpatent:Page/Pagecolumn48
[22]Patent:WO2008/33747,2008,A2,.Locationinpatent:Page/Pagecolumn254-255
[23]EuropeanJournalofMedicinalChemistry,2009,vol.44,#3,p.1369-1376
[24]Patent:WO2013/12915,2013,A1,.Locationinpatent:Paragraph00664
[25]JournalofMedicinalChemistry,2014,vol.57,#5,p.1770-1776
[26]Patent:WO2014/205223,2014,A1,.Locationinpatent:Paragraph0149-0150
[27]Synlett,2015,vol.26,#3,p.393-403
[28]LettersinDrugDesignandDiscovery,2014,vol.11,#10,p.1143-1148
[29]NewJournalofChemistry,2017,vol.41,#5,p.1918-1924
[30]Patent:WO2017/189553,2017,A1,.Locationinpatent:Paragraph0341;0342
[1]Patent:WO2015/89327,2015,A1,.Locationinpatent:Paragraph0413
[1]ChemischeBerichte,1962,vol.95,p.281-294
[2]JournaloftheAmericanChemicalSociety,1983,vol.105,p.3824-3832
[1]IlFarmaco,1993,vol.48,p.357-374
[2]EuropeanJournalofMedicinalChemistry,2009,vol.44,p.1369-1376
[1]Kiseleva,V.V.;Gakh,A.A.;Fainzil'berg,A.A.[BulletinoftheAcademyofSciencesoftheUSSRDivisionofChemicalScience,1990,vol.39,#9.2.,p.1888-1895][IzvestiyaAkademiiNaukSSSR,SeriyaKhimicheskaya,1990,vol.39,#9,p.2075-2084]
[2]Ahad,AliMd.;Zuohe,Song;Du-Cuny,Lei;Moses,SylvestorA.;Zhou,LiLi;Zhang,Shuxing;Powis,Garth;Meuillet,EmmanuelleJ.;Mash,EugeneA.[BioorganicandMedicinalChemistry,2011,vol.19,#6,p.2046-2054]
[3]CurrentPatentAssignee:THEUNIVERSITYOFTEXASSYSTEM;ARIZONABOARDOFREGENTS-WO2009/129267,2009,A2Locationinpatent:Page/Pagecolumn88
[1]BulletinoftheAcademyofSciencesoftheUSSRDivisionofChemicalScience,1990,vol.39,p.1888-1895 IzvestiyaAkademiiNaukSSSR,SeriyaKhimicheskaya,1990,vol.39,p.2075-2084