2020-02-18 20:05:49
STRUCTURAL AND STEREOCHEMICAL ASPECTS OF TADALAFIL AND ITS ANALOGUES
Phosphodiesterases (PDEs) are hydrolyzing enzymes, the PDEs comprises 11 families that hydrolyze cyclic nucleotides. They regulate cAMP and cGMP intracellular levels, the levels of those second mes- sengers influence all cell functions. PDEs inhibitors block the degradative action of this class of enzymes.
Upon superimposing of all reported inhibitors bound to PDE4D, PDE4B, and PDE5A, it was clear that all PDE inhibitors bind to the binding cavity of PDEs in conservative manner, despite their major dif- ferences in their chemotypes. All these inhibitors possess a planar ring fitted by a hydrophobic clamp (one jaw is a conserved phenylalanine(F) in all PDEs whereas the other jaw is always a hydrophobic residue). These inhibitors form monodentate or bidentate H-bond with an invari- ant glutamine (Q), a residue that is preserved among all PDE enzymes. The selectivity of PDE enzymes toward cAMP or cGMP relies on the ability of essential amino acids lining the active site to anchor the invari- ant Q in different orientations; a glutamine switch takes place to allow binding to either cAMP or cGMP. The active site of PDEs is the com- mon binding site for all reported PDEs inhibitors; primarily they bind to the Q (core pocket) and may extend to the relatively large M pocket (metal-binding site) depending on the size of their substituents. There is no direct binding between PDEs inhibitors and metal ions of the M pocket; they rather bind indirectly via a network of water molecules (Zhang et al., 2004). Tadalafil binds to PDE5A in a unique manner; tada- lafil forms a single hydrogen bond between carbonyl of Q817 and het- erocyclic nitrogen in its indole ring. Q817 amide group rotates nearly 90○ compared to its orientation with sildenafil and vardenafil, such flip allows accommodation of a single H-bond donor from tadalafil. The C-6 pendant aryl ring of tadalafil appears fitting in the Q2 hydrophobic pocket, this pocket is occupied by ethoxy group of sildenafil and varde- nafil. Tadalafil does not show any interaction with the metal ions in the M pocket, not even water mediated interactions. Moreover, the rela- tively rigid chemical structure of tadalafil has increased its binding affin- ity, this may be attributed to the fact that it has only one nonterminal rotatable bond, upon binding to PDE5 enzyme tadalafil can form more stable complex than sildenafil and vardenafil due to loss of little entropy (Figures 1 and 2) (Sung et al., 2003).
Tadalafil was co-crystallized with human PDE5 (PDB: 1UDU) with 2.8 Å resolution, while sildenafil was co-crystallized with human PDE5 (PDB: 2H42) with 2.3 Å resolution. The analysis of this PDB data showed that some essential residues near the binding site are not resolved in 1UDU. In 2011, Mohamed et al. superimposed the two crystal structures of PDE5 and highlighted the differences between the two crystal structures; they deduced that the I665 residue appears in 2H42 but is missing 1UDU. In their work, they assumed substantial hydrophobic interactions between Ile665 and the N-alkyl substituent of tadalafil and its analogues. They concluded that the inversion of the 6R chiral center of tadalafil to 6S induces a 180○ flip of the central scaffold. This flip leads to the loss of essential H-bond between car- bonyl of Q817 and the indole NH- of tadalafil. Additionally, the 6S con- figuration shows a steric clash between one carbonyl group of piperazinedione and the carbonyl of Q817, this clash has a significant deteriorating effect on binding affinity. The stereoinversion had no effect on the π-π stacking interaction with P820. Their work highlighted an important finding; the chiral center at C-6 rather than at C-12a is the determinant factor of activity. More analogues were thus prepared to focus on producing only the 6R analogues starting with the less expensive L-tryptophan (Mohamed et al., 2011).
1.1| Tadalafil analogues with modification on C-6 pendant aryl
All active tadalafil analogues bear an aryl or heteroaryl ring at C-6, this ring is reported to fit into the Q pocket of the PDE5 enzyme via hydro- phobic interaction. The size, lipophilicity, and position of the substituents on the aryl ring play a role on both potency and selectivity. The pendant aryl moiety occupies the narrow Q2 pocket, where the pocket governs the selectivity of the ligands toward the enzyme. Substi- tution on the aromatic ring with mild electron withdrawing groups (6) (IC50 = 15 nM) improved the PDE5 inhibition compared with compound (7) (IC50 = 90 nM) bearing, whereas the introduction of 4-cyano group (8) (IC50 = 765 nM) resulted in a marked decrease in PDE5 inhibition, it seems that substitution with strong electron withdrawing groups lowers the electron density on the C-6 aromatic ring, this can potentially influ- ence the essential π-π interaction. (Daugan et al., 2003b).
A para chloro substitution on the pendant aryl yielded an equipo- tent tadalafil analog (9) (IC50 = 3 nM) with remarkable selectivity toward PDE5 versus PDE11(Ahmed et al., 2011). The modification of benzodioxol of tadalafil to dichlorophenyl derivatives was adopted, 2,4-Dichlorophenyl (10) showed equipotent PDE5 inhibitory activity compared to tadalafil (IC50 = 2 nM) whereas 2,6-Dichlorophenyl ana- logues (11) lacked PDE5 inhibitory activity. Docking studies proved that structural isomers (11) and (12) may be sterically locked in a con- formation that prevents binding to PDE5 (Mohamed et al., 2011). It is worth mentioning that compound (10) showed remarkable activity against PDE 11A (IC50 = 11 nM), the exact mechanism of this inhibi- tory activity is still unknown. More recent studies adopted in silico approaches including molecular modeling and virtual screening proto- cols to design tadalafil analogues with better PDE5/PDE11 selectivity profile (Kayık, Tüzün, & Durdagi, 2017). Findings of those studies went along with Abadi et al. results indicating PDE5/PDE11 selectiv- ity is governed by the tetracycle terminal ring interactions rather than the substitution on the pendant aryl (Mohamed et al., 2011).
Changing the para chloro substituent of the pendant aryl to para bromo yielded an equipotent analog (13) (IC50 = 3 nM) (Ahmed et al., 2012). Conversely, changing the bromine position from para to ortho led to analog (14) which is 100 times less potent than (13) (IC50 = 320 nM) (Abadi, Gary, Tinsley, Piazza, & Abdel-Halim, 2010). 4-methoxyphenyl analog (15) showed potent PDE5 inhibition (IC50 = 5 nM) (Daugan et al., 2003b). 3,4 dimethoxyphenyl analog (16) showed no PDE5 inhibitory activity. The 3,4 dimethoxy group is sterically compact when compared with 3,4-methylenedioxy of tadalafil. The constrained conformation of 3,4-methylenedioxy group seems to be an essential determinant for activity. The pres- ence of 3,4 dimethoxy free rotating groups was assumed to prevent the optimal orientation of the C-6 aromatic ring (Ahmed et al., 2010).
1.2| Tadalafil analogues with modifications at C-5
Several tadalafil analogues were prepared to bear a hydantoin (Imidazolidine-1,3-dione)/thiohydantoin (2-Thioxo-imidazolidin-4-one) ring instead of the piperazinedione ring. C-5 of the analogues was designed to bear different aryl/heteroaryl rings including 2,5-dimethoxyphenyl (Abadi et al., 2009), 2,4-dimethoxyphenyl (Abadi, Lehmann, Piazza, Abdel-Halim, & Ali, 2011), 2-bromopheny (Abadi et al., 2010), 3-pyridinyl (Ahmed et al., 2010), 5-bromothienyl (El-Gamil et al., 2013), 5-thienyl furanyl rings (Daugan et al., 2003a). Analogues with either a para methoxy or para chloro group at the phe- nyl ring showed improved potency, whereas the cyano derivatives lacked activity. Substitution at position 2 with either chloro or methoxy groups prevented the optimal orientation of the phenyl ring. It is obvious that same substituents on pendant C-5 or C-6 enhance PDE5 inhibition activity in piperazinedione and hydantoin analogues. A remarkable difference between piperazinedione derivatives and their hydantoin congeners is that both cis- and trans-isomers are nearly equipotent. PDE5 inhibition showed no diastereoslectivity preference, hydantoin analogues (17) (IC50 = 5 nM) and its diastereo- mers (18) (IC50 = 8 nM) are of nearly equal activity. For hydantoin derivatives, deletion of one carbonyl group (19) (IC50 = 60 nM) caused a marginal decrease in inhibitory activity, whereas the deletion of both of carbonyls caused a remarkable decrease in activity (20) (IC50 > 10,000 nM; Daugan et al., 2003a).
The fusion of tetrahydro-β-carboline skeleton to thiohydantoin ring was also investigated; this modification abolished PDE5 inhibitory activity (21–23). Loss in activity can be attributed to the lower electronegativity of the sulfur atom compared with the oxygen atom (Abadi et al., 2009, 2010, 2011).
The presence of two carbonyl groups is essential for activity, while the size of the fused ring is highly tolerated.
1,3 disubstituted tetrahydro-β-carboline derivative (24) demon- strated no PDE5 inhibitory activity. Compounds bearing two carbonyl groups at position C-1 and N-2 (Ahmed et al., 2010), or analogues with two carbonyl groups at position C-1 and N-2 with an additional terminal N-alkyl substituents showed no inhibitory activities as well (26–29) (Ahmed, 2010). This indicates the need for a fused tetracyclic ring to ensure proper binding to PDE5 active site. Even Introduction of one carbonyl group (24) or two carbonyl groups (25–29) was not sufficient to yield compounds with inhibitory activity even in the pres- ence of terminal N-alkyl substituent suggesting that a rigidification of the carbonyls in either a hydantoin or piperazinedione ring is a requirement of activity. The rigidity offers an essential interaction because the carbonyl groups can interact via water-bridging with H613/N662 as well as with backbone of M816 (Ahmed et al., 2012; Zoraghi, Francis, & Corbin, 2007).
The fusion of THBC to a diazepane-dione (30) was reported only once, where only the trans-isomer was successfully synthesized to yield a 7-membered ring hybrid, which retained PDE5 inhibitory activity in the nanomolar range (Jiang et al., 2004). All those findings indicated that the catalytic domain in PDE5 can tolerate tetracyclic-β-carboline moieties with 5, 6, or even 7 membered fused rings. The presence of the carbonyl groups is more essential than the size of the rings and the rigidity is a determinant factor for proper PDE5 inhibition.
1.3| Tadalafil analogues with modification on N-substituent on the terminal ring Daugan et al. suggested that piperazinedione ring of tadalafil can tolerate a wide range of N-alkyl and aryl substituents (Daugan et al., 2003b). In 2007, new analogues of tadalafil bearing various aryl groups were explored (Beghyn et al., 2007). The obtained results showed that the aryl substituents are generally well-accepted. Both electron-donating and elec- tron withdrawing groups at the meta or para positions of the N-phenyl ring yielded active analogues. Lipophilic substituents showed diminished activity (31) IC50 = 377 nM, whereas heterocycles like thiophene (32), pyr- idine (33), and pyrimidine (34) showed an improved IC50 = 18, 28, 53 nM, respectively. Despite the fact that all analogues were weaker than tadalafil yet this work offered a ground for the design and synthesis of more selec- tive PDE5 inhibitors with better solubility (Beghyn et al., 2007).
Abadi et al. proved via ensemble docking studies that both the size and steric properties of the N-substituents of hydantoin or piperazine- dione rings must be optimized to improve activity and selectivity parame- ters. The results of the ensemble docking showed that some essential residues that are close to the binding site are not resolved in PDB 1UDU mainly I665 and T664.
The results showed that N-substituents on both hydantoin and piperazinedione of tadalafil and its analogues occupied a hydrophobic pocket formed by Y612, L765, A767, I768, Q775, I778, and V782 and therefore the stability of the PDE5 inhibitor complex was strongly dependent on the size and bulkiness of the terminal N-substituent (Ahmed et al., 2012). Furthermore, the ensemble docking revealed that the N-alkyl substituents are extending nearby to the H-loop residues N661, S663, and I665 for the 5/6 R-isomers as well as to Y12 and Q775 for the 5/6 S-isomers. Such findings suggested that substituting the com- monly adopted N-alkyl group with N-polar groups having acceptor atoms or halogen atoms might lead to more potent and more selective tadalafil analogues. Another study based on the previous findings prepared tada- lafil analogues with N-alkylhydroxy or alkylamino substituents on the piperazinedione nitrogen, the polar substituent are designed to interact with some of the hydrophilic residues in the binding pocket. Moderately bearing 5S or 6S configuration and polar terminal N-substituent can serve as basis for active tadalafil analogues (Elhady et al., 2015).
The N-substituents are crucial structural determinants for PDE5 inhibition, the versatile nature of the amino acids lining PDE5 H-loop offers a large chemical space from which novel tadalafil analogues bear- ing alkyl, aryl, hydrophobic, or hydrophilic substituents can be designed.
In summary, it is essential to have a 6R configuration, cisanalogues are more potent than trans-analogues yet an R absolute configuration at position 6 is more crucial than an R absolute configuration at position 12a. The size of the terminal fused ring is well-tolerated as long as at least one carbonyl group is present. N-substituents on the terminal fused dione ring range from small alkyl to bulky aromatic rings with no deleterious effect on activity. Replacing the lipophilic groups with polar groups still needs further optimization. Substitution on the indole ring mostly lowered the potency. The pendant aryl ring at C-6 plays a role in both potency and selectivity. Lipophilic, electron donating, or mild electron withdrawing are highly favored at para position.
1-Decylpiperazine dihydrochlorideCatalog No.:AA01EQNZ CAS No.:110027-41-3 MDL No.:MFCD00958135 MF:C14H32Cl2N2 MW:299.3233 |
4-(Nitrooxy)butyl butanoateCatalog No.:AA007E12 CAS No.:1100273-14-0 MDL No.:MFCD18157662 MF:C8H15NO5 MW:205.2084 |
Pyridinium,4-[(4S)-4-amino-4-carboxybutyl]-1-[(5S)-5-amino-5-carboxypentyl]-3,5-bis[(3S)-3-amino-3-carboxypropyl]-Catalog No.:AA0084FJ CAS No.:11003-57-9 MDL No.:MFCD02179314 MF:C24H41N5O8 MW:527.6110 |
4-Iodo-7h-pyrrolo[2,3-d]pyrimidineCatalog No.:AA003A1P CAS No.:1100318-96-4 MDL No.:MFCD17016136 MF:C6H4IN3 MW:245.0205 |
5-bromo-2,3,4,9-tetrahydro-1H-carbazol-1-oneCatalog No.:AA01ELIQ CAS No.:1100345-72-9 MDL No.:MFCD31665246 MF:C12H10BrNO MW:264.1179 |
{4-[(Methoxy-methyl-amino)-methyl]-thiazol-2-yl}-carbamic acid tert-butyl esterCatalog No.:AA01DO0I CAS No.:1100350-49-9 MDL No.:MFCD11982863 MF:C11H19N3O3S MW:273.3519 |
1-(tert-Butyl)-4-chloro-1H-pyrazolo[3,4-d]pyrimidineCatalog No.:AA01A2QX CAS No.:1100365-45-4 MDL No.:MFCD11104747 MF:C9H11ClN4 MW:210.6634 |
2-Amino-N-phenylethanesulfonamide hydrochlorideCatalog No.:AA0097YE CAS No.:1100424-69-8 MDL No.:MFCD11858079 MF:C8H13ClN2O2S MW:236.7190 |
Mg-101Catalog No.:AA007VVC CAS No.:110044-82-1 MDL No.:MFCD00065505 MF:C20H37N3O4 MW:383.5255 |
N-(5-amino-1,3,4-thiadiazol-2-yl)methanesulfonamideCatalog No.:AA019VCK CAS No.:1100459-09-3 MDL No.:MFCD12197148 MF:C3H6N4O2S2 MW:194.2353 |
4-[(4-methylbenzenesulfonyl)methyl]benzoic acidCatalog No.:AA019V0H CAS No.:110046-36-1 MDL No.:MFCD11650663 MF:C15H14O4S MW:290.3343 |
5-Bromo-2,2'-bithiophene-5'-carboxaldehydeCatalog No.:AA003MDG CAS No.:110046-60-1 MDL No.:MFCD01321134 MF:C9H5BrOS2 MW:273.1694 |
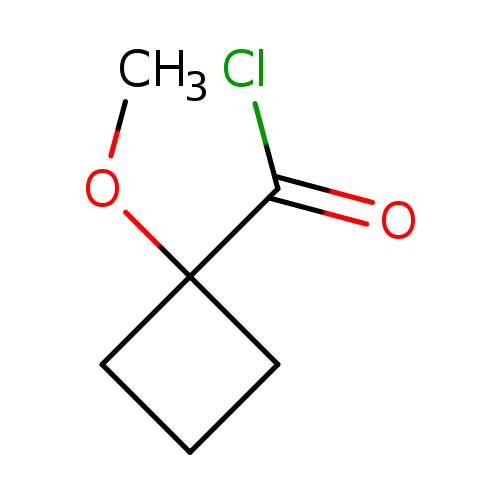
Cyclobutanecarbonylchloride, 1-methoxy-Catalog No.:AA007DZD CAS No.:110046-66-7 MDL No.:MFCD13173515 MF:C6H9ClO2 MW:148.5875 |
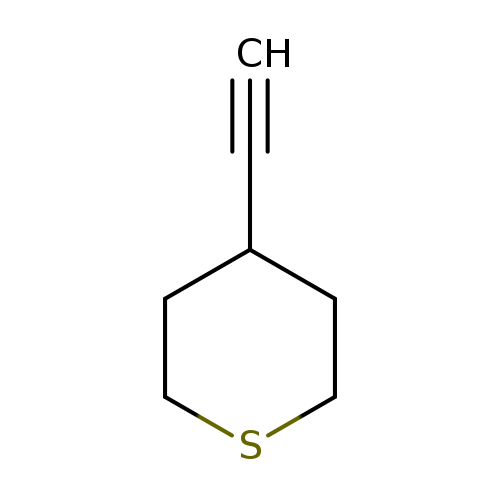
4-Ethynyltetrahydro-2h-thiopyranCatalog No.:AA00HBL8 CAS No.:1100509-34-9 MDL No.:MFCD23106385 MF:C7H10S MW:126.2193 |
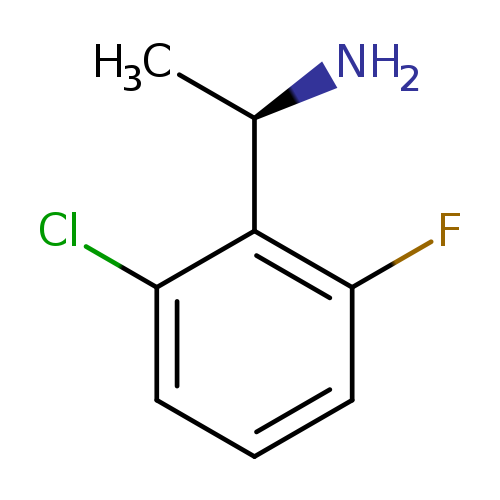
(R)-1-(2-Chloro-6-fluorophenyl)ethanamineCatalog No.:AA00963G CAS No.:1100575-44-7 MDL No.:MFCD12757993 MF:C8H9ClFN MW:173.6152 |
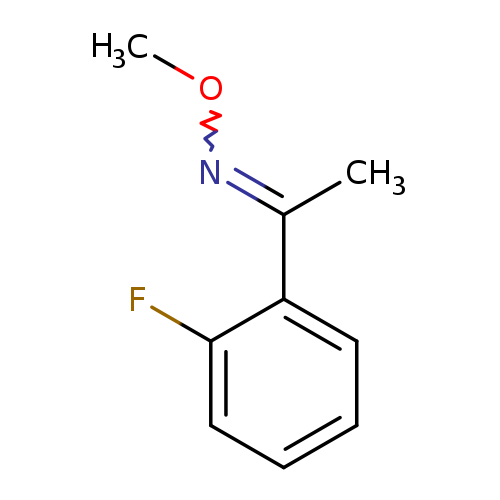
[1-(2-fluorophenyl)ethylidene](methoxy)amineCatalog No.:AA01AL46 CAS No.:1100595-43-4 MDL No.:MFCD28954269 MF:C9H10FNO MW:167.1802 |
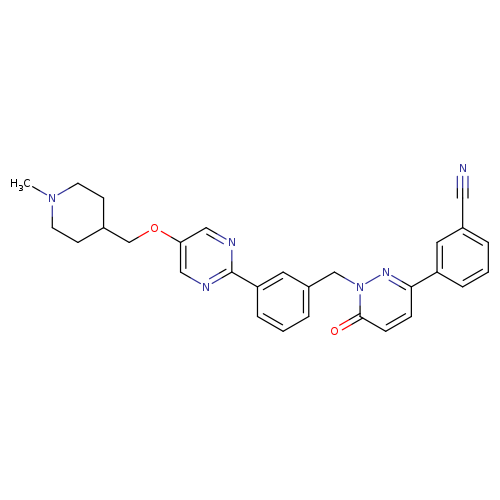
Emd-1214063Catalog No.:AA0039I7 CAS No.:1100598-32-0 MDL No.:MFCD18452823 MF:C29H28N6O2 MW:492.5716 |
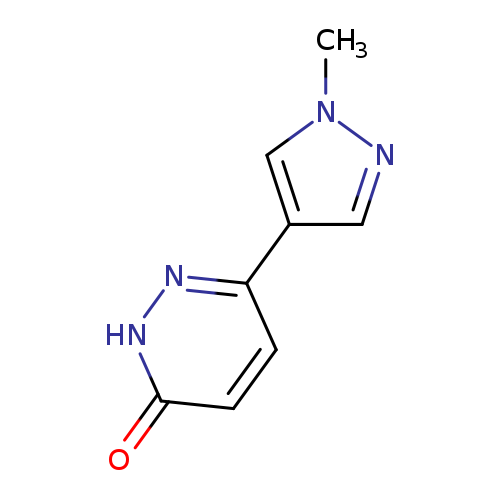
6-(1-Methyl-1h-pyrazol-4-yl)-2,3-dihydropyridazin-3-oneCatalog No.:AA01E7XO CAS No.:1100598-49-9 MDL No.:MFCD16109164 MF:C8H8N4O MW:176.1753 |
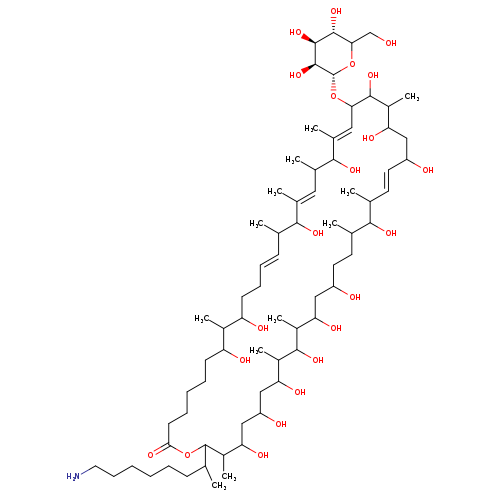
monazomycinCatalog No.:AA0094B5 CAS No.:11006-31-8 MDL No.:MFCD01714647 MF:C72H133NO22 MW:1364.8199 |
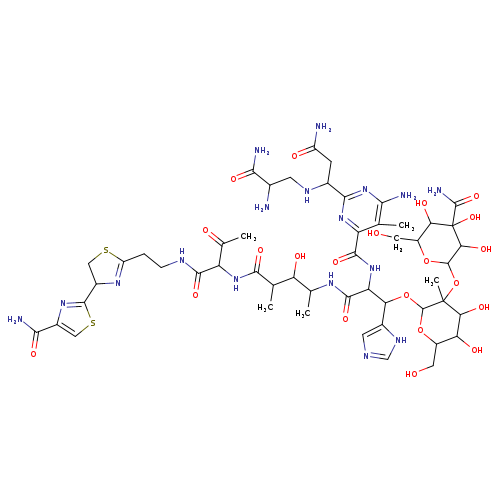
PhleomycinCatalog No.:AA00HBL9 CAS No.:11006-33-0 MDL No.:MFCD30738212 MF:C51H75N17O21S2 MW:1326.3725 |
Cu(ii) chlorin e6 trisodium saltCatalog No.:AA0034IW CAS No.:11006-34-1 MDL No.:MFCD00012149 MF:C34H43CuN4Na3O6 MW:736.2437 |
Sodium tauroglycocholateCatalog No.:AA00JKGZ CAS No.:11006-55-6 MDL No.:MFCD00036243 MF:C28H48N2NaO8S MW:595.7441 |
Virginiamycin ComplexCatalog No.:AA007DZ4 CAS No.:11006-76-1 MDL No.:MFCD01778146 MF:C28H35N3O7 MW:525.5934 |
5-Mercapto-4-phenethyl-4H-1,2,4-triazol-3-olCatalog No.:AA008U3D CAS No.:110062-45-8 MDL No.:MFCD06655228 MF:C10H11N3OS MW:221.2788 |
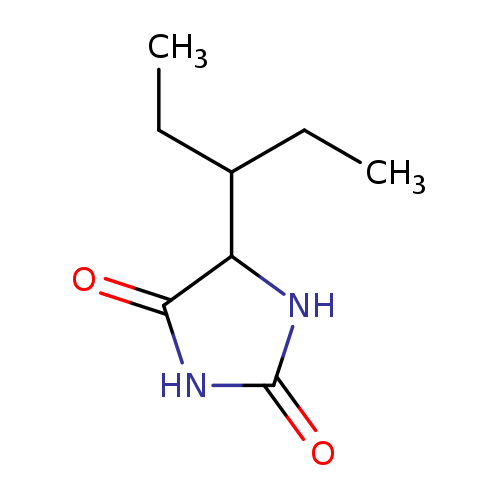
2,4-Imidazolidinedione, 5-(1-ethylpropyl)-Catalog No.:AA007VV6 CAS No.:110072-96-3 MDL No.:MFCD07369630 MF:C8H14N2O2 MW:170.2090 |
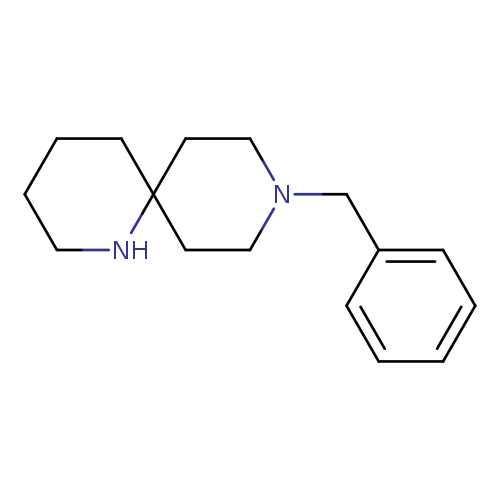
9-Benzyl-1,9-diazaspiro[5.5]undecaneCatalog No.:AA00998Q CAS No.:1100748-66-0 MDL No.:MFCD12406521 MF:C16H24N2 MW:244.3752 |
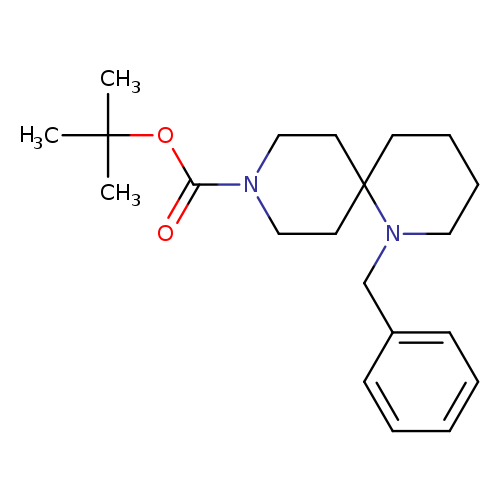
tert-Butyl 1-benzyl-1,9-diazaspiro[5.5]undecane-9-carboxylateCatalog No.:AA00998R CAS No.:1100748-67-1 MDL No.:MFCD13184378 MF:C21H32N2O2 MW:344.4910 |
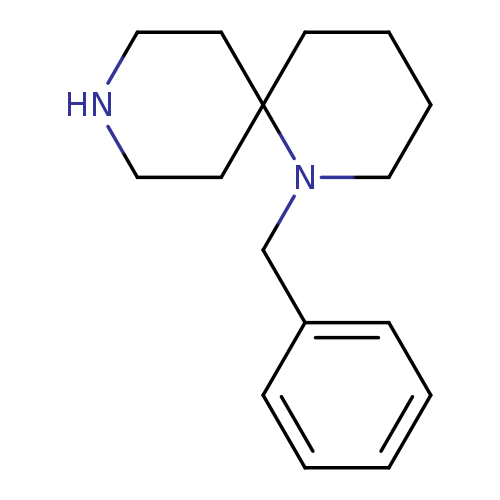
1-Benzyl-1,9-diazaspiro[5.5]undecane 2hclCatalog No.:AA008U29 CAS No.:1100748-68-2 MDL No.:MFCD12406522 MF:C16H24N2 MW:244.3752 |
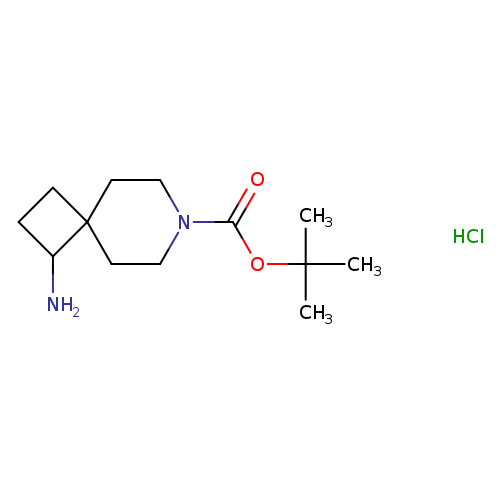
tert-Butyl 1-amino-7-azaspiro[3.5]nonane-7-carboxylate hydrochlorideCatalog No.:AA008TU4 CAS No.:1100748-78-4 MDL No.:MFCD19439666 MF:C13H25ClN2O2 MW:276.8028 |
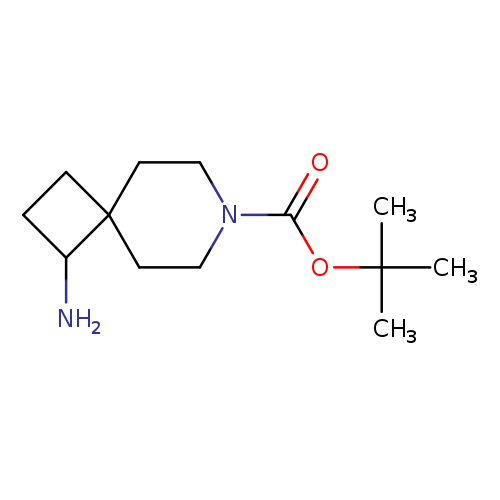
1-Amino-7-azaspiro[3.5]nonane-7-carboxylic acid tert-butyl esterCatalog No.:AA008TQ9 CAS No.:1100748-84-2 MDL No.:MFCD17016205 MF:C13H24N2O2 MW:240.3419 |
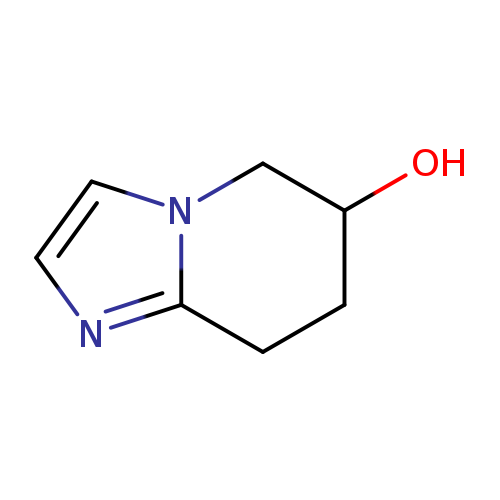
5,6,7,8-Tetrahydroimidazo[1,2-a]pyridin-6-olCatalog No.:AA008UA4 CAS No.:1100750-16-0 MDL No.:MFCD21336103 MF:C7H10N2O MW:138.1671 |
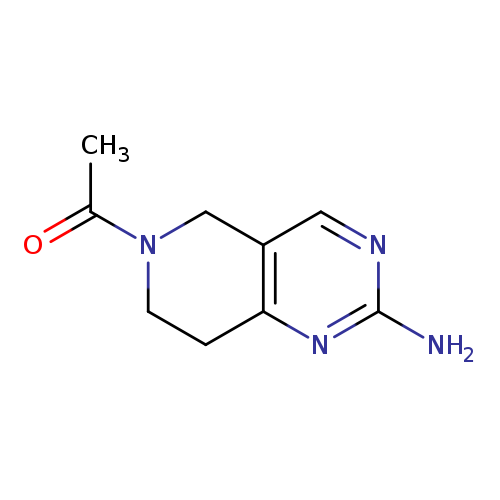
1-(2-Amino-5h,6h,7h,8h-pyrido[4,3-d]pyrimidin-6-yl)ethan-1-oneCatalog No.:AA008XGF CAS No.:1100752-63-3 MDL No.:MFCD11655916 MF:C9H12N4O MW:192.2178 |
3-(Benzyloxy)-5-chloroanilineCatalog No.:AA0084F1 CAS No.:1100752-67-7 MDL No.:MFCD11655923 MF:C13H12ClNO MW:233.6935 |
5-Bromo-2-fluoro-benzamidineCatalog No.:AA0092GX CAS No.:1100752-70-2 MDL No.:MFCD09832178 MF:C7H6BrFN2 MW:217.0383 |
4-Bromo-2-fluoro-benzamidineCatalog No.:AA0093JG CAS No.:1100752-71-3 MDL No.:MFCD13561984 MF:C7H6BrFN2 MW:217.0383 |
7-Oxa-4-azaspiro[2.5]octan-5-oneCatalog No.:AA0095RC CAS No.:1100753-07-8 MDL No.:MFCD11655976 MF:C6H9NO2 MW:127.1412 |
Tetrahydrofuran-2-carbothioamideCatalog No.:AA01AJCF CAS No.:1100753-12-5 MDL No.:MFCD05662455 MF:C5H9NOS MW:131.1961 |
3-bromo-1-(4-fluorophenyl)pyrrolidin-2-oneCatalog No.:AA01C32Q CAS No.:1100756-25-9 MDL No.:MFCD18886488 MF:C10H9BrFNO MW:258.0870 |
5-{[(3-methoxyphenyl)methyl]sulfanyl}-1,3,4-thiadiazol-2-amineCatalog No.:AA00IQWY CAS No.:110076-51-2 MDL No.:MFCD00925754 MF:C10H11N3OS2 MW:253.3438 |
5-bromo-4-methyl-2-(trifluoromethyl)pyrimidineCatalog No.:AA01DX8P CAS No.:1100767-04-1 MDL No.:MFCD23133295 MF:C6H4BrF3N2 MW:241.0086 |
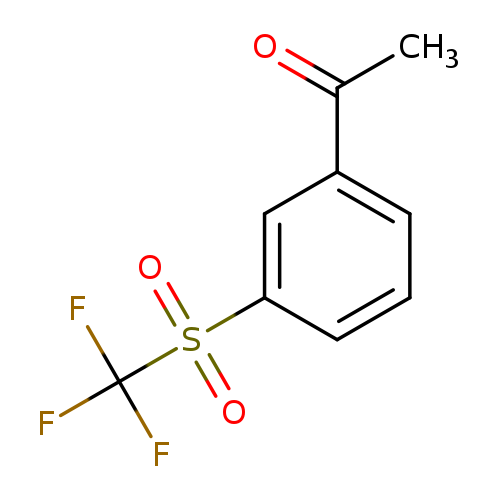
1-(3-Trifluoromethanesulfonylphenyl)ethan-1-oneCatalog No.:AA01BJ5O CAS No.:1100768-01-1 MDL No.:MFCD25460316 MF:C9H7F3O3S MW:252.2103 |
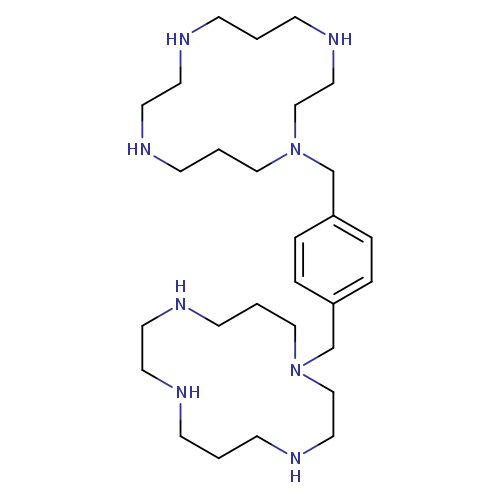
1-{[4-(1,4,8,11-tetraazacyclotetradecan-1-ylmethyl)phenyl]methyl}-1,4,8,11-tetraazacyclotetradecaneCatalog No.:AA007VV1 CAS No.:110078-46-1 MDL No.:MFCD05662218 MF:C28H54N8 MW:502.7820 |
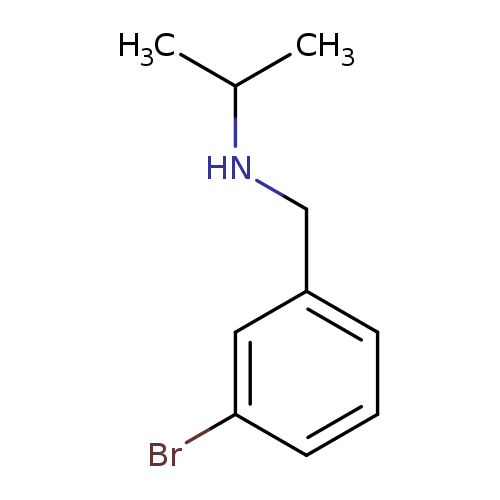
[(3-Bromophenyl)methyl](propan-2-yl)amineCatalog No.:AA009TTK CAS No.:110079-41-9 MDL No.:MFCD08757430 MF:C10H14BrN MW:228.1289 |
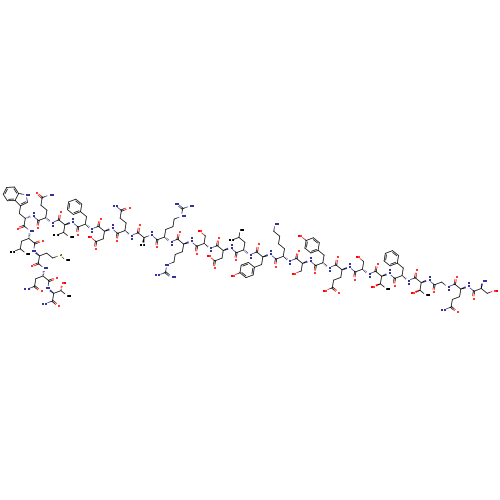
(Des-His1,Glu9)-Glucagon (1-29) amide (human, rat, porcine) H-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Glu-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-NH2Catalog No.:AA01EAVZ CAS No.:110084-95-2 MDL No.:MFCD00080353 MF:C148H221N41O47S MW:3358.6498 |
ALPHA-CHLORO-3-NITROBENZALDOXIMECatalog No.:AA01DMFY CAS No.:1100844-81-2 MDL No.:MFCD02183502 MF:C7H5ClN2O3 MW:200.5792 |
3-(heptan-3-yl)-1H-pyrazol-5-amineCatalog No.:AA019S0N CAS No.:110086-14-1 MDL No.:MFCD11194935 MF:C10H19N3 MW:181.2780 |
5-Hydroxy-2-methylpentan-2-yl acetateCatalog No.:AA00HBLH CAS No.:110086-92-5 MDL No.:MFCD28015551 MF:C8H16O3 MW:160.2108 |
4-Acetoxy-4-methyl-1-pentanalCatalog No.:AA007VUX CAS No.:110086-93-6 MDL No.:MFCD06658283 MF:C8H14O3 MW:158.1950 |
Prasugrel alpha-Hydroxy IMpurityCatalog No.:AA0091TQ CAS No.:1100905-45-0 MDL No.: MF:C11H11FO2 MW:194.2022 |
3-EthynyltetrahydrofuranCatalog No.:AA00HBLI CAS No.:1100987-19-6 MDL No.:MFCD22566104 MF:C6H8O MW:96.1271 |
2-(Methylthio)pyrimidine-5-carboxylic acidCatalog No.:AA007DYO CAS No.:110099-94-0 MDL No.:MFCD07394096 MF:C6H6N2O2S MW:170.1890 |
1-(2-Chloropyrimidin-5-yl)ethanoneCatalog No.:AA008UA9 CAS No.:110100-00-0 MDL No.:MFCD10697144 MF:C6H5ClN2O MW:156.5697 |
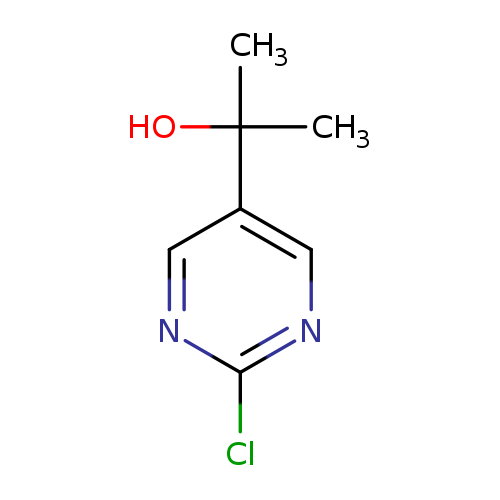
2-(2-Chloropyrimidin-5-yl)propan-2-olCatalog No.:AA01A2HE CAS No.:110100-09-9 MDL No.:MFCD19441124 MF:C7H9ClN2O MW:172.6122 |
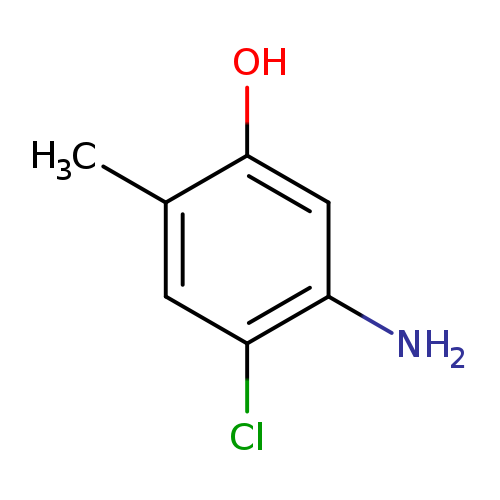
5-Amino-4-chloro-2-methylphenolCatalog No.:AA003MB6 CAS No.:110102-86-8 MDL No.:MFCD09744010 MF:C7H8ClNO MW:157.5975 |
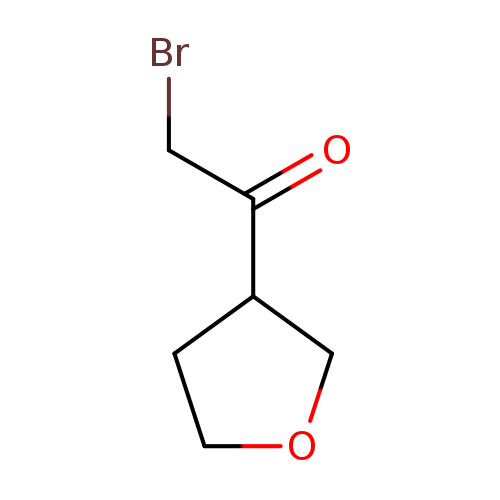
2-bromo-1-(oxolan-3-yl)ethan-1-oneCatalog No.:AA01ABSM CAS No.:1101023-98-6 MDL No.:MFCD23845094 MF:C6H9BrO2 MW:193.0385 |
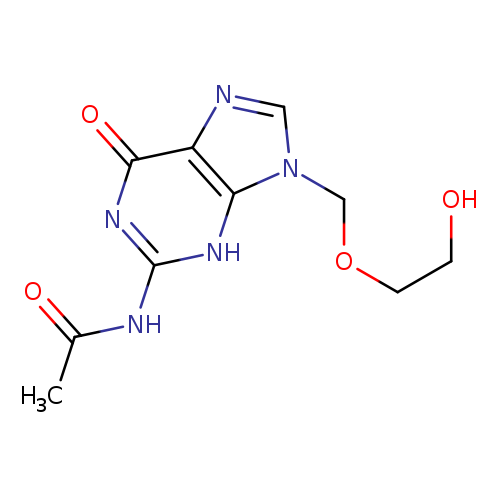
N-(9-((2-Hydroxyethoxy)methyl)-6-oxo-6,9-dihydro-1H-purin-2-yl)acetamideCatalog No.:AA008SER CAS No.:110104-37-5 MDL No.:MFCD01078801 MF:C10H13N5O4 MW:267.2413 |
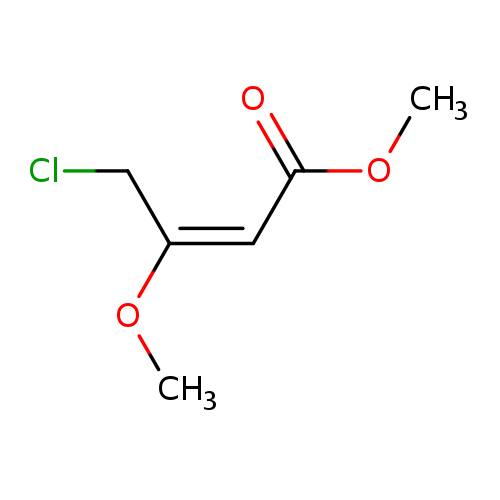
Methyl E-4-chloro-3-methoxy-2-butenoateCatalog No.:AA003RG0 CAS No.:110104-60-4 MDL No.:MFCD00071562 MF:C6H9ClO3 MW:164.5869 |
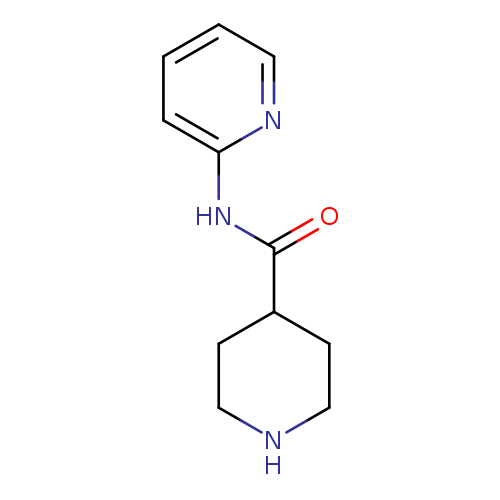
N-(Pyridin-2-yl)piperidine-4-carboxamideCatalog No.:AA007VP4 CAS No.:110105-31-2 MDL No.:MFCD08272001 MF:C11H15N3O MW:205.2563 |
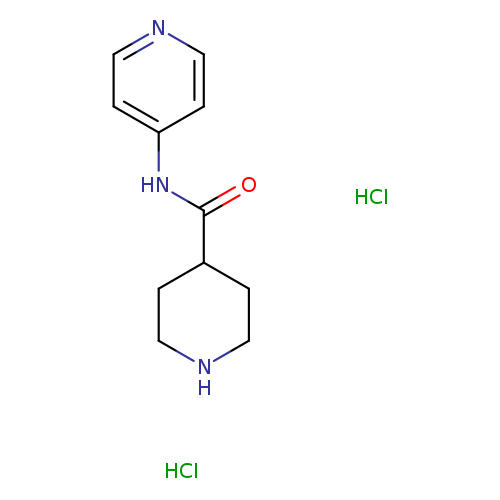
N-Pyridin-4-ylpiperidine-4-carboxamide dihydrochlorideCatalog No.:AA019MCC CAS No.:110105-33-4 MDL No.:MFCD10686814 MF:C11H17Cl2N3O MW:278.1782 |
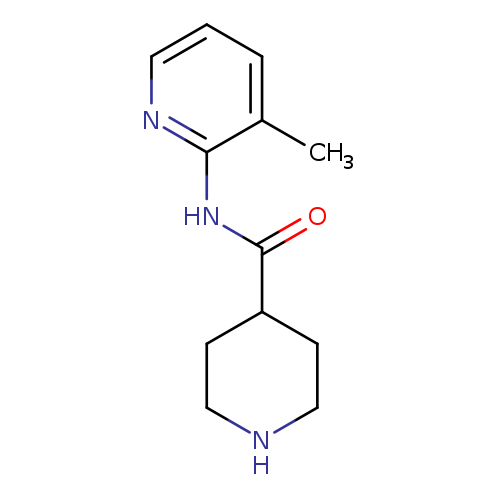
N-(3-Methylpyridin-2-yl)piperidine-4-carboxamideCatalog No.:AA007VP3 CAS No.:110105-98-1 MDL No.:MFCD11128699 MF:C12H17N3O MW:219.2829 |
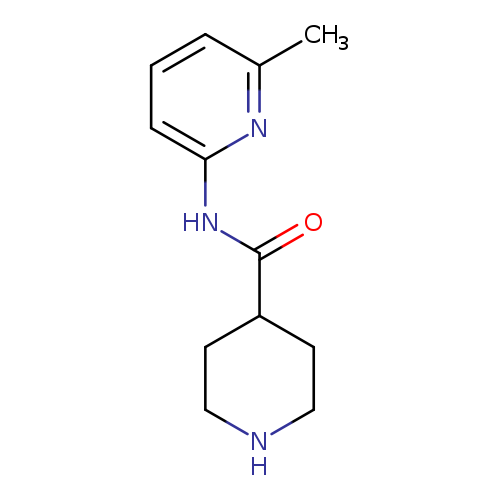
N-(6-Methylpyridin-2-yl)piperidine-4-carboxamideCatalog No.:AA008495 CAS No.:110105-99-2 MDL No.:MFCD08272004 MF:C12H17N3O MW:219.2829 |
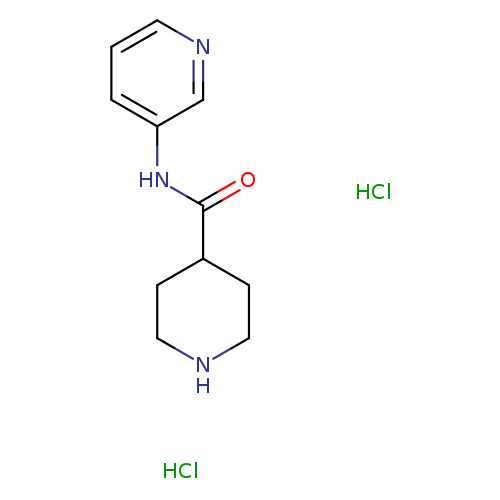
N-(pyridin-3-yl)piperidine-4-carboxamide dihydrochlorideCatalog No.:AA01A01W CAS No.:110106-25-7 MDL No.:MFCD22378620 MF:C11H17Cl2N3O MW:278.1782 |
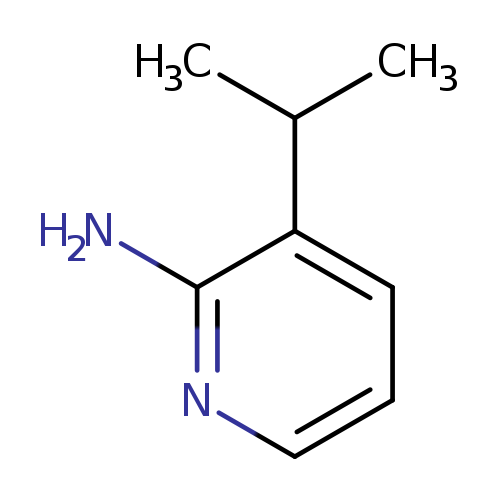
3-(Propan-2-yl)pyridin-2-amineCatalog No.:AA00IKXZ CAS No.:1101060-79-0 MDL No.:MFCD19648929 MF:C8H12N2 MW:136.1943 |
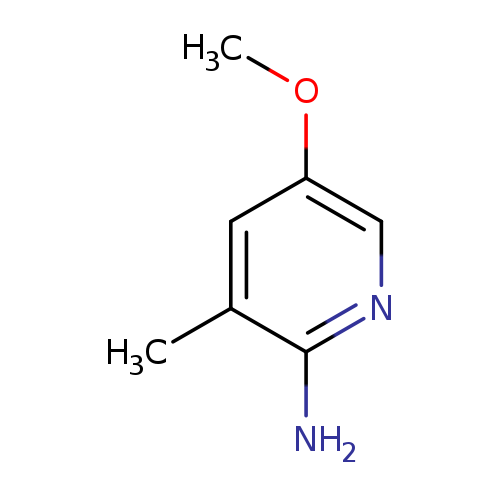
5-METHOXY-3-METHYLPYRIDIN-2-AMINECatalog No.:AA01FMFR CAS No.:1101060-84-7 MDL No.:MFCD18250734 MF:C7H10N2O MW:138.1671 |
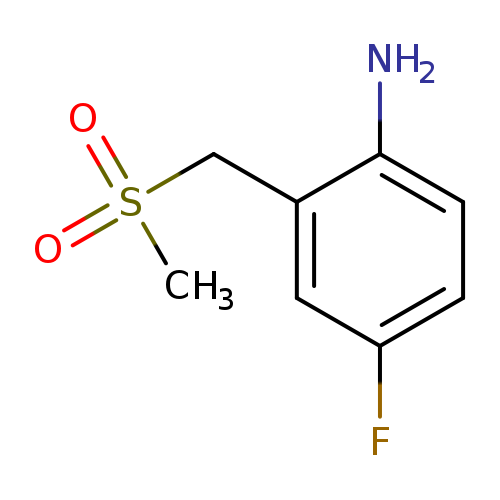
4-Fluoro-2-(methanesulfonylmethyl)anilineCatalog No.:AA00HBLJ CAS No.:1101063-17-5 MDL No.:MFCD12779627 MF:C8H10FNO2S MW:203.2339 |
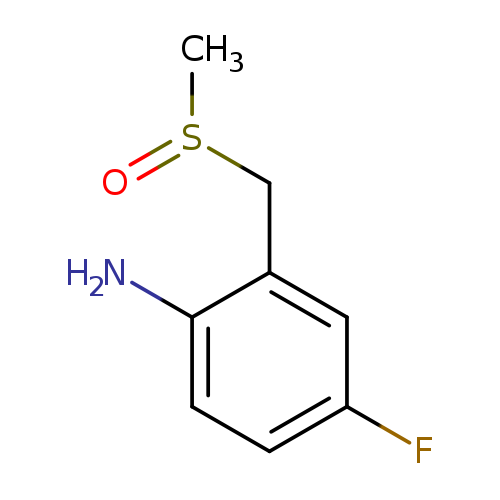
4-fluoro-2-(methanesulfinylmethyl)anilineCatalog No.:AA01ABMR CAS No.:1101063-45-9 MDL No.:MFCD14613587 MF:C8H10FNOS MW:187.2345 |
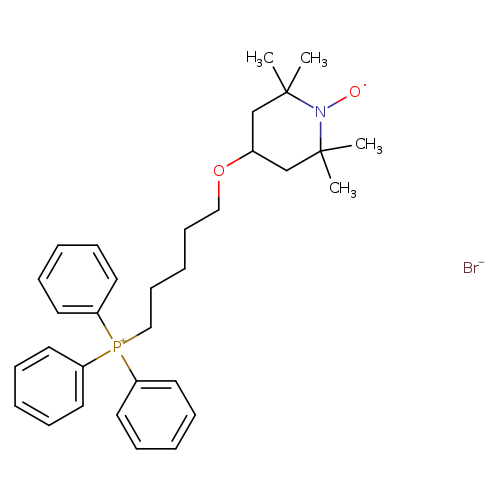
MitoTEMPOLCatalog No.:AA0097CX CAS No.:1101113-39-6 MDL No.: MF:C32H42BrNO2P MW:583.5591 |
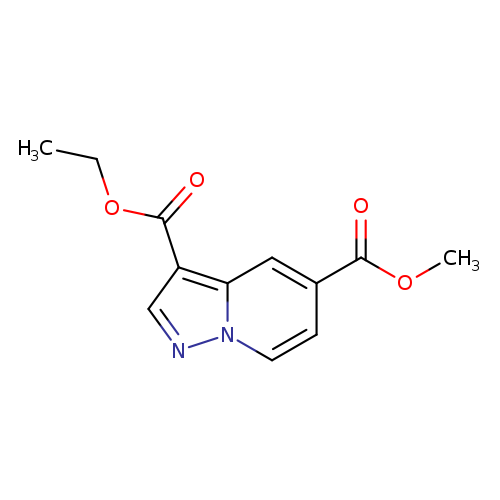
3-Ethyl 5-methyl pyrazolo[1,5-a]pyridine-3,5-dicarboxylateCatalog No.:AA019EY4 CAS No.:1101120-00-6 MDL No.:MFCD20528906 MF:C12H12N2O4 MW:248.2347 |
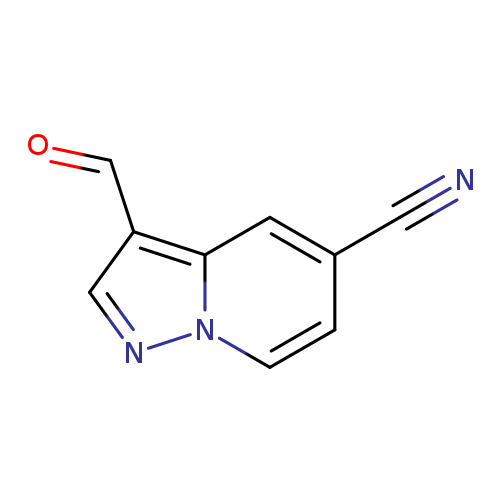
3-Formylpyrazolo[1,5-a]pyridine-5-carbonitrileCatalog No.:AA00HBLL CAS No.:1101120-05-1 MDL No.:MFCD22067049 MF:C9H5N3O MW:171.1555 |
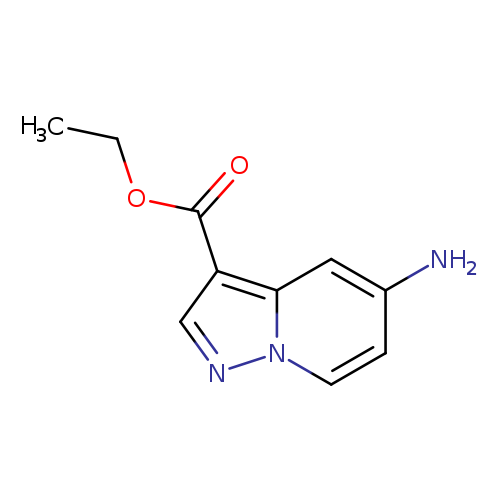
Ethyl 5-aminopyrazolo[1,5-a]pyridine-3-carboxylateCatalog No.:AA008U6R CAS No.:1101120-35-7 MDL No.:MFCD20923175 MF:C10H11N3O2 MW:205.2132 |
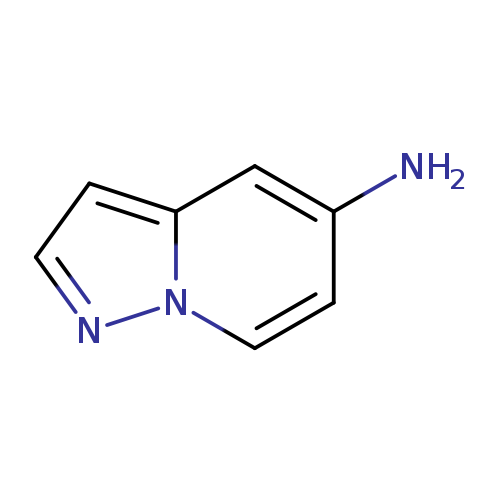
Pyrazolo[1,5-a]pyridin-5-amineCatalog No.:AA008U6S CAS No.:1101120-37-9 MDL No.:MFCD11924930 MF:C7H7N3 MW:133.1506 |
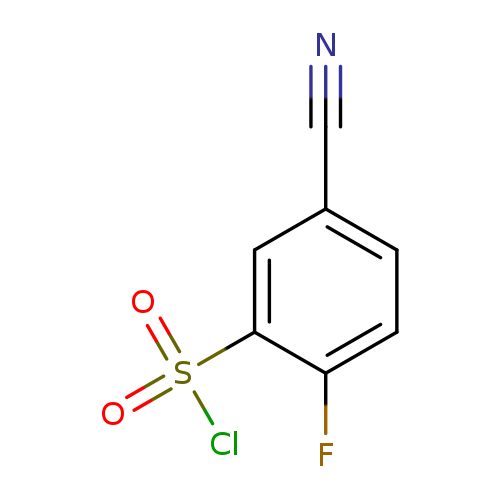
5-Cyano-2-fluorobenzene-1-sulfonyl chlorideCatalog No.:AA007VP0 CAS No.:1101120-80-2 MDL No.:MFCD18394026 MF:C7H3ClFNO2S MW:219.6206 |
5-Bromopyrazolo[1,5-a]pyridine-3-carboxylic acidCatalog No.:AA0092H6 CAS No.:1101121-05-4 MDL No.:MFCD18837595 MF:C8H5BrN2O2 MW:241.0415 |
tert-butyl N-(4-ethoxybutyl)carbamateCatalog No.:AA019VYK CAS No.:1101136-17-7 MDL No.:MFCD12912805 MF:C11H23NO3 MW:217.3052 |
4-[(1-oxo-7-phenylheptyl)amino]-(4R)-octanoic acidCatalog No.:AA007DTJ CAS No.:1101136-50-8 MDL No.:MFCD18382098 MF:C21H33NO3 MW:347.4916 |
CALPAIN INHIBITOR IICatalog No.:AA008RIN CAS No.:110115-07-6 MDL No.:MFCD00065506 MF:C19H35N3O4S MW:401.5639 |
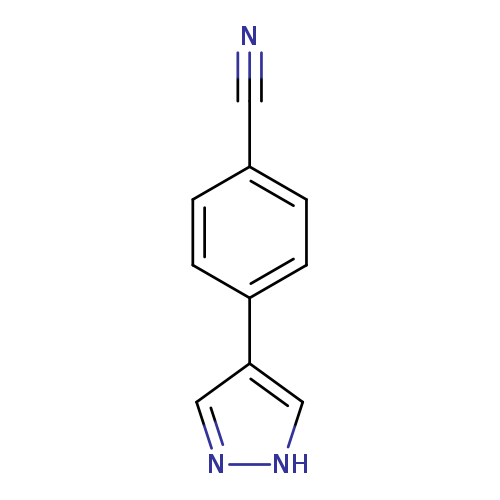
4-(1H-Pyrazol-4-yl)benzonitrileCatalog No.:AA00HBLO CAS No.:1101167-56-9 MDL No.:MFCD11933267 MF:C10H7N3 MW:169.1827 |
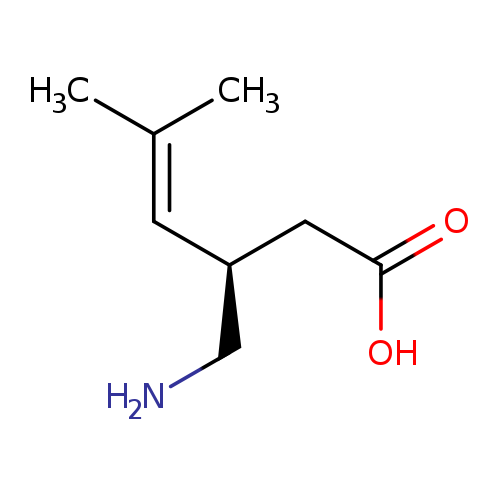
(R)-4,5-DehydroPregabalinCatalog No.:AA01DZIW CAS No.:1101167-84-3 MDL No.: MF:C8H15NO2 MW:157.2102 |
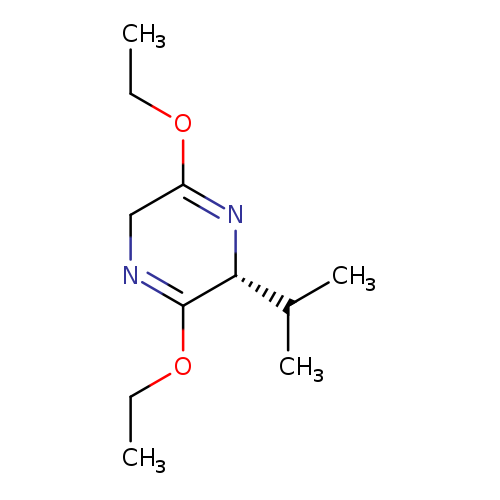
(R)-2,5-Dihydro-3,6-diethoxy-2-isopropylpyrazineCatalog No.:AA0038WR CAS No.:110117-71-0 MDL No.:MFCD09836052 MF:C11H20N2O2 MW:212.2887 |
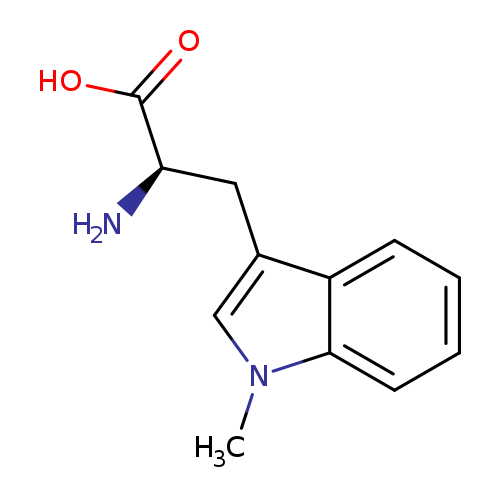
1-Methyl-D-tryptophanCatalog No.:AA0032QF CAS No.:110117-83-4 MDL No.:MFCD00274271 MF:C12H14N2O2 MW:218.2518 |
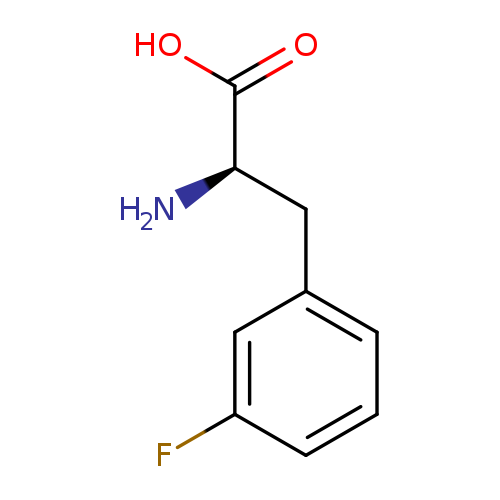
3-Fluoro-d-phenylalanineCatalog No.:AA00HBLP CAS No.:110117-84-5 MDL No.:MFCD00066449 MF:C9H10FNO2 MW:183.1796 |
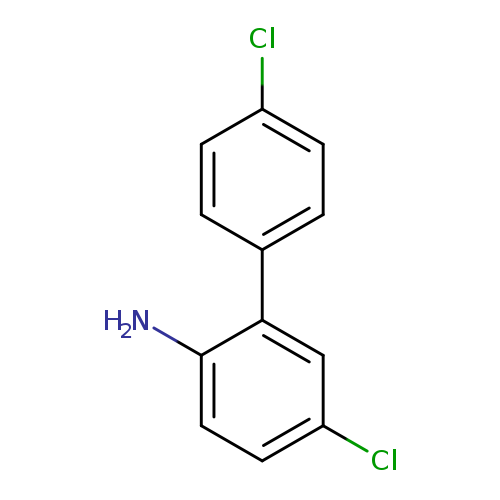
4-Chloro-2-(4-chlorophenyl)anilineCatalog No.:AA01BGG5 CAS No.:1101170-85-7 MDL No.:MFCD24078169 MF:C12H9Cl2N MW:238.1126 |
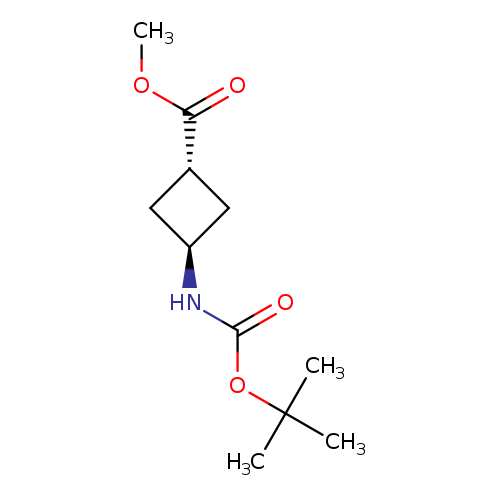
Methyl trans-3-(boc-amino)cyclobutanecarboxylateCatalog No.:AA00998T CAS No.:1101173-77-6 MDL No.:MFCD20922912 MF:C11H19NO4 MW:229.2729 |
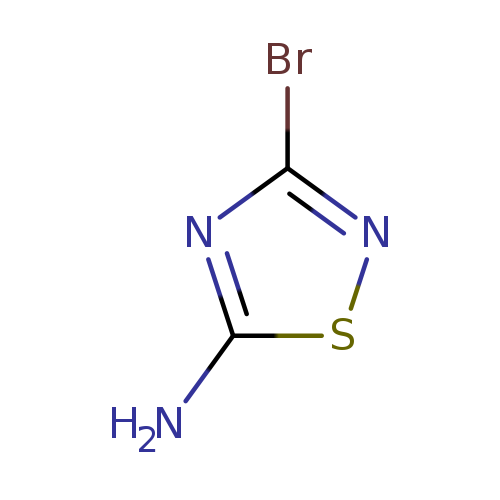
3-Bromo-1,2,4-thiadiazol-5-amineCatalog No.:AA00848W CAS No.:1101173-93-6 MDL No.:MFCD13193591 MF:C2H2BrN3S MW:180.0264 |
N-(2,3-dimethylphenyl)-1,3-thiazolidine-4-carboxamideCatalog No.:AA01A9XF CAS No.:1101181-60-5 MDL No.:MFCD09940252 MF:C12H16N2OS MW:236.3332 |
2-(2-propyl-1H-benzimidazol-1-yl)propanoic acid hydrochlorideCatalog No.:AA00J1L0 CAS No.:1101183-40-7 MDL No.:MFCD11506554 MF:C13H17ClN2O2 MW:268.7393 |
1-(3,5-Dimethylbenzoyl)piperidine-2-carboxylic acidCatalog No.:AA01A3KI CAS No.:1101193-09-2 MDL No.:MFCD09049183 MF:C15H19NO3 MW:261.3163 |
2-Ethyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid hydrochlorideCatalog No.:AA0090QQ CAS No.:1101199-16-9 MDL No.:MFCD06800715 MF:C12H16ClNO2 MW:241.7139 |
5-Methylpyridine-2-boronic acid, pinacol esterCatalog No.:AA007VOW CAS No.:1101205-22-4 MDL No.:MFCD07368874 MF:C12H18BNO2 MW:219.0878 |
1-(2-methylbenzoyl)piperidine-2-carboxylic acidCatalog No.:AA01A3O8 CAS No.:1101227-14-8 MDL No.:MFCD08442141 MF:C14H17NO3 MW:247.2897 |
Chlorocarbonylhydrido[4,5-bis-(di-i-propylphosphinomethyl)acridine]ruthenium(II)Catalog No.:AA008WNA CAS No.:1101230-25-4 MDL No.:MFCD19443487 MF:C28H41ClNOP2Ru++ MW:606.1018 |
(11bR)-(+)-4,4-Di-t-butyl-2,6-bis[3,5-bis(trifluoroMethyl)phenyl]-4,5-dihydro-3H-dinaphtho[2,1-cCatalog No.:AA008VR1 CAS No.:1101230-28-7 MDL No.:MFCD18827624 MF:C27H39NP2 MW:439.5528 |
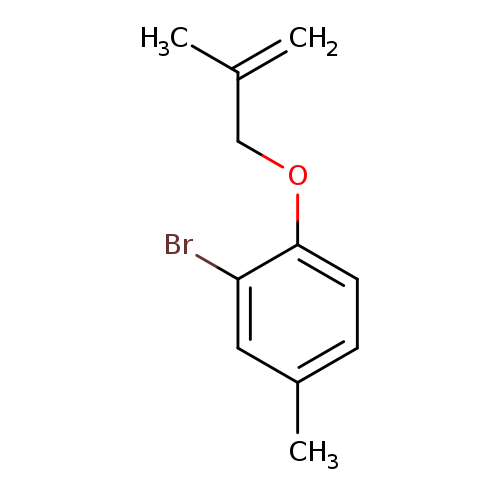
2-bromo-4-methyl-1-[(2-methylprop-2-en-1-yl)oxy]benzeneCatalog No.:AA01A2OW CAS No.:110124-11-3 MDL No.:MFCD11156059 MF:C11H13BrO MW:241.1243 |
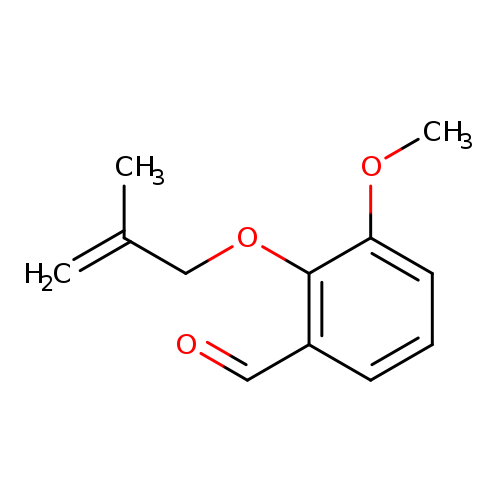
3-Methoxy-2-[(2-methyl-2-propen-1-yl)oxy]benzaldehydeCatalog No.:AA007DTG CAS No.:110124-13-5 MDL No.:MFCD08691807 MF:C12H14O3 MW:206.2378 |
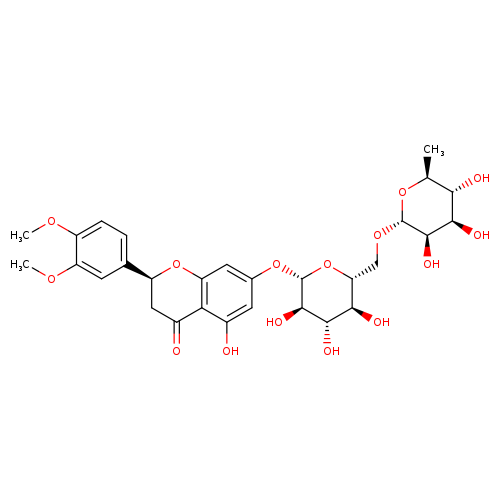
Methyl hesperidinCatalog No.:AA003RXN CAS No.:11013-97-1 MDL No.:MFCD01741310 MF:C29H36O15 MW:624.5871 |
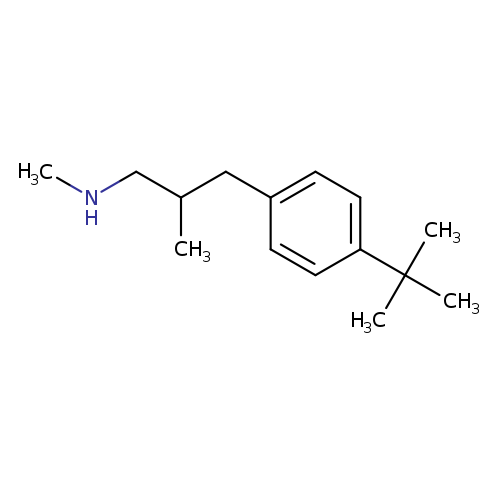
[3-(4-tert-Butylphenyl)-2-methylpropyl](methyl)amineCatalog No.:AA01DUWD CAS No.:110135-01-8 MDL No.:MFCD16781963 MF:C15H25N MW:219.3657 |