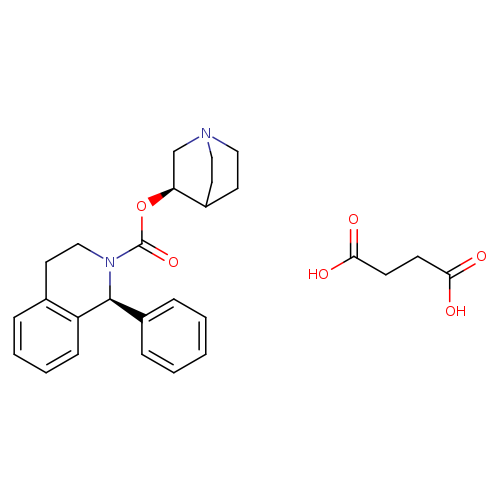
[1]CurrentPatentAssignee:DRREDDY'SLABORATORIESLIMITED-WO2008/11462,2008,A2Locationinpatent:Page/Pagecolumn25;26
[1]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[2]CurrentPatentAssignee:BEIJINGLABWORLDBIOMEDICALTECHNOLOGYCOLTD-CN108314681,2018,ALocationinpatent:Paragraph0012;0014
[3]CurrentPatentAssignee:WEIHAIDISUPHARM;DISHAPHARMACEUTICALGROUP;SHANDONGDISHAPHARMACEUTICALDISHAPHAMACEUTICAL-CN103450183,2017,BLocationinpatent:Paragraph0018;0037-0039
[4]CurrentPatentAssignee:ZAKLADYFARMACEUTYCZNEPOLPHARMAS.A.-WO2009/142522,2009,A1Locationinpatent:Page/Pagecolumn12
[5]CurrentPatentAssignee:MEDICHEM,S.A.-US2010/29944,2010,A1Locationinpatent:Page/Pagecolumn7
[6]CurrentPatentAssignee:CHONGQINGKERUIPHARMACEUTICALGROUP-CN103896938,2016,BLocationinpatent:Paragraph0071
[7]CurrentPatentAssignee:KYUNGDONGPHARM.CO.,LTD.-US2015/112072,2015,A1Locationinpatent:Paragraph0084-0108
[8]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn13
[9]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[10]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[11]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn13
[12]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn10;11
[13]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[14]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn111
[15]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[16]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[17]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn12
[18]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn11
[19]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn10
[20]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn12
[21]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn9-10;11
[22]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn12
[23]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn10
[24]CurrentPatentAssignee:ASTELLASPHARMAINC.-EP1714965,2006,A1Locationinpatent:Page/Pagecolumn10
[25]CurrentPatentAssignee:DRREDDY'SLABORATORIESLIMITED-WO2008/11462,2008,A2Locationinpatent:Page/Pagecolumn24
[26]CurrentPatentAssignee:DRREDDY'SLABORATORIESLIMITED-WO2008/11462,2008,A2Locationinpatent:Page/Pagecolumn26;27
[27]CurrentPatentAssignee:DRREDDY'SLABORATORIESLIMITED-WO2008/11462,2008,A2Locationinpatent:Page/Pagecolumn24;25
[28]CurrentPatentAssignee:MEDICHEM,S.A.-US2008/242697,2008,A1Locationinpatent:Page/Pagecolumn4
[29]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-WO2009/11844,2009,A1Locationinpatent:Page/Pagecolumn16
[30]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-WO2009/11844,2009,A1Locationinpatent:Page/Pagecolumn16-17;21;21-22;22-23
[31]CurrentPatentAssignee:DIPHARMAFRANCISS.R.L.-US2009/203915,2009,A1Locationinpatent:Page/Pagecolumn3-4
[32]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn9
[33]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-US2008/114028,2008,A1Locationinpatent:Page/Pagecolumn12
[34]CurrentPatentAssignee:TEVAPHARMACEUTICALINDUSTRIESLTD.-WO2007/76116,2007,A2Locationinpatent:Page/Pagecolumn13-14
[35]CurrentPatentAssignee:KRKADDNOVOMESTO-WO2011/3624,2011,A1Locationinpatent:Page/Pagecolumn22
[36]CurrentPatentAssignee:ZAKLFARMACEUTYCZNEPOLPHARMASPOLKAAKCYJNA;ZAKLADYFARMACEUTYCZNEPOLPHARMAS.A.-WO2011/86003,2011,A1Locationinpatent:Page/Pagecolumn13
[37]Locationinpatent:experimentalpartNiphade,NavnathC.;Jagtap,KunalM.;Mali,AnilC.;Solanki,PavankumarV.;Jachak,MadhukarN.;Mathad,VijayavitthalT.[MonatsheftefurChemie,2011,vol.142,#11,p.1181-1186]
[38]CurrentPatentAssignee:CHENGDUHUAYUPHARMACEUTICAL-CN105566315,2016,ALocationinpatent:Paragraph0062;0063
[39]CurrentPatentAssignee:DRREDDY'SLABORATORIESLIMITED-WO2008/128028,2008,A2Locationinpatent:Page/Pagecolumn30-31
[1]CurrentPatentAssignee:ASTELLASPHARMAINC.-EP1714965,2006,A1Locationinpatent:Page/Pagecolumn11
 740780-79-4
740780-79-4
 110-15-6
110-15-6

 242478-37-1
242478-37-1
 862207-71-4
862207-71-4
 862207-70-3
862207-70-3


[1]CurrentPatentAssignee:ADVENTINTERNATIONALCORPORATION-WO2008/77357,2008,A2Locationinpatent:Page/Pagecolumn7-8
Title: Pharmacokinetics and toxicity of antimuscarinic drugs for overactive bladder treatment in females.
Journal: Expert opinion on drug metabolism & toxicology 20121101
Title: Efficacy and safety of solifenacin to treat overactive bladder symptoms in patients with idiopathic normal pressure hydrocephalus: an open-label, multicenter, prospective study.
Journal: Neurourology and urodynamics 20120901
Title: Solifenacin for overactive bladder: a systematic review and meta-analysis.
Journal: International urogynecology journal 20120801
Title: Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review.
Journal: Annals of internal medicine 20120619
Title: Urinary urgency: a review of its assessment as the key symptom of the overactive bladder syndrome.
Journal: World journal of urology 20120601
Title: Solifenacin objectively decreases urinary sensation in women with overactive bladder syndrome.
Journal: International urology and nephrology 20120401
Title: Improvement of urinary dysfunction after kidney transplantation by administration of the antimuscarinic agent--prospective randomized controlled study.
Journal: Transplantation 20120327
Title: The efficacy and safety of solifenacin in patients with overactive bladder syndrome.
Journal: Collegium antropologicum 20120301
Title: Which anticholinergic drug for overactive bladder symptoms in adults.
Journal: The Cochrane database of systematic reviews 20120118
Title: Efficacy of solifenacin for overactive bladder symptoms, symptom bother, and health-related quality of life in patients by duration of self-reported symptoms: a secondary analysis of the VIBRANT study.
Journal: Urologic nursing 20120101
Title: Adding to the evidence base: efficacy of solifenacin for overactive bladder symptoms, symptom bother, and health-related quality of life in patients by duration of self-reported symptoms: a secondary analysis of the VIBRANT study.
Journal: Urologic nursing 20120101
Title: Co-administration of an α(1) -blocker improves the efficacy and safety of antimuscarinic agents in rats with detrusor overactivity.
Journal: International journal of urology : official journal of the Japanese Urological Association 20111201
Title: Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol.
Journal: Urology 20111201
Title: Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
Journal: PLoS computational biology 20111201
Title: Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy.
Journal: Neurourology and urodynamics 20111101
Title: [Treatment for overactive bladder].
Journal: Der Urologe. Ausg. A 20111001
Title: Solifenacin may improve sleep quality in patients with overactive bladder and sleep disturbance.
Journal: Urology 20110901
Title: A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder.
Journal: British journal of clinical pharmacology 20110801
Title: The effect of solifenacin on urethral sphincter morphology.
Journal: International urogynecology journal 20110801
Title: Comparisons of urodynamic effects, therapeutic efficacy and safety of solifenacin versus tolterodine for female overactive bladder syndrome.
Journal: The journal of obstetrics and gynaecology research 20110801
Title: Efficacy of solifenacin on nocturia in Japanese patients with overactive bladder: impact on sleep evaluated by bladder diary.
Journal: The Journal of urology 20110701
Title: Tadalafil versus solifenacin for persistent storage symptoms after prostate surgery in patients with erectile dysfunction: a prospective randomized study.
Journal: International journal of urology : official journal of the Japanese Urological Association 20110701
Title: Solifenacin as add-on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms--ASSIST, randomized controlled study.
Journal: Urology 20110701
Title: Discovery of novel quaternary ammonium derivatives of (3R)-quinuclidinyl carbamates as potent and long acting muscarinic antagonists.
Journal: Bioorganic & medicinal chemistry letters 20110601
Title: Which single-item measures of overactive bladder symptom treatment correlate best with patient satisfaction?
Journal: Neurourology and urodynamics 20110401
Title: Evaluation of brain anticholinergic activities of urinary spasmolytic drugs using a high-throughput radio receptor bioassay.
Journal: Journal of the American Geriatrics Society 20110301
Title: Tolerability of solifenacin and oxybutynin immediate release in older (> 65 years) and younger (≤ 65 years) patients with overactive bladder: sub-analysis from a Canadian, randomized, double-blind study.
Journal: Current medical research and opinion 20110201
Title: Relationships between symptoms, symptom bother, and health-related quality of life in patients with overactive bladder taking solifenacin or placebo in the VIBRANT study.
Journal: International journal of clinical practice 20110201
Title: [Treatment with solifenacin reduces urinary urgency and improves quality of life. Results of the non-interventional CAP-study].
Journal: MMW Fortschritte der Medizin 20110113
Title: Efficacy and tolerability of solifenacin in patients aged ≥ 65 years with overactive bladder: post-hoc analysis of 2 open-label studies.
Journal: Postgraduate medicine 20110101
Title: [Impact of overactive bladder on sexual function in women].
Journal: Urologia 20110101
Title: Overactive bladder syndrome: what is the role of evidence of detrusor overactivity in the cystometric study?
Journal: Minerva urologica e nefrologica = The Italian journal of urology and nephrology 20101201
Title: Determination of solifenacin in human plasma by liquid chromatography-tandem mass spectrometry.
Journal: Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20101201
Title: A validated rapid stability-indicating method for the determination of related substances in solifenacin succinate by ultra-fast liquid chromatography.
Journal: Journal of chromatographic science 20101101
Title: Impact of solifenacin on diary-recorded and patient-reported urgency in patients with severe overactive bladder (OAB) symptoms.
Journal: Current medical research and opinion 20101001
Title: Loss of muscarinic and purinergic receptors in urinary bladder of rats with hydrochloric acid-induced cystitis.
Journal: Urology 20101001
Title: Prospective open label study of solifenacin for overactive bladder in children.
Journal: The Journal of urology 20101001
Title: Solifenacin and tolterodine are equally effective in the treatment of overactive bladder symptoms.
Journal: Journal of the Formosan Medical Association = Taiwan yi zhi 20101001
Title: Urodynamic effects of solifenacin in untreated female patients with symptomatic overactive bladder.
Journal: International journal of urology : official journal of the Japanese Urological Association 20100901
Title: The cost-effectiveness of solifenacin vs fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release and tolterodine immediate-release in the treatment of patients with overactive bladder in the UK National Health Service.
Journal: BJU international 20100801
Title: Solifenacin for overactive bladder: secondary analysis of data from VENUS based on baseline continence status.
Journal: International urogynecology journal 20100701
Title: New strategies for medical management of overactive bladder in children.
Journal: Current opinion in urology 20100701
Title: Solifenacin treatment in men with overactive bladder: effects on symptoms and patient-reported outcomes.
Journal: The aging male : the official journal of the International Society for the Study of the Aging Male 20100601
Title: Tolerability of 5 mg solifenacin once daily versus 5 mg oxybutynin immediate release 3 times daily: results of the VECTOR trial.
Journal: The Journal of urology 20100501
Title: Efficacy of simplified bladder training in patients with overactive bladder receiving a solifenacin flexible-dose regimen: results from a randomized study.
Journal: BJU international 20100401
Title: Solifenacin: scientific evidence in the treatment of overactive bladder.
Journal: Archivos espanoles de urologia 20100401
Title: Pediatrics: combining antimuscarinics shows promise for overactive bladder.
Journal: Nature reviews. Urology 20100201
Title: The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: ameliorative effect of solifenacin succinate (Vesicare), a bladder-selective antimuscarinic agent, on overactive bladder symptoms, especially urgency episodes.
Journal: Journal of pharmacological sciences 20100101
Title: Solifenacin pharmacology.
Journal: Archivos espanoles de urologia 20100101
Title: Comparison of risk of neurovascular and cardiovascular side effects between tiotropium and other anticholinergic agents.
Journal: Quality in primary care 20100101
Title: Canadian cost-effectiveness analysis of solifenacin compared to oxybutynin immediate-release in patients with overactive bladder.
Journal: Journal of medical economics 20100101
Title: [Solifenacin in treatment of the overactive bladder syndrome--diagnosis, clinical management and results].
Journal: Akusherstvo i ginekologiia 20100101
Title: [Treatment of overactive urinary bladder with imperative urinary incontinence in women].
Journal: Urologiia (Moscow, Russia : 1999) 20100101
Title: The add-on effect of solifenacin for patients with remaining overactive bladder after treatment with tamsulosin for lower urinary tract symptoms suggestive of benign prostatic obstruction.
Journal: Advances in urology 20100101
Title: Safety and tolerability of solifenacin add-on therapy to alpha-blocker treated men with residual urgency and frequency.
Journal: The Journal of urology 20091201
Title: Effects of solifenacin on overactive bladder symptoms, symptom bother and other patient-reported outcomes: results from VIBRANT - a double-blind, placebo-controlled trial.
Journal: International journal of clinical practice 20091201
Title: Impact of solifenacin on quality of life, medical care use, work productivity, and health utility in the elderly: an exploratory subgroup analysis.
Journal: The American journal of geriatric pharmacotherapy 20091201
Title: Anticholinergic drug use for overactive bladder in Sweden: a nationwide pharmacoepidemiological study.
Journal: International urogynecology journal and pelvic floor dysfunction 20091101
Title: Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10 mg.
Journal: Expert opinion on drug safety 20091101
Title: Listening to the patient: a flexible approach to the use of antimuscarinic agents in overactive bladder syndrome.
Journal: BJU international 20091001
Title: Solifenacin for therapy resistant overactive bladder.
Journal: The Journal of urology 20091001
Title: Solifenacin for overactive bladder: patient-reported outcomes from a large placebo-controlled trial.
Journal: Postgraduate medicine 20090901
Title: Comparison of muscarinic receptor selectivity of solifenacin and oxybutynin in the bladder and submandibular gland of muscarinic receptor knockout mice.
Journal: European journal of pharmacology 20090801
Title: Patient-reported most bothersome symptoms in OAB: post hoc analysis of data from a large, open-label trial of solifenacin.
Journal: International urogynecology journal and pelvic floor dysfunction 20090601
Title: Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa.
Journal: The Journal of pharmacology and experimental therapeutics 20090301
Title: In vivo and in vitro pharmacological characterization of SVT-40776, a novel M3 muscarinic receptor antagonist, for the treatment of overactive bladder.
Journal: British journal of pharmacology 20090301
Title: Solifenacin succinate for the treatment of overactive bladder.
Journal: Expert opinion on drug metabolism & toxicology 20090301
Title: Solifenacin in the treatment of overactive bladder syndrome in Italian patients: pharmacoeconomic evaluation.
Journal: Journal of medical economics 20090301
Title: Solifenacin in the treatment of urgency and other symptoms of overactive bladder: results from a randomized, double-blind, placebo-controlled, rising-dose trial.
Journal: BJU international 20090201
Title: Urodynamic parameters after solifenacin treatment in men with overactive bladder symptoms and detrusor underactivity.
Journal: Neurourology and urodynamics 20090101
Title: The cost utility of solifenacin in the treatment of overactive bladder.
Journal: International urology and nephrology 20090101
Title: Treatment with solifenacin increases warning time and improves symptoms of overactive bladder: results from VENUS, a randomized, double-blind, placebo-controlled trial.
Journal: Urology 20090101
Title: Solifenacin for overactive bladder in women unsuccessfully treated with immediate release oxybutynin: a pilot study.
Journal: Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 20090101
Title: Diary and patient-reported outcomes in patients with severe overactive bladder switching from tolterodine extended release 4 mg/day to solifenacin treatment: An open-label, flexible-dosing, multicentre study.
Journal: Clinical drug investigation 20090101
Title: Cost-effectiveness analysis of solifenacin flexible dosing in patients with overactive bladder symptoms in four Nordic countries.
Journal: Acta obstetricia et gynecologica Scandinavica 20090101
Title: Clinical pharmacokinetics and pharmacodynamics of solifenacin.
Journal: Clinical pharmacokinetics 20090101
Title: Response to 'Suspected differential interactions of digoxin with imidafenacin and propantheline; some thoughts for introspection'.
Journal: Drug metabolism and pharmacokinetics 20090101
Title: Highly sensitive and rapid LC-ESI-MS/MS method for the simultaneous quantification of uroselective alpha1-blocker, alfuzosin and an antimuscarinic agent, solifenacin in human plasma.
Journal: Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20081215
Title: QT prolongation and torsade de pointes associated with solifenacin in an 81-year-old woman.
Journal: British journal of clinical pharmacology 20081201
Title: [Efficacy and safety of solifenacin in daily clinical practice--clinical study phase IV].
Journal: Ceska gynekologie 20081201
Title: Solifenacin in the treatment of urgency and other symptoms of overactive bladder: results from a randomized, double-blind, placebo-controlled, rising-dose trial.
Journal: BJU international 20081101
Title: Efficacy and safety of solifenacin succinate in Korean patients with overactive bladder: a randomised, prospective, double-blind, multicentre study.
Journal: International journal of clinical practice 20081101
Title: Human urine with solifenacin intake but not tolterodine or darifenacin intake blocks detrusor overactivity.
Journal: International urogynecology journal and pelvic floor dysfunction 20081001
Title: Efficacy of solifenacin in patients previously treated with tolterodine extended release 4 mg: results of a 12-week, multicenter, open-label, flexible-dose study.
Journal: Clinical therapeutics 20081001
Title: A cost-utility analysis of once daily solifenacin compared to tolterodine in the treatment of overactive bladder syndrome.
Journal: Current medical research and opinion 20080801
Title: [Urinary urgency].
Journal: MMW Fortschritte der Medizin 20080731
Title: Solifenacin at 3 years: a review of efficacy and safety.
Journal: Postgraduate medicine 20080701
Title: Impact of solifenacin on resource utilization, work productivity and health utility in overactive bladder patients switching from tolterodine ER.
Journal: Current medical research and opinion 20080601
Title: [Overactive bladder. When it's pressing, immediate help is indicated].
Journal: MMW Fortschritte der Medizin 20080515
Title: Photodistributed lichenoid drug eruption secondary to solifenacin.
Journal: Clinical and experimental dermatology 20080501
Title: Solifenacin-induced small bowel pseudo-obstruction.
Journal: Journal of hospital medicine 20080301
Title: Solifenacin treatment for overactive bladder in Hispanic patients: patient-reported symptom bother and quality of life outcomes from the VESIcare Open-Label Trial.
Journal: International journal of clinical practice 20080101
Title: Pharmacological effects of solifenacin on human isolated urinary bladder.
Journal: Pharmacology 20080101
Title: Cardiovascular safety and overall tolerability of solifenacin in routine clinical use: a 12-week, open-label, post-marketing surveillance study.
Journal: Drug safety 20080101
Title: [Assessment of symptoms severity in patients with overactive bladder].
Journal: Urologiia (Moscow, Russia : 1999) 20080101
Title: Effects of intravenously and orally administered solifenacin succinate (YM905) on carbachol-induced intravesical pressure elevation and salivary secretion in mice.
Journal: Biological & pharmaceutical bulletin 20071201
Title: Determination of solifenacin succinate, a novel muscarinic receptor antagonist, and its major metabolite in rat plasma by semi-micro high performance liquid chromatography.
Journal: Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20071115
Title: Comparative in vivo uroselectivity profiles of anticholinergics, tested in a novel anesthetized rabbit model.
Journal: European journal of pharmacology 20071031
Title: An unusual cause of postoperative detrusor overactivity.
Journal: International urogynecology journal and pelvic floor dysfunction 20071001
Title: Treatment outcomes in the STAR study: a subanalysis of solifenacin 5 mg and tolterodine ER 4 mg.
Journal: European urology 20071001
Title: Comparison of the efficacy and tolerability of solifenacin succinate with or without previous use of trospium chloride.
Journal: International urogynecology journal and pelvic floor dysfunction 20070901
Title: Randomized, double-blind, placebo- and propiverine-controlled trial of the once-daily antimuscarinic agent solifenacin in Japanese patients with overactive bladder.
Journal: BJU international 20070901
Title: Pharmacologic management of overactive bladder.
Journal: Clinical interventions in aging 20070901
Title: Number of daytime micturitions and volume voided per micturition in the evaluation of efficacy of drugs for overactive bladder: findings from randomized clinical trials.
Journal: European urology 20070801
Title: Nocturnal polyuria and nocturia relief in patients treated with solifenacin for overactive bladder symptoms.
Journal: International urogynecology journal and pelvic floor dysfunction 20070701
Title: Overactive bladder treatments in early phase clinical trials.
Journal: Expert opinion on investigational drugs 20070701
Title: Update on drugs for overactive bladder syndrome.
Journal: Drug and therapeutics bulletin 20070601
Title: [Comment on the STAR study: Comparison of the efficacy and tolerance of solifenacin and tolterodine retard in the treatment of overactive bladder].
Journal: Der Urologe. Ausg. A 20070401
Title: Solifenacin treatment for overactive bladder in black patients: patient-reported symptom bother and health-related quality of life outcomes.
Journal: Current medical research and opinion 20070401
Title: Solifenacin for overactive bladder with incontinence: symptom bother and health-related quality of life outcomes.
Journal: The Annals of pharmacotherapy 20070301
Title: Pharmacological characterization of a new antimuscarinic agent, solifenacin succinate, in comparison with other antimuscarinic agents.
Journal: Biological & pharmaceutical bulletin 20070101
Title: Redefining response in overactive bladder syndrome.
Journal: BJU international 20070101
Title: Pharmacokinetics, safety, and tolerability of solifenacin in patients with renal insufficiency.
Journal: Journal of pharmacological sciences 20070101
Title: [Solifenacin in the treatment of patients with hyperactive urinary bladder].
Journal: Urologiia (Moscow, Russia : 1999) 20070101
Title: [A placebo-controlled, double-blind, randomized trial of single daily dose of anti-muscarinic drug solifenacin succinate in patients with overactive bladder].
Journal: Akusherstvo i ginekologiia 20070101
Title: Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.
Journal: Expert opinion on pharmacotherapy 20061201
Title: [Pharmacological and clinical profile of solifenacin succinate (Vesicare) developed as a new therapeutic agent for overactive bladder].
Journal: Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20061201
Title: Open-label study of the safety and pharmacokinetics of solifenacin in subjects with hepatic impairment.
Journal: Journal of pharmacological sciences 20061201
Title: Solifenacin succinate (VESIcare): overactive bladder therapy.
Journal: Urologic nursing 20061201
Title: New developments in the treatment of urinary incontinence.
Journal: Minerva urologica e nefrologica = The Italian journal of urology and nephrology 20061201
Title: Solifenacin.
Journal: The Urologic clinics of North America 20061101
Title: Treatment of the overactive bladder syndrome with muscarinic receptor antagonists: a matter of metabolites?
Journal: Naunyn-Schmiedeberg's archives of pharmacology 20061101
Title: Symptom bother and health-related quality of life outcomes following solifenacin treatment for overactive bladder: the VESIcare Open-Label Trial (VOLT).
Journal: Clinical therapeutics 20061101
Title: Reductions in overactive bladder-related incontinence from pooled analysis of phase III trials evaluating treatment with solifenacin.
Journal: International urogynecology journal and pelvic floor dysfunction 20060901
Title: Solifenacin succinate for the treatment of symptoms of overactive bladder.
Journal: Clinical therapeutics 20060901
Title: Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: A pooled analysis.
Journal: The American journal of geriatric pharmacotherapy 20060901
Title: Multiple doses of the antimuscarinic agent solifenacin do not affect the pharmacodynamics or pharmacokinetics of warfarin or the steady-state pharmacokinetics of digoxin in healthy subjects.
Journal: British journal of clinical pharmacology 20060801
Title: Solifenacin significantly improves all symptoms of overactive bladder syndrome.
Journal: International journal of clinical practice 20060801
Title: Patient-reported outcomes in overactive bladder: importance for determining clinical effectiveness of treatment.
Journal: Urology 20060801
Title: [Comment on the STAR study: Comparison of the efficacy and tolerance of solifenacin and tolterodine retard in the treatment of overactive bladder].
Journal: Der Urologe. Ausg. A 20060701
Title: Comparative evaluation of exocrine muscarinic receptor binding characteristics and inhibition of salivation of solifenacin in mice.
Journal: Biological & pharmaceutical bulletin 20060701
Title: Pharmacokinetic effect of ketoconazole on solifenacin in healthy volunteers.
Journal: Basic & clinical pharmacology & toxicology 20060701
Title: Solifenacin: as effective in mixed urinary incontinence as in urge urinary incontinence.
Journal: International urogynecology journal and pelvic floor dysfunction 20060601
Title: Short- and long-term efficacy of solifenacin treatment in patients with symptoms of mixed urinary incontinence.
Journal: BJU international 20060601
Title: Using anticholinergics to treat overactive bladder: the issue of treatment tolerability.
Journal: The American journal of medicine 20060301
Title: The emergence of new drugs for overactive bladder.
Journal: Expert opinion on emerging drugs 20060301
Title: Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.
Journal: The American journal of geriatric pharmacotherapy 20060301
Title: New drugs 06, part I.
Journal: Nursing 20060201
Title: Re: Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, Wright DM, Bolodeoku J. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol 2005;48:464-70.
Journal: European urology 20060101
Title: Efficacy of solifenacin in patients with severe symptoms of overactive bladder: a pooled analysis.
Journal: Current medical research and opinion 20060101
Title: Solifenacin in overactive bladder syndrome.
Journal: Drugs 20060101
Title: Solifenacin versus tolterodine--a head-to-head study: finally! But not final?
Journal: Current urology reports 20051101
Title: [Drug discovery in the fields of urology: tamsulosin and solifenacin].
Journal: Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20051101
Title: Synthesis and antimuscarinic properties of quinuclidin-3-yl 1,2,3,4-tetrahydroisoquinoline-2-carboxylate derivatives as novel muscarinic receptor antagonists.
Journal: Journal of medicinal chemistry 20051020
Title: Solifenacin in the management of the overactive bladder syndrome.
Journal: International journal of clinical practice 20051001
Title: A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial.
Journal: European urology 20050901
Title: Solifenacin is effective for the treatment of OAB dry patients: a pooled analysis.
Journal: European urology 20050901
Title: Pharmacokinetic interaction of solifenacin with an oral contraceptive containing ethinyl estradiol and levonorgestrel in healthy women: a double-blind, placebo-controlled study.
Journal: Clinical therapeutics 20050901
Title: Solifenacin and darifenacin for overactive bladder.
Journal: Obstetrics and gynecology 20050801
Title: New treatment options for overactive bladder.
Journal: South Dakota journal of medicine 20050601
Title: Muscarinic receptor binding, plasma concentration and inhibition of salivation after oral administration of a novel antimuscarinic agent, solifenacin succinate in mice.
Journal: British journal of pharmacology 20050501
Title: Effect of age on the pharmacokinetics of solifenacin in men and women.
Journal: International journal of clinical pharmacology and therapeutics 20050501
Title: Effects of solifenacin succinate (YM905) on detrusor overactivity in conscious cerebral infarcted rats.
Journal: European journal of pharmacology 20050404
Title: Solifenacin and darifenacin for overactive bladder.
Journal: The Medical letter on drugs and therapeutics 20050314
Title: Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome.
Journal: European urology 20050301
Title: Improved quality of life in patients with overactive bladder symptoms treated with solifenacin.
Journal: BJU international 20050101
Title: New molecular entity: Vesicare, Yamanouchi/GlaxoSmithKline solifenacin.
Journal: Geriatrics 20050101
Title: New drugs: acamprosate calcium and solifenacin succinate.
Journal: Journal of the American Pharmacists Association : JAPhA 20050101
Title: Recent developments in the management of overactive bladder: focus on the efficacy and tolerability of once daily solifenacin succinate 5 mg.
Journal: Current medical research and opinion 20050101
Title: [Neurological aspect of the hyperactive urinary bladder syndrome].
Journal: Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova 20050101
Title: Solifenacin in overactive bladder syndrome.
Journal: Drugs & aging 20050101
Title: Solifenacin in overactive bladder syndrome: a viewpoint by Scott Serels.
Journal: Drugs & aging 20050101
Title: Solifenacin in overactive bladder: a viewpoint by Hashim Hashim.
Journal: Drugs & aging 20050101
Title: Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.
Journal: The Journal of urology 20041101
Title: The emerging role of solifenacin in the treatment of overactive bladder.
Journal: Expert opinion on investigational drugs 20041001
Title: Preview of new drugs for overactive bladder and incontinence: darifenacin, solifenacin, trospium, and duloxetine.
Journal: Current urology reports 20041001
Title: Elevating our therapeutic expectations in overactive bladder.
Journal: Journal of the American Academy of Nurse Practitioners 20041001
Title: Pharmacokinetics and safety of solifenacin succinate in healthy young men.
Journal: Journal of clinical pharmacology 20040901
Title: [Overactive bladder. New anticholinergic drug controls urinary urge].
Journal: MMW Fortschritte der Medizin 20040708
Title: Food does not affect the pharmacokinetics of solifenacin, a new muscarinic receptor antagonist: results of a randomized crossover trial.
Journal: British journal of clinical pharmacology 20040701
Title: [In overactive bladder, above all urgency is stressful. The patients know the site of each toilet].
Journal: MMW Fortschritte der Medizin 20040527
Title: In vitro and in vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats.
Journal: European journal of pharmacology 20040525
Title: Solifenacin: treatment of overactive bladder.
Journal: Drugs of today (Barcelona, Spain : 1998) 20040401
Title: Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.
Journal: BJU international 20040201
Title: Comparison of in vitro selectivity profiles of solifenacin succinate (YM905) and current antimuscarinic drugs in bladder and salivary glands: a Ca2+ mobilization study in monkey cells.
Journal: Life sciences 20040102
Title: Solifenacin appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a placebo- and tolterodine-controlled phase 2 dose-finding study.
Journal: BJU international 20040101
Title: Solifenacin demonstrates high absolute bioavailability in healthy men.
Journal: Drugs in R&D 20040101
Title: [Urinary incontinence: new pharmacologic therapies].
Journal: Revista de medicina de la Universidad de Navarra 20040101
Title: M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland.
Journal: Naunyn-Schmiedeberg's archives of pharmacology 20020801
Title: Effects of YM905, a novel muscarinic M3-receptor antagonist, on experimental models of bowel dysfunction in vivo.
Journal: Japanese journal of pharmacology 20010701
Title: Krishna SR, Rao BM, Rao NS.A validated rapid stability-indicating method for the determination of related substances in solifenacin succinate by ultra-fast liquid chromatography.J Chromatogr Sci. 2010 Nov;48(10):807-10.
Title: Ohtake A, Sato S, Sasamata M, Miyata K.The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: ameliorative effect of solifenacin succinate (Vesicare), a bladder-selective antimuscarinic agent, on overactive bladder symptoms, especially urgency episodes.J Pharmacol Sci. 2010;112(2):135-41. Epub 2010 Feb 4.
Title: Hoffstetter S, Leong FC.Solifenacin succinate for the treatment of overactive bladder.Expert Opin Drug Metab Toxicol. 2009 Mar;5(3):345-50.
Title: Choo MS, Lee JZ, Lee JB, Kim YH, Jung HC, Lee KS, Kim JC, Seo JT, Paick JS, Kim HJ, Na YG, Lee JG.Efficacy and safety of solifenacin succinate in Korean patients with overactive bladder: a randomised, prospective, double-blind, multicentre study.Int J Clin Pract. 2008 Nov;62(11):1675-83.
Title: Imamura T, et al.Combined treatment with a β3 -adrenergic receptor agonist and a muscarinic receptor antagonist inhibits detrusor overactivity induced by cold stress in spontaneously hypertensive rats. Neurourol Urodyn. 2017 Apr;36(4):1026-1033.