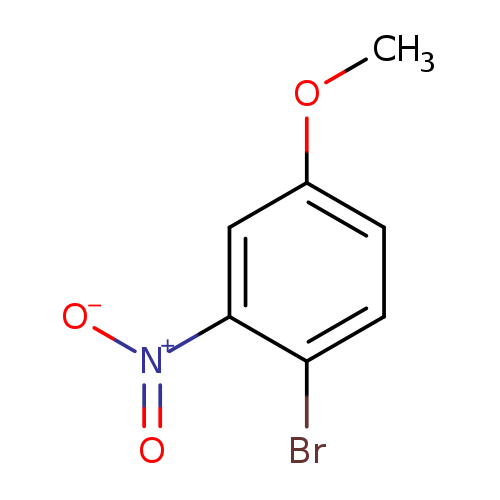
[1]Patent:WO2017/60906,2017,A1,.Locationinpatent:Paragraph00138
[1]AngewandteChemie-InternationalEdition,2014,vol.53,#52,p.14451-14455
[2]Angew.Chem.,2014,vol.126,#52,p.14679-14683,5
[1]TetrahedronLetters,2004,vol.45,#4,p.817-819
[1]EuropeanJournalofMedicinalChemistry,2013,vol.59,p.283-295
[1]Chemistry-AEuropeanJournal,2017,vol.23,p.9996-10000
[2]JournalofMedicinalChemistry,1995,vol.38,p.950-957
[3]OrganicLetters,2015,vol.17,p.802-805
[4]TetrahedronLetters,2017,vol.58,p.4559-4562
[5]EuropeanJournalofOrganicChemistry,2019,vol.2019,p.6697-6701
[6]JournaloftheAmericanChemicalSociety,1994,vol.116,p.11797-11810
[7]Patent:WO2009/62285,2009,A1.Locationinpatent:Page/Pagecolumn116
[8]AngewandteChemie-InternationalEdition,2014,vol.53,p.7349-7353
[9]Tetrahedron,2009,vol.65,p.8542-8555
[10]Patent:US2011/98311,2011,A1
[11]Patent:US2012/309758,2012,A1.Locationinpatent:Page/Pagecolumn61
[12]Patent:US2015/231142,2015,A1.Locationinpatent:Paragraph0355
[13]OrganicLetters,2010,vol.12,p.2258-2261
[14]Patent:US2011/152246,2011,A1.Locationinpatent:Page/Pagecolumn156-158
[15]Patent:WO2012/58125,2012,A1.Locationinpatent:Page/Pagecolumn124
[16]JournaloftheAmericanChemicalSociety,2008,vol.130,p.472-480
[17]JournaloftheChemicalSociety,1935,p.946
[18]JournalofgeneralchemistryoftheUSSR,1962,vol.32,p.2325-2331 ZhurnalObshcheiKhimii,1962,vol.32,p.2358-2365
[19]JournalofOrganicChemistry,1989,vol.54,p.5856-5866
[20]JournalofOrganicChemistry,1992,vol.57,p.6380-6382
[21]TetrahedronLetters,2006,vol.47,p.2739-2742
[22]Tetrahedron,2010,vol.66,p.9779-9784
[23]JournaloftheAmericanChemicalSociety,2011,vol.133,p.6061-6071
[24]JournaloftheAmericanChemicalSociety,2011,vol.133,p.6166-6169
[25]Patent:US9408907,2016,B2.Locationinpatent:Page/Pagecolumn61
[26]Patent:WO2009/111337,2009,A1.Locationinpatent:Page/Pagecolumn167
[27]Patent:WO2010/130842,2010,A1.Locationinpatent:Page/Pagecolumn236-237
[1]Kasahara,Akira;Izumi,Taeko;Murakami,Satoshi;Miyamoto,Kazuhiro;Hino,Toshimi[JournalofHeterocyclicChemistry,1989,vol.26,p.1405-1413]
[1]Narayanan,Krishnaswamy;Schindler,Liesl;Cook,JamesM.[JournalofOrganicChemistry,1991,vol.56,#1,p.359-365]
[2]Locationinpatent:experimentalpartPehlivan,Leyla;Métay,Estelle;Laval,Stéphane;Dayoub,Wissam;Demonchaux,Patrice;Mignani,Gérard;Lemaire,Marc[Tetrahedron,2011,vol.67,#10,p.1971-1976]
[1]Patent:US2007/78147,2007,A1.Locationinpatent:Page/Pagecolumn68
[2]Patent:WO2007/56582,2007,A1.Locationinpatent:Page/Pagecolumn77-78
[3]TetrahedronLetters,2011,vol.52,p.3376-3378
[4]Patent:WO2012/73146,2012,A1.Locationinpatent:Page/Pagecolumn24-25
[5]TetrahedronLetters,1996,vol.37,p.7189-7192
[6]BioorganicandMedicinalChemistry,2003,vol.11,p.521-528
[7]Patent:WO2014/210255,2014,A1.Locationinpatent:Page/Pagecolumn264
[8]Patent:US2002/165275,2002,A1
[1]Söderberg,BjörnC.;Shriver,JamesA.[JournalofOrganicChemistry,1997,vol.62,#17,p.5838-5845]