2019-11-05 14:52:13
B. Marco-Dufort, M.W. Tibbitt*
Macromolecular Engineering Laboratory, Department of Mechanical and Process Engineering, ETH Zürich, Zürich 8092, Switzerland
As mentioned above, dynamic covalent chemistries, including boronic ester bonds, enable the formation of responsive and moldable materials as the bonds can rearrange by breaking and reforming upon application of external stimuli on experimental timescales [43]. The main considerations in the design of these adaptable polymer networks from dynamic covalent chemistries are the specific chemistry of the binding pair and the mechanism of network formation. The specific boronic acid-diol chemistry defines the thermodynamics and kinetics of boronic ester bond formation, breaking, and reformation, which controls the dynamics of network strand rearrangement. The mechanism of network formation governs the topology of the network or how the network strands are linked together, which in turn influences the mechanical properties (such as gel point, plateau modulus, relaxation time, and swelling) of the resultant viscoelastic material. Simply put, the properties of polymer networks and gels are controlled by how the molecules in the network are connected [49]. Broad use of dynamic covalent networks and gels requires a robust understanding of how these factors influence emergent network properties. In this section, we discuss how network formation and topology relate to the mechanical properties of dynamic covalent networks.
Network formation and gel point
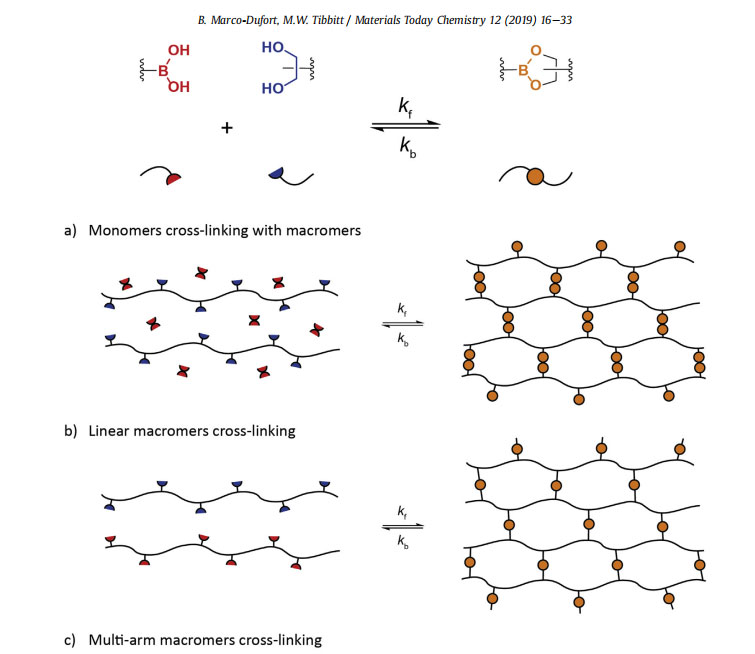
Polymer network formation occurs by cross-linking multifunctional monomer or macromer precursors [50]. Here, a functional group refers to a chemical moiety that can participate in the formation of a single dynamic covalent bond. Boronic ester crosslinking occurs via complementary bonding between boronic acid and diol moieties, as shown in Fig. 4. If each precursor molecule has a functionality of exactly two, the reaction will lead to chain extension and growth of individual polymer chains. On the other hand, if the average functionality is greater than two, branching can occur which bonds polymer chains together. At a critical extent of reaction pc, the cross-linking of disparate polymer chains through the branch points induces a phase transition from liquid-like behavior to solid-like behavior as a three-dimensional polymer network percolates across the entire system [50]. The critical point pc is referred to as the gel point. Just below the gel point, the system exists as a solution of polydisperse branched polymers, and beyond the gel point, at least some fraction of the system exists as an ‘infinite’ molecular weight polymer network that spans the entire volume. The gel point can be calculated based on the functionality and structure of the network forming precursors, using mean-field models of gelation via the Flory [51] or Flory-Stockmayer [52,53] approaches that calculate pc as the conversion at which the weight-average molecular weight of the growing, branched polymer chains diverges.
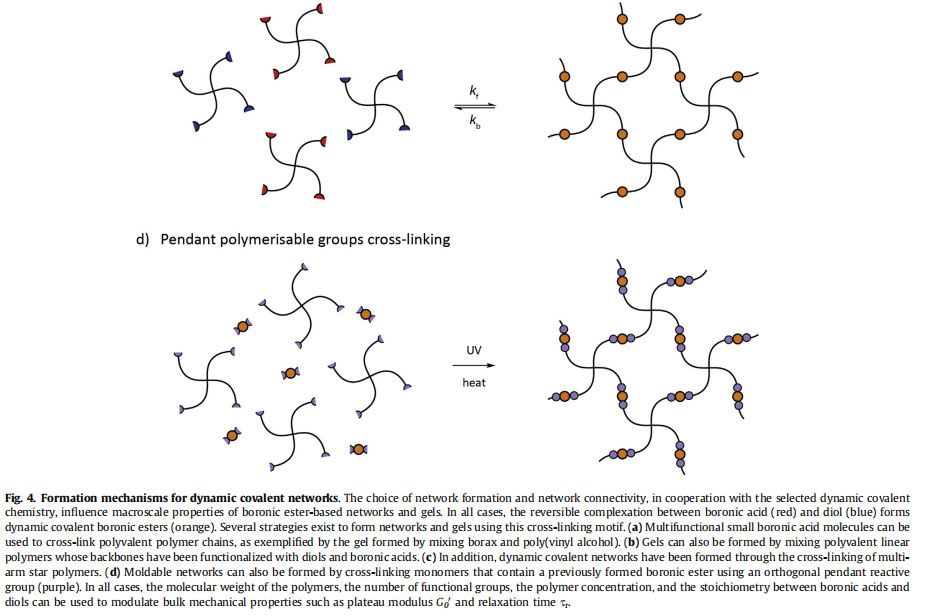
To exploit the reversible nature of the boronic ester bond to form dynamic covalent networks, boronic esters moieties need to be installed into the backbone of the forming polymer network. Two main mechanisms can be employed to introduce boronic ester bonds into the network backbone [4]. First, networks can be formed via direct cross-linking of multifunctional boronic acid and diol monomers or macromers, as shown in Fig. 4a, b, and c. In this manner, the cross-links themselves are composed of reversible and dynamic boronic esters during formation. Alternatively, networks can be formed from monomers or macromers that contain one or multiple boronic esters in their core and pendant polymerizable functional groups (such as (meth)acrylates, thiols and alkenes, or azides and alkynes), as shown in Fig. 4d. Here, the cross-linking chemistry and conditions must maintain the integrity of the boronic ester bond. As most boronic esters are highly dynamic in aqueous solution at standard pH [116], the former mechanism that uses the boronic ester as the cross-linking chemistry itself is more common for the formation of boronic esterebased hydrogels and will be the focus of the rest of this section. In covalently cross-linked networks and gels, a formed bond is permanent, and calculation of the gel point is relatively straightforward. For example, the critical extent of reaction pc based on the Flory approach can be calculated for multifunctional precursors as:

where f is the average functionality of the precursors [51,54]. In the Flory-Stockmayer approach, the critical extent of reaction pc for a step-growth polymerization between precursor A with functionality fA and precursor B with functionality fB can be calculated as:

where r is the stoichiometric ratio between A and B with r 2½0; 1[53]. Note, the two approaches converge for r ¼ 1 and fA ¼ fB.
For dynamic covalent bonds, such as boronic esters, the reacted bonds are not permanent; the bonds are in flux as they continually rearrange. Therefore, gelation in dynamic covalent networks relates
also to the equilibrium constant Keq and the concentration of the functional groups [C]. At sufficiently long times after mixing, one can assume that the system has reached thermodynamic equilibrium and that the number of formed bonds or ‘extent of reaction’ is determined solely by the equilibrium constant for the dynamic covalent chemistry and the concentration of functional groups. Thus, the number of formed bonds p ~ f(Keq,[C]) must remain above pc to maintain a gel. As discussed above, Keq for boronic ester bonds varies with changes in environmental factors, including pH, temperature, or stress. Modulating Keq provides a facile handle to reverse gelation in dynamic covalent networks and enables easy processing of the networks. External stimuli can transform the network from a solid to a liquid and back again repeatedly, allowing for recycling, healing, as well as shaping and reforming through subtle shifts in the external conditions.
A thorough treatment of the gel point for associating polymers was developed by Semenov and Rubinstein [55]. The guidelines here provide descriptive indicators for what systems will or will not
form a gel and how external parameters can be used to transition between sol and gel, reversibly. This understanding has enabled the design and synthesis of multifunctional precursors that have been
used to engineer responsive materials exploiting the dynamic covalent nature of boronic ester bonds [43]. This has been demonstrated in the design of moldable materials from borax an polysaccharides, glucose-responsive gels, and self-healing polymer networks that will be discussed further below.
Dynamic mechanical analysis
Engineering properties of major interest in the design of polymeric materials, including dynamic covalent networks, are the plateau modulus and the relaxation time. The modulus of elasticity E for a material defines the ratio between the stress applied to a material and the resultant deformation in the linear elastic region. The relaxation time tr characterizes the timescale within which strain-induced stresses within the material are dissipated [50]. A standard technique for quantifying the linear mechanical properties of viscoelastic materials is to measure the stress in the material in response to small sinusoidal deformations. The application of cyclic strain, called dynamic mechanical analysis, is employed commonly for soft materials (E ~ 101 e108 Pa) characterization using a shear rheometer, which measures the shear modulus G of the material, or the ratio of shear stress to shear strain. G and E are related via the Poisson's ratio [56].
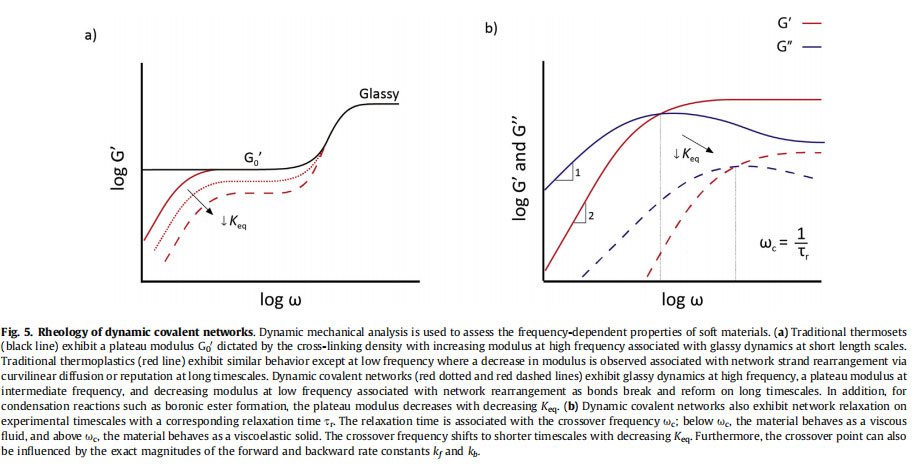
Shear rheometry probes materials properties with an applied oscillatory shear strain. It measures the frequency-dependent dynamic or complex modulus G* of the material, which can be expressed in terms of the storage modulus G0(u)da measure of the stored elastic energydand the loss modulus G''(u)da representation of the viscous energy dissipation [56]. Tuning the frequency of the applied strain enables investigation of different timescales or relaxation modes within the material. Owing to the many hierarchical length scales present in polymeric materials that are inherently coupled to different timescales of molecular motion, mechanical properties are dependent on the frequency of analysis. High frequency measurements probe short times and thus small motions at the atom or monomer level while low frequency measurements probe long times and thus large motions at the chain or material level. As such, the frequency-dependent modulus of all polymeric materials increases at high frequency, indicative of the glassy dynamics associated with relaxation on the smallest length scales [4]. At lower frequencies, polymer segments and chains are able to relax, and the frequencydependent modulus decreases to a plateau modulus G0'. Both the plateau modulus and the glassy region are illustrated in Fig. 5a, which plots the frequency-dependent storage modulus G0(u) for model polymer networks.
Classic polymeric materials are subdivided into thermoplasticsdpolymer melts that flow with increasing temperature or dissolve upon addition of a good solventdand thermosetsd covalently cross-linked networks that do not change state with temperature prior to combustion and swell without dissolving upon addition of a good solvent. In thermosets, the topology of the network strands is fixed by the permanent covalent cross-links. These can fluctuate in three-dimensional space but prevent flow or dissolution of the network. In thermoplastics, the topology of the polymer strands is constrained by other polymer chains via physical interactions, entanglements, and excluded volume; however, at sufficiently long times, chains can overcome these physical constraints and move or reptate to relax internal stress.
Therefore, as their network topology is fixed, thermosets behave as ideal, elastic materials. They possess a plateau modulus associated with the cross-linking density of the material and a non-finite
relaxation time. A representative curve for their ideal behavior is shown in Fig. 5a. Thermoplastics, on the other hand, also demonstrate a plateau modulus, as polymer diffusion is constrained by
topological entanglements with other chains that act as effective cross-links at certain timescales. However, with decreasing frequency or longer timescales, the frequency-dependent modulus
decays as the polymer chains are able to reptate via curvilinear diffusion, and thus thermoplastics possess a finite relaxation time, as shown in Fig. 5a.
Viscoelastic dynamic covalent networks exhibit rheological behavior that mirrors aspects of both thermosets and thermoplastics. Their behavior is characterized by the Maxwell model of viscoelasticity, which combines in series an elastic element with modulus GM and a viscous element with viscosity hM [50]. In the maxwell model, a critical timescale is defined as tr ¼ hM/GM. The material will behave as a solid at timescales shorter than tr and flows like a viscous liquid on timescales longer than tr. That is, dynamic covalent networks possess a plateau storage modulus defined by the network cross-link density as well as a finite relaxation time defined by the chemistry of the dynamic covalent cross-links, as illustrated in Fig. 5a. A complete treatment of the
polymer physics and scaling laws for the behavior of reversible polymer networks was developed by Rubinstein and Semenov, and we refer the reader to these studies for a more thorough discussion of the theoretical foundations of associating polymer networks [57]. Here, we restrict our discussion to general engineering design criteria for the design of dynamic covalent
networks.
Plateau modulus
As previously discussed, the elastic modulus is a critical parameter in the design of soft materials. The theory of rubber elasticity for cross-linked polymer networks relates the molecular details and topology of the network to the modulus of the material[50]. At its core, rubber elasticity states that E ~ nT, where n is the number density of network strands and T is the temperature of the material. The number density of network strands is directly related to the cross-link density rx and further depends on the molecular properties of the network precursors, e.g., molecular weight, persistence length, and concentration. The recursive method of Miller and Macosko is commonly used to calculate the number density of network strands based on the properties of the precursor molecules [58].
The molecular foundation of rubber elasticity is the entropic penalty caused by deforming individual polymer chains away from their relaxed state, which leads to a restoring force of kBT per network strand. Here, kB is the Boltzmann constant and T is the temperature. Thus, the modulus scales linearly with the number density of network strands with each strand contributing kBT to the modulus. Based on rubber elasticity, the shear modulus can be calculated directly as G ¼ nkBT. Thus, the plateau modulus from shear rheometry G00 can be related to the number density of network strands in the cross-linked material. Rubber elasticity is based on the underlying assumptions that the network strands are Gaussian, and the cross-links are points in free space [50]. The model stated above, G ¼ nkBT, is based on the affine network model, which assumes that each network strand experiences the same deformation as the bulk material. The phantom network model accounts for the fact that the cross-links are not fixed in space but fluctuate around an average location. This leads to an overall decrease of the predicted modulus of the material as given by G ¼ nkBT (1e2/f), where f is the functionality at the cross-link junctions. Recently, more advanced versions of rubber elasticity have been developed that account for more complicated network topology that includes network loops and entanglements including the real elastic network theory or RENT [49]. In all models, the general scaling that G ~ E ~ nT holds is inherent to the thermoelastic nature of rubbery materials.
As alluded to above, several design elements provide control over the network strand density and thus the elasticity in polymer networks and gels. Consider the step-growth polymerization of 4-arm star-polymers functionalized with boronic acid or diol derivatives, as depicted in Fig. 4c. Here, the network strand density can be tuned by altering the molecular weight of the individual polymer molecules and thereby the molecular weight of the individual arms. Additionally, the overall polymer concentration will affect the density of network strands, as well as the equilibrium bound fraction and the extent of network defects. Selecting backbone polymers with different rigidities or extents of hydration will also influence the modulus for a fixed polymer concentration. The network connectivity, or how many network strands link together at each cross-linking junction, also changes the density of active network strands for a given polymer concentration and can be increased by interchanging the 4-arm star-polymers for 8-arm starpolymers or polyfunctional linear polymers.
Relaxation time
The time-dependent viscoelastic properties of dynamic covalent networks can also be engineered by the cross-linking chemistry and network topology. As discussed above, these materials exhibit frequency-dependent storage G0(u) and loss Gʺ(u) moduli, owing to the breaking and reformation of boronic ester bonds that occurs on experimental timescales. At high frequencies or short timescales,
their properties are analogous to an elastic network; at low frequencies or long timescales, the material behaves like a viscous polymer solution. Oscillatory shear rheometry across a range of
frequencies is used to quantify the viscoelastic behavior of dynamic covalent networks. In general, a critical frequency uc is observed when G0(u) ¼ Gʺ(u), which is related to the relaxation time tr of the
material, tr ¼ 2p=uc. At frequencies below uc, the viscoelastic moduli should follow the classic scaling rules for terminal relaxation in a Maxwell model, where G0(u) ~ u2 and Gʺ(u) ~ u1 [57]. Fig. 5b illustrates these behaviors and the effect of varying Keq or the magnitude of the forward and reverse rate constants.
The characteristic timescale of the viscoelastic behavior in dynamic covalent networks and gels is controlled by the dynamic covalent chemistry that comprises the reversible cross-links, which ultimately dictates the timescale of relaxation of the network strands. The timescale of the individual bond tB is related to the relaxation time tr of the network. Tuning the specific chemistry of the boronic acid-diol pair, the environmental conditions and the network topology provide control over the time-dependent properties for this class of materials [57,62]. For example, bonds with short tB timescales produce materials that behave as viscous polymer melts whereas bonds with high tB generate materials that can be indistinguishable from covalent networks [63].
In practice, dynamic covalent networks are not characterized by a single relaxation time but display a distribution of relaxation times. This is related both to the variance in dynamic covalent bond properties and cross-link topology in the network. The distribution of relaxation times can be determined by converting the frequencydependent storage and loss moduli calculated from rheometry into a relaxation spectrum H(t), as described by Grindy et al. for metal ligandebased dynamic polymer networks [64]. The relaxation spectrum models the material as an infinite number of Maxwell models in parallel rather than the single Maxwell model mentioned previously. It can be interpreted as how much energy is stored or dissipated by the material during dynamic loading [64]. A more complete understanding of how network topology and binding thermodynamics and kinetics control the relaxation behavior in dynamic covalent networks and gels is a focus of current research and will be necessary to provide a priori design of materials with precise relaxation behaviors for targeted applications.
Materials assembly from boronic ester cross-linking
As described above, the reversible nature of the boronic ester bond can be exploited to build dynamic covalent networks and gels with time-dependent viscoelastic properties that are moldable and
self-healing. Excellent reviews of boronic ester-based materials have been published, and we direct the reader to these works for a more comprehensive discussion of boronic esterebased materials
design [43,65]. Here, we restrict our discussion first to some of the foundational work that demonstrates how boronic acid-diol chemistry and network topology can be used to engineer dynamic covalent networks from boronic ester bonds and then highlight some of the emerging biomedical applications of boronic esterebased materials. The earliest examples of engineering materials with boronic
esters involved the combination of borax with polyhydroxy compounds. Deuel and Neukom demonstrated in their seminal paper that borax could be used in aqueous solution to cross-link gels with
suitable hydroxyl functional polysaccharides, including poly(vinyl alcohol) (PVA) [66]. The cross-linking of PVA with borax (Fig. 4a) is now commonly used to make moldable and self-healing ‘slime’
based on these original recipes. In their original article, Deuel and Neukom already demonstrated that materials properties depend on pH, providing evidence for an ‘optimal’ pH for gel formation and
highlighted that the addition of small molecule diols, including fructose, glycerin, and sucrose, influenced materials properties by disrupting the formed cross-links. This work also explored altering the ratio of borax to polyhydroxy, which indicated that the materials properties depend on the extent of cross-linking. As a whole, this manuscript laid the foundation for the myriad of boronic esterebased materials that are now made and how they can be engineered based on their cross-linking chemistry, environmental factors, and network topology.
Building upon these concepts, Pezron et al. investigated the
properties of reversible gels formed from borax and galactomannan, guaran, and (hydroxypropyl)guaran [67,68]. In these studies, 11B NMR was used to probe the extent of free borax as compared to mono-diol and di-diol complexation, providing insights into the molecular structure of the gels and the thermodynamics of binding. This work was extended by Leibler et al. to construct physical models of the properties and dynamics of reversible networks based on the underlying structure and chemistry [60]. Building on these findings, Kesavan and Prud'homme studied the linear viscoelastic properties of guaran and (hydroxypropyl)guaran, corroborating with these data the theory developed by Leibler et al. for the rheology of reversible networks [69].
A major focus for the modern use of boronic esterebased materials has been on the sensing of carbohydrates. Kitano et al. incorporated PBA moieties into poly(N-vinyl-2-pyrrolidone) [poly(NVP-co-PBA)], which was combined with PVA to assemble glucose-responsive gels. These responded to glucose by undergoing a gel-sol transition, envisioning applications as glucose-responsive drug delivery platforms [70]. In this work, they demonstrated the influence of polymer molecular weight, stoichiometry, and diol chemistry on materials rheology. The general concept of using boronic ester bonds for the assembly of materials that respond to the presence of carbohydrates has been explored by many other groups. For example, Kataoka et al. engineered a range of glucosesensing materials from PBA-based materials [71,72]. An additional emphasis in the community has been on the general design of moldable and self-healing networks and gels using the dynamic covalent boronic ester bond. Roberts et al. synthesized linear polymers of acrylic acid or 2- hydroxypropylmethacrylamide containing 10 mol% of PBA or salicylhydroxamic acid (SHA) [73]. Upon mixing in aqueous solutions, the PBA and SHA containing polymers assembled to form dynamically restructuring hydrogel networks, best illustrated by the scheme in Fig. 4b, whose properties were tuned by the volume fraction of polymer and pH. Similarly, as depicted in Fig. 4c, He et al. assembled reversible hydrogels from catechol-functionalized 4-arm poly(ethylene glycol) (PEG) and 1,3-benzenediboronic acid, exploiting the high affinity binding between PBA and catechol to engineer pH-responsive and self-healing gels [74]. In this work, they demonstrated that higher concentrations of the multi-arm PEG increased the plateau modulus of the formed network. Cash et al. installed dynamic covalent boronic ester bonds directly into polymer networks through the direct thiol-ene photopolymerization of 4-((allyloxy)methyl)-2-(4-vinylphenyl)-1,3,2-dioxaborolane, a boronic ester containing divinyl monomers, with multifunctional small molecule thiols [75]. The installation of the reversible boronic ester bond into the network backbone during photopolymerization delivered tough and self-healing polymer networks, as illustrated in Fig. 4d. In an analogous approach, Theato et al. built moldable and self-healing gels by incorporating borax into the monomer mixture during network formation via Michaeltype addition of PEG diacrylate and dithiothreitol, a 1,2-diol-containing dithiol [76]. Cromwell et al. designed dynamic networks through the chemical synthesis of 1,2-diol-containing polycyclooctene and diphenylboronic ester linkers [15]. In this manner, they fabricated solvent-free polymer networks that were selfhealing and reprocessable, with the self-healing rate controlled by the small molecule PBA-diol binding kinetics.
Advancing from these early demonstrations, Yesilyurt et al. fabricated dynamic covalent networks from 4-arm PEG molecules functionalized with 1,2-diols or PBA derivatives (Fig. 4c), which highlighted the ability to tune materials properties with boronic acid-diol chemistry and network topology [7,77]. In these welldefined networks, they explored the influence of altering the boronic acid chemistry, which modified the optimal pH of network formation and the crossover frequency uc [7]. In addition, they showed that tuning the weight percent of polymer controlled the plateau modulus but did not influence significantly the relaxation time of the gel [77]. Network dissolution and release of entrapped molecules was modulated by the presence of free diols, including glucose, and the gels were designed as injectable materials based on their shear-thinning and self-healing properties. In total, the studies presented here on the design of dynamic covalent networks and gels from boronic ester bonds illustrate that control of materials properties is enabled by tailoring both the chemistry of the reversible bond and the network topology.
AA Blocks offers a comprehensive range of building blocks and specially designed scaffolds to support your R&D like spectrum chemical
4-(Dimethylamino)benzaldehydeCatalog No.:AA000036 CAS No.:100-10-7 MDL No.:MFCD00003381 MF:C9H11NO MW:149.1897 |
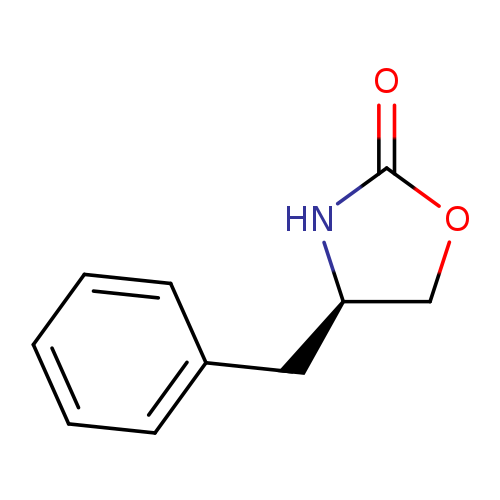
(R)-4-Benzyl-2-oxazolidinoneCatalog No.:AA0006BM CAS No.:102029-44-7 MDL No.:MFCD00010846 MF:C10H11NO2 MW:177.1998 |
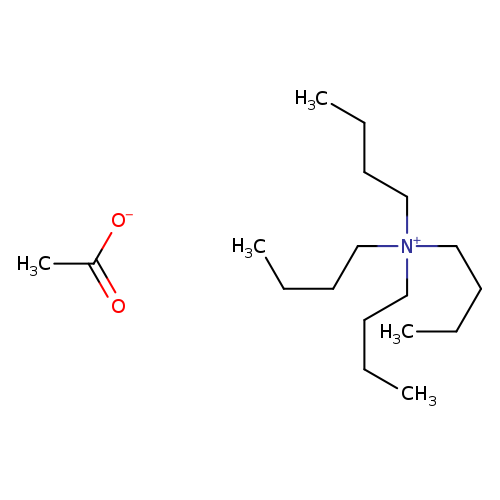
Tetrabutylammonium acetateCatalog No.:AA0035KW CAS No.:10534-59-5 MDL No.:MFCD00043208 MF:C18H39NO2 MW:301.5078 |
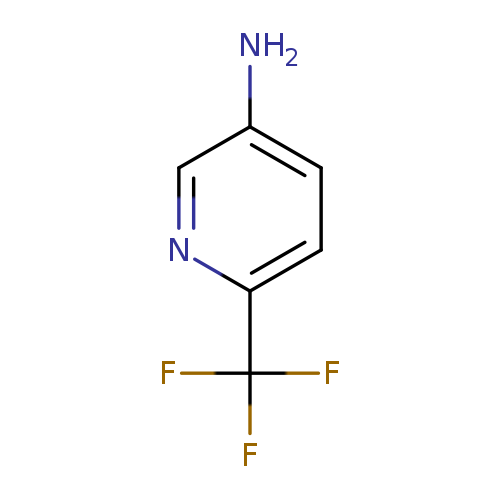
5-Amino-2-trifluoromethylpyridineCatalog No.:AA00343U CAS No.:106877-33-2 MDL No.:MFCD01862656 MF:C6H5F3N2 MW:162.1125 |
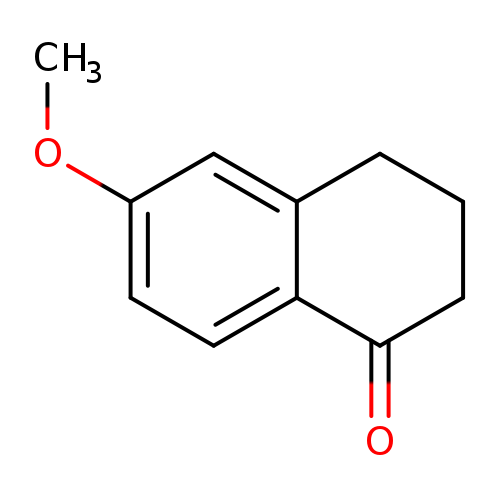
6-Methoxy-1-tetraloneCatalog No.:AA003N6K CAS No.:1078-19-9 MDL No.:MFCD00001695 MF:C11H12O2 MW:176.2118 |
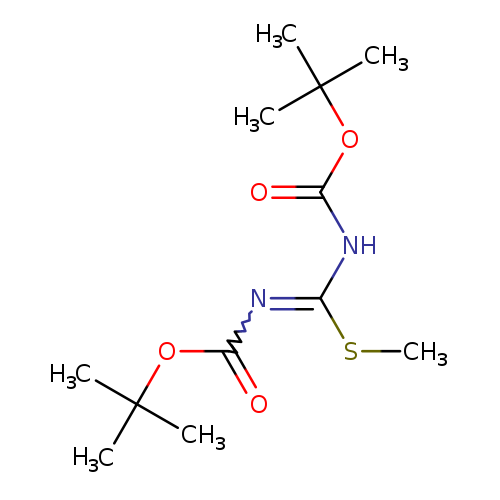
1,3-Di-boc-2-methylisothioureaCatalog No.:AA0038WP CAS No.:107819-90-9 MDL No.:MFCD00239356 MF:C12H22N2O4S MW:290.3791 |

(R)-1-N-Boc-3-hydroxypyrrolidineCatalog No.:AA003C4D CAS No.:109431-87-0 MDL No.:MFCD01317838 MF:C9H17NO3 MW:187.2362 |
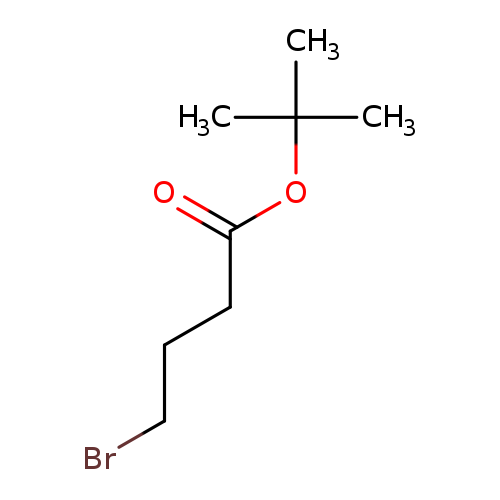
tert-Butyl 4-bromobutanoateCatalog No.:AA008S5F CAS No.:110661-91-1 MDL No.:MFCD02179416 MF:C8H15BrO2 MW:223.1075 |
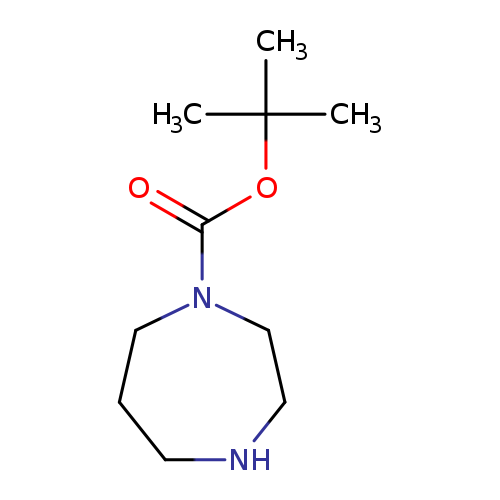
tert-Butyl 1,4-diazepane-1-carboxylateCatalog No.:AA0032MD CAS No.:112275-50-0 MDL No.:MFCD00276987 MF:C10H20N2O2 MW:200.2780 |
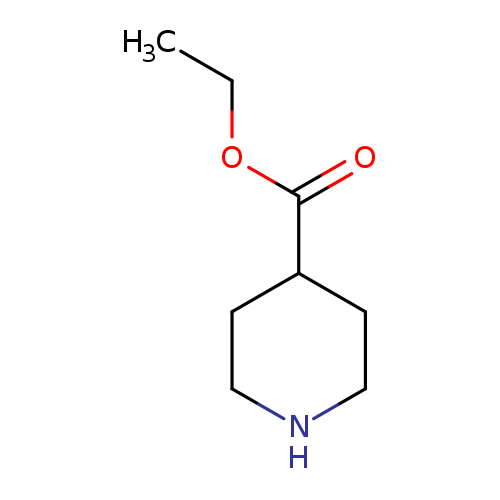
Ethyl piperidine-4-carboxylateCatalog No.:AA003429 CAS No.:1126-09-6 MDL No.:MFCD00006003 MF:C8H15NO2 MW:157.2102 |
5-Amino-1-methyl-1H-pyrazoleCatalog No.:AA000I93 CAS No.:1192-21-8 MDL No.:MFCD00068156 MF:C4H7N3 MW:97.1185 |
2-MethylbenzothiazoleCatalog No.:AA000PYU CAS No.:120-75-2 MDL No.:MFCD00005794 MF:C8H7NS MW:149.2129 |
Pyrazine-2,5-dicarboxylic acidCatalog No.:AA0035GV CAS No.:122-05-4 MDL No.:MFCD27920502 MF:C6H4N2O4 MW:168.1070 |
4-AcetamidobenzaldehydeCatalog No.:AA0016H2 CAS No.:122-85-0 MDL No.:MFCD00003380 MF:C9H9NO2 MW:163.1733 |
4,4-Difluorocyclohexanecarboxylic acidCatalog No.:AA0033RZ CAS No.:122665-97-8 MDL No.:MFCD03788493 MF:C7H10F2O2 MW:164.1499 |
1-Amino-1-cyclopropanecarbonitrile, HClCatalog No.:AA000XIK CAS No.:127946-77-4 MDL No.:MFCD04114063 MF:C4H7ClN2 MW:118.5648 |
2,6-DiaminoanthraquinoneCatalog No.:AA000V7Q CAS No.:131-14-6 MDL No.:MFCD00001234 MF:C14H10N2O2 MW:238.2414 |
2-Bromo-4-nitroanilineCatalog No.:AA0011DA CAS No.:13296-94-1 MDL No.:MFCD00025152 MF:C6H5BrN2O2 MW:217.0201 |
4-Bromo-3-fluorobenzonitrileCatalog No.:AA001435 CAS No.:133059-44-6 MDL No.:MFCD07782069 MF:C7H3BrFN MW:200.0078 |
6-IodoquinolineCatalog No.:AA0014GB CAS No.:13327-31-6 MDL No.:MFCD08457906 MF:C9H6IN MW:255.0551 |
1,3-Diethyl 2-aminopropanedioate, HClCatalog No.:AA0034NC CAS No.:13433-00-6 MDL No.:MFCD00012510 MF:C7H14ClNO4 MW:211.6434 |
2,4-Dichloro-5-iodopyrimidineCatalog No.:AA0032W9 CAS No.:13544-44-0 MDL No.:MFCD01898087 MF:C4HCl2IN2 MW:274.8746 |
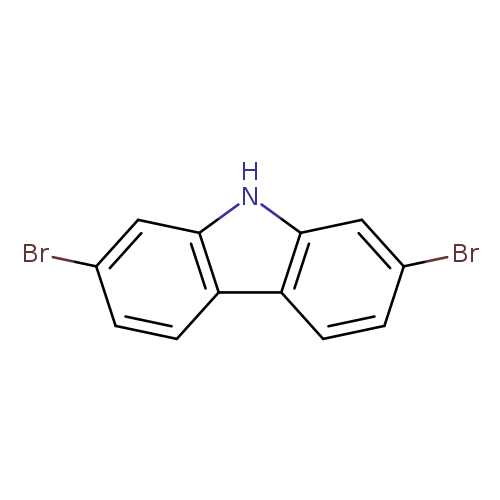
2,7-Dibromo-9H-carbazoleCatalog No.:AA0032ZF CAS No.:136630-39-2 MDL No.:MFCD09033507 MF:C12H7Br2N MW:324.9987 |
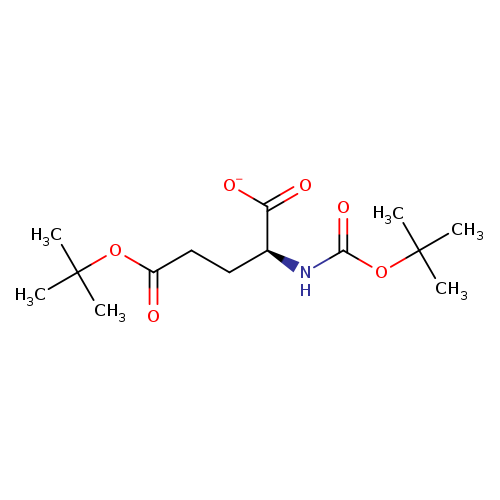
Boc-L-Glu(OtBu)-OHCatalog No.:AA0012YN CAS No.:13726-84-6 MDL No.:MFCD00038257 MF:C14H24NO6- MW:302.3435 |
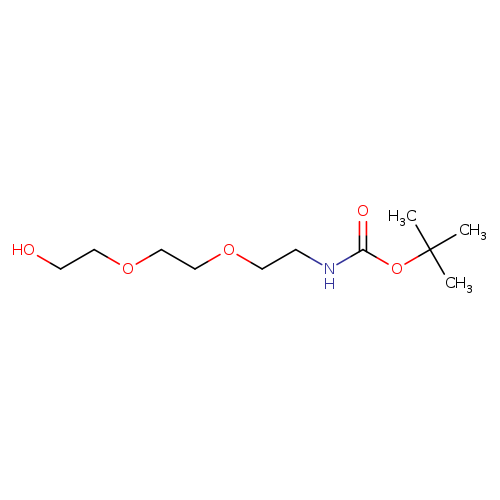
tert-Butyl (2-(2-(2-hydroxyethoxy)ethoxy)ethyl)carbamateCatalog No.:AA001A89 CAS No.:139115-92-7 MDL No.:MFCD07357496 MF:C11H23NO5 MW:249.3040 |
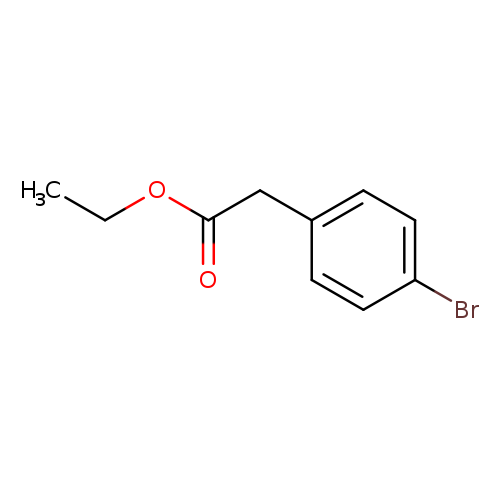
Ethyl 4-bromophenylacetateCatalog No.:AA001CIM CAS No.:14062-25-0 MDL No.:MFCD00016333 MF:C10H11BrO2 MW:243.0971 |
2'-Hydroxy-5'-methylacetophenoneCatalog No.:AA001KDK CAS No.:1450-72-2 MDL No.:MFCD00002380 MF:C9H10O2 MW:150.1745 |

N-Boc-hexahydro-5-oxocyclopenta[c]pyrroleCatalog No.:AA001KTG CAS No.:148404-28-8 MDL No.:MFCD11616063 MF:C12H19NO3 MW:225.2842 |
Alpha-aminoisobutyric acid methyl ester HCl, tech gradeCatalog No.:AA00APFI CAS No.:15028-41-8 MDL No.:MFCD00214247 MF:C5H12ClNO2 MW:153.6073 |
4-Hydroxy-3-methylbenzaldehydeCatalog No.:AA003LFB CAS No.:15174-69-3 MDL No.:MFCD00012360 MF:C8H8O2 MW:136.1479 |
1H-Indazol-5-olCatalog No.:AA001O48 CAS No.:15579-15-4 MDL No.:MFCD01938124 MF:C7H6N2O MW:134.1353 |
4-Amino-3-nitrobenzoic acidCatalog No.:AA001QT0 CAS No.:1588-83-6 MDL No.:MFCD00017009 MF:C7H6N2O4 MW:182.1335 |
2,4,6-TrichloropyridineCatalog No.:AA001S01 CAS No.:16063-69-7 MDL No.:MFCD00971928 MF:C5H2Cl3N MW:182.4351 |
4-Bromo-3-hydroxypyridineCatalog No.:AA001SDL CAS No.:161417-28-3 MDL No.:MFCD03093021 MF:C5H4BrNO MW:173.9954 |
1-Boc-3-aminomethylpiperidineCatalog No.:AA001SQO CAS No.:162167-97-7 MDL No.:MFCD01317792 MF:C11H22N2O2 MW:214.3046 |
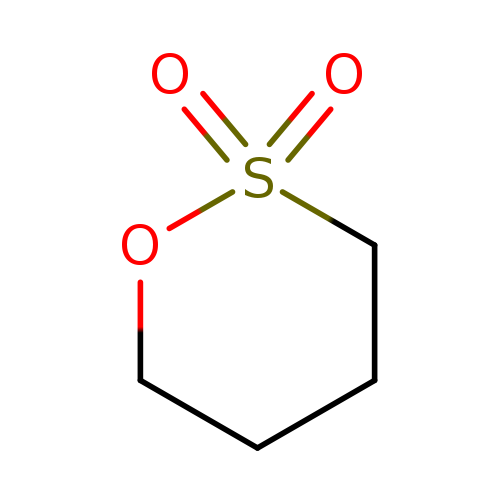
1,4-Butane sultoneCatalog No.:AA001TXI CAS No.:1633-83-6 MDL No.:MFCD00006584 MF:C4H8O3S MW:136.1695 |
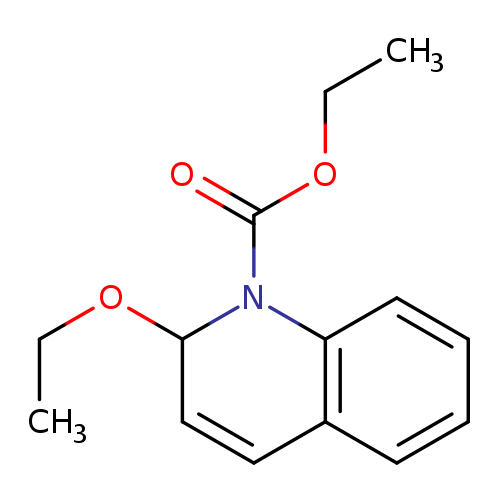
N-Ethoxycarbonyl-2-ethoxy-1,2-dihydroquinolineCatalog No.:AA001U5Y CAS No.:16357-59-8 MDL No.:MFCD00006703 MF:C14H17NO3 MW:247.2897 |
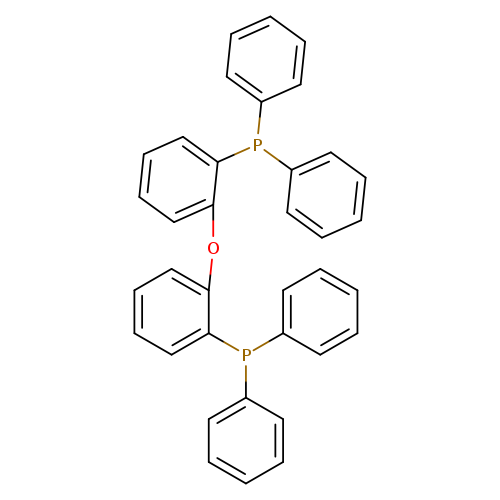
Bis(2-diphenylphosphinophenyl)etherCatalog No.:AA001WKV CAS No.:166330-10-5 MDL No.:MFCD00233863 MF:C36H28OP2 MW:538.5544 |
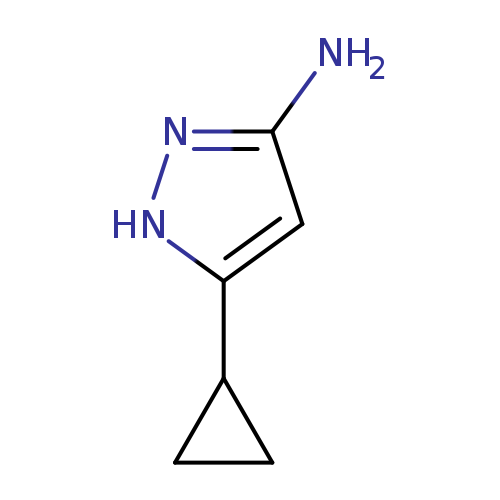
3-Cyclopropyl-1h-pyrazol-5-amineCatalog No.:AA00208X CAS No.:175137-46-9 MDL No.:MFCD00067985 MF:C6H9N3 MW:123.1558 |
8-Bromooctanoic acidCatalog No.:AA0024XK CAS No.:17696-11-6 MDL No.:MFCD00004430 MF:C8H15BrO2 MW:223.1075 |
(3-Bromophenyl)acetic acidCatalog No.:AA002GF6 CAS No.:1878-67-7 MDL No.:MFCD00004330 MF:C8H7BrO2 MW:215.0440 |
1-ChloroisoquinolineCatalog No.:AA007RBZ CAS No.:19493-44-8 MDL No.:MFCD00024134 MF:C9H6ClN MW:163.6036 |
1-CyclopropylpiperazineCatalog No.:AA0028PD CAS No.:20327-23-5 MDL No.:MFCD06254805 MF:C7H14N2 MW:126.1995 |
2-BromostyreneCatalog No.:AA00294J CAS No.:2039-88-5 MDL No.:MFCD00000076 MF:C8H7Br MW:183.0452 |
Bis(diphenylphosphino)methaneCatalog No.:AA002IS0 CAS No.:2071-20-7 MDL No.:MFCD00003537 MF:C25H22P2 MW:384.3897 |
N-HydroxyacetamidineCatalog No.:AA007PVS CAS No.:22059-22-9 MDL No.:MFCD00603514 MF:C2H6N2O MW:74.0818 |
2-Naphthylhydrazine, HClCatalog No.:AA00BBIU CAS No.:2243-58-5 MDL No.:MFCD04109481 MF:C10H11ClN2 MW:194.6607 |
1-Iodo-3,5-dimethylbenzeneCatalog No.:AA0032PO CAS No.:22445-41-6 MDL No.:MFCD00060659 MF:C8H9I MW:232.0615 |
2-Chloro-4-methyl-5-nitropyridineCatalog No.:AA002LZ5 CAS No.:23056-33-9 MDL No.:MFCD00010688 MF:C6H5ClN2O2 MW:172.5691 |
Ethyl 3-ethoxy-3-iminopropionate, HClCatalog No.:AA0034S3 CAS No.:2318-25-4 MDL No.:MFCD00051405 MF:C7H14ClNO3 MW:195.6440 |
(1R,2S)-(-)-2-Amino-1,2-diphenylethanolCatalog No.:AA003BEI CAS No.:23190-16-1 MDL No.:MFCD00074960 MF:C14H15NO MW:213.2750 |
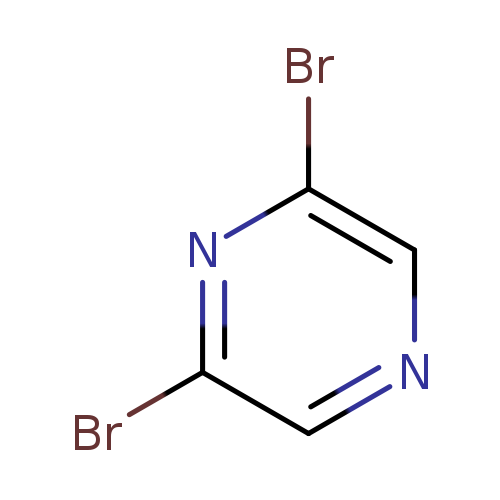
2,6-DibromopyrazineCatalog No.:AA002MIR CAS No.:23229-25-6 MDL No.:MFCD09834804 MF:C4H2Br2N2 MW:237.8801 |
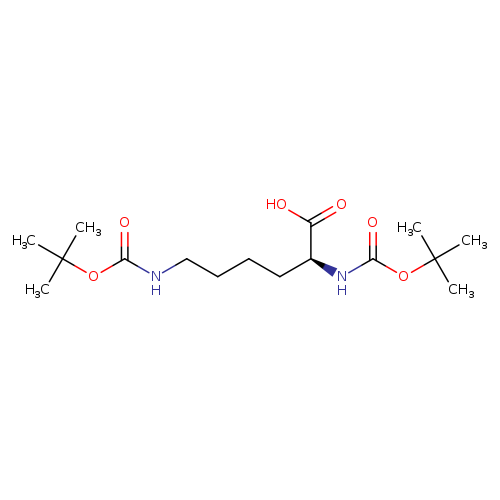
(S)-2,6-Bis-tert-butoxycarbonylaminohexanoic acidCatalog No.:AA00381W CAS No.:2483-46-7 MDL No.:MFCD00038515 MF:C16H30N2O6 MW:346.4192 |
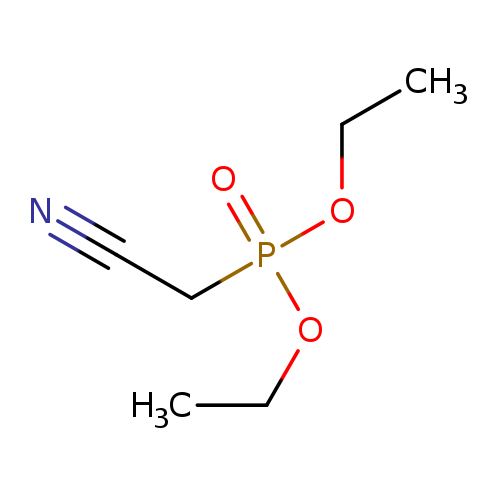
Diethyl cyanomethylphosphonateCatalog No.:AA0034NG CAS No.:2537-48-6 MDL No.:MFCD00001893 MF:C6H12NO3P MW:177.1381 |
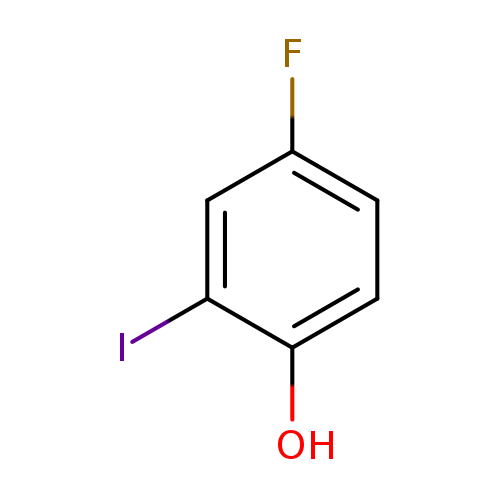
4-Fluoro-2-iodophenolCatalog No.:AA0037N9 CAS No.:2713-29-3 MDL No.:MFCD15527539 MF:C6H4FIO MW:237.9982 |
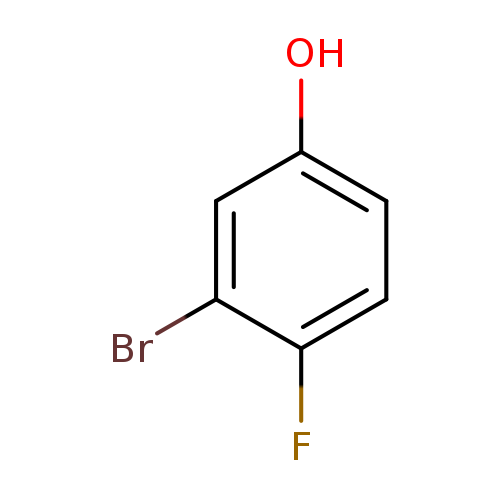
3-Bromo-4-fluorophenolCatalog No.:AA0033JG CAS No.:27407-11-0 MDL No.:MFCD03425884 MF:C6H4BrFO MW:190.9978 |
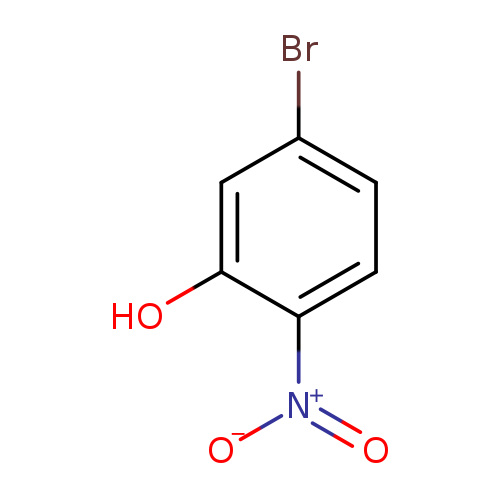
5-Bromo-2-nitrophenolCatalog No.:AA002UD7 CAS No.:27684-84-0 MDL No.:MFCD03095027 MF:C6H4BrNO3 MW:218.0049 |
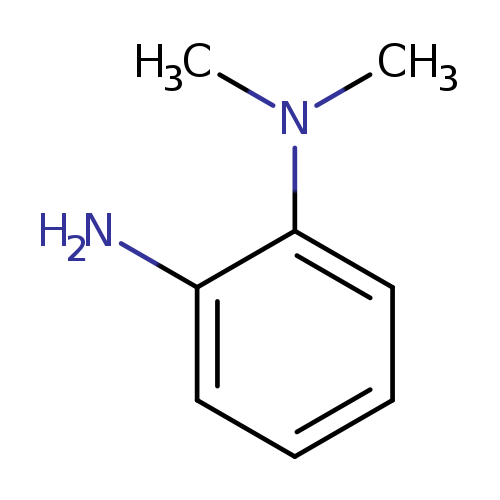
2-Amino-N,N-dimethylanilineCatalog No.:AA00BG0G CAS No.:2836-03-5 MDL No.:MFCD01706706 MF:C8H12N2 MW:136.1943 |
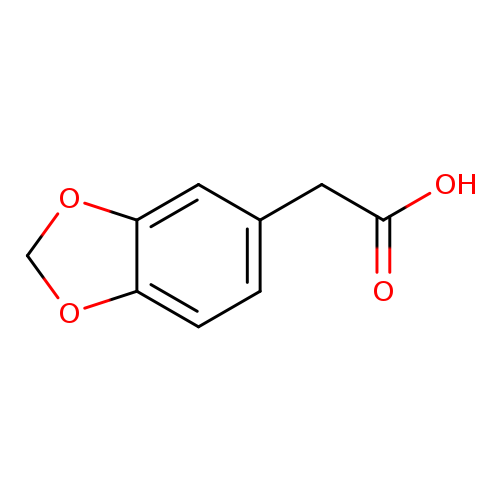
2-(Benzo[d][1,3]dioxol-5-yl)acetic acidCatalog No.:AA002VSN CAS No.:2861-28-1 MDL No.:MFCD00014576 MF:C9H8O4 MW:180.1574 |
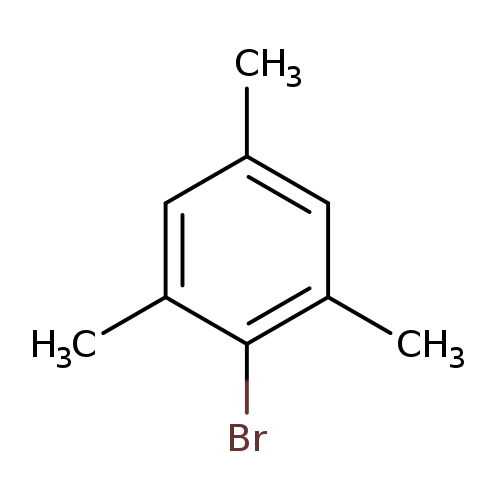
2,4,6-TrimethylbromobenzeneCatalog No.:AA003GJ5 CAS No.:576-83-0 MDL No.:MFCD00000073 MF:C9H11Br MW:199.0876 |
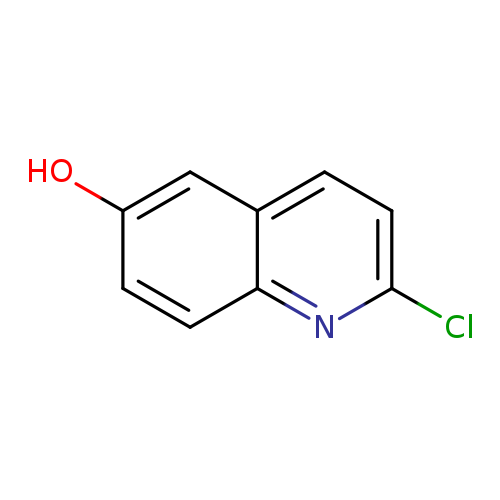
2-Chloroquinolin-6-olCatalog No.:AA0036YO CAS No.:577967-89-6 MDL No.:MFCD12405093 MF:C9H6ClNO MW:179.6030 |
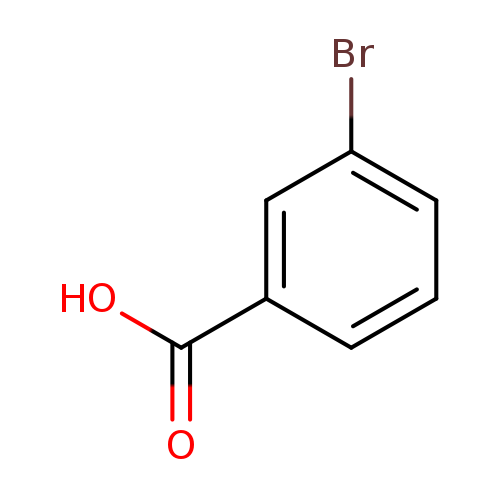
3-Bromobenzoic acidCatalog No.:AA0033JY CAS No.:585-76-2 MDL No.:MFCD00002487 MF:C7H5BrO2 MW:201.0174 |
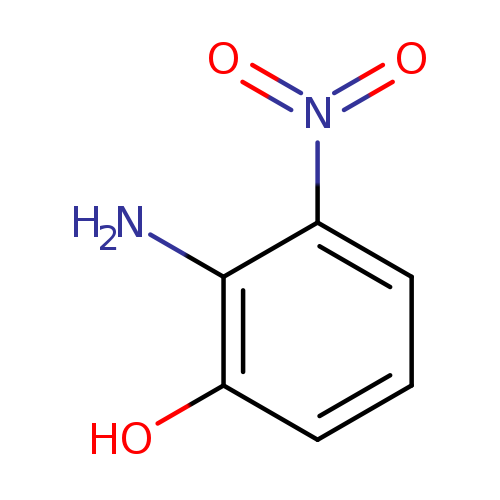
2-Amino-3-nitrophenolCatalog No.:AA00330B CAS No.:603-85-0 MDL No.:MFCD00010875 MF:C6H6N2O3 MW:154.1234 |
Ethyl 2-ethylacetoacetateCatalog No.:AA003Q19 CAS No.:607-97-6 MDL No.:MFCD00039898 MF:C8H14O3 MW:158.1950 |
Methyl 3-hydroxypropanoateCatalog No.:AA003RPQ CAS No.:6149-41-3 MDL No.:MFCD00272293 MF:C4H8O3 MW:104.1045 |
2-Amino-5-nitrobenzoic acidCatalog No.:AA003GET CAS No.:616-79-5 MDL No.:MFCD00017039 MF:C7H6N2O4 MW:182.1335 |
4-(Dimethylamino)phenolCatalog No.:AA00IAV3 CAS No.:619-60-3 MDL No.:MFCD01707548 MF:C8H11NO MW:137.1790 |
Cinnamic acidCatalog No.:AA0036XC CAS No.:621-82-9 MDL No.:MFCD00004369 MF:C9H8O2 MW:148.1586 |
2-Amino-6-nitrobenzothiazoleCatalog No.:AA003N8K CAS No.:6285-57-0 MDL No.:MFCD00005786 MF:C7H5N3O2S MW:195.1985 |
Boc-l-propargylglycineCatalog No.:AA00IB23 CAS No.:63039-48-5 MDL No.:MFCD01320855 MF:C10H15NO4 MW:213.2304 |
7-Methoxy-1-tetraloneCatalog No.:AA003ND5 CAS No.:6836-19-7 MDL No.:MFCD00001696 MF:C11H12O2 MW:176.2118 |
4-Bromo-3-methylanilineCatalog No.:AA00F9TR CAS No.:6933-10-4 MDL No.:MFCD00007828 MF:C7H8BrN MW:186.0491 |
Methyl 4-(chlorosulfonyl)benzoateCatalog No.:AA00354B CAS No.:69812-51-7 MDL No.:MFCD00627554 MF:C8H7ClO4S MW:234.6568 |
Ethyl 3-amino-4-pyrazolecarboxylateCatalog No.:AA0034S0 CAS No.:6994-25-8 MDL No.:MFCD00005238 MF:C6H9N3O2 MW:155.1546 |
4-Chloro-2-fluoronitrobenzeneCatalog No.:AA003L0G CAS No.:700-37-8 MDL No.:MFCD00042211 MF:C6H3ClFNO2 MW:175.5449 |
5-Fluoro-1-indanoneCatalog No.:AA003MNY CAS No.:700-84-5 MDL No.:MFCD00041031 MF:C9H7FO MW:150.1497 |
6-Fluoro-1-tetraloneCatalog No.:AA0034A6 CAS No.:703-67-3 MDL No.:MFCD09031370 MF:C10H9FO MW:164.1763 |
Fmoc-Asp(OtBu)-OHCatalog No.:AA0034UZ CAS No.:71989-14-5 MDL No.:MFCD00037131 MF:C23H25NO6 MW:411.4477 |
2,4-Dimethoxybenzyl alcoholCatalog No.:AA003B26 CAS No.:7314-44-5 MDL No.:MFCD00004614 MF:C9H12O3 MW:168.1898 |
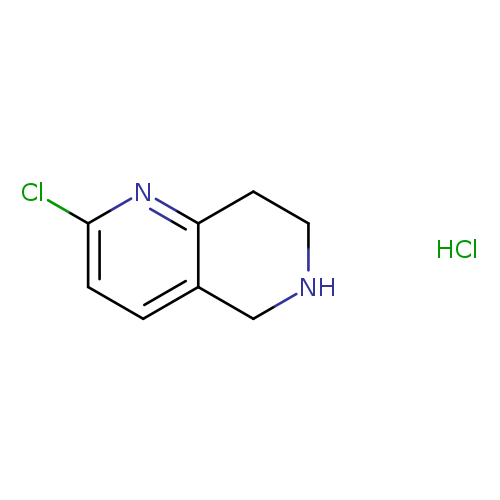
2-Chloro-5,6,7,8-tetrahydro-1,6-naphthyridine, HClCatalog No.:AA0036LM CAS No.:766545-20-4 MDL No.:MFCD09835503 MF:C8H10Cl2N2 MW:205.0844 |
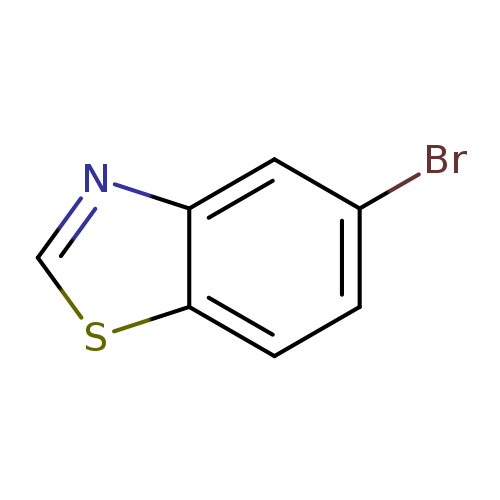
5-BromobenzothiazoleCatalog No.:AA00364E CAS No.:768-11-6 MDL No.:MFCD03001442 MF:C7H4BrNS MW:214.0824 |
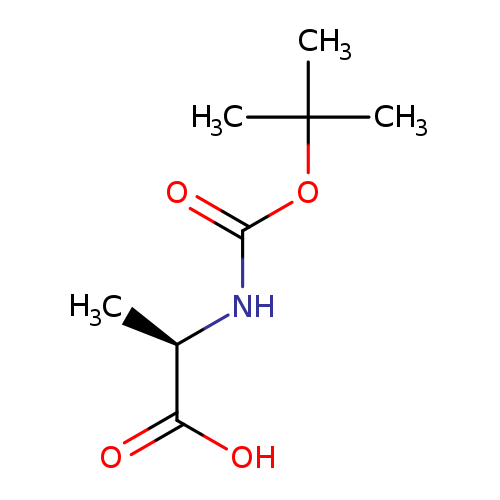
(R)-2-((tert-Butoxycarbonyl)amino)propanoic acid hydrateCatalog No.:AA003ODH CAS No.:7764-95-6 MDL No.:MFCD26383924 MF:C8H15NO4 MW:189.2090 |
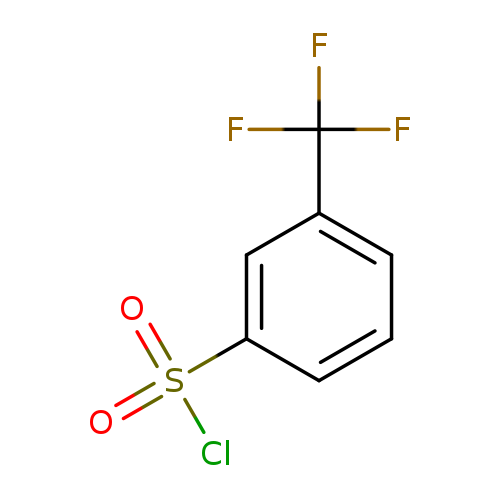
3-(Trifluoromethyl)benzenesulfonyl chlorideCatalog No.:AA003I7W CAS No.:777-44-6 MDL No.:MFCD00014724 MF:C7H4ClF3O2S MW:244.6187 |
4-Fluorobenzenesulfinic acid sodium saltCatalog No.:AA00G2DM CAS No.:824-80-6 MDL No.:MFCD08448302 MF:C6H4FNaO2S MW:182.1479 |
tert-Butyl 2,4-dioxopiperidine-1-carboxylateCatalog No.:AA004VGE CAS No.:845267-78-9 MDL No.:MFCD10566071 MF:C10H15NO4 MW:213.2304 |
4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-2-amineCatalog No.:AA00330I CAS No.:84955-31-7 MDL No.:MFCD22576107 MF:C6H5ClN4 MW:168.5837 |

(S)-(+)-3-HydroxytetrahydrofuranCatalog No.:AA0036C5 CAS No.:86087-23-2 MDL No.:MFCD00064327 MF:C4H8O2 MW:88.1051 |
Indole-3-acetic acidCatalog No.:AA003JIB CAS No.:87-51-4 MDL No.:MFCD00005636 MF:C10H9NO2 MW:175.1840 |
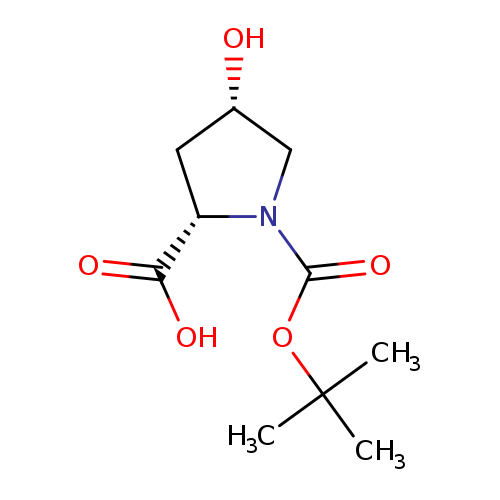
N-Boc-cis-4-hydroxy-L-ProlineCatalog No.:AA003OXC CAS No.:87691-27-8 MDL No.:MFCD02094406 MF:C10H17NO5 MW:231.2457 |
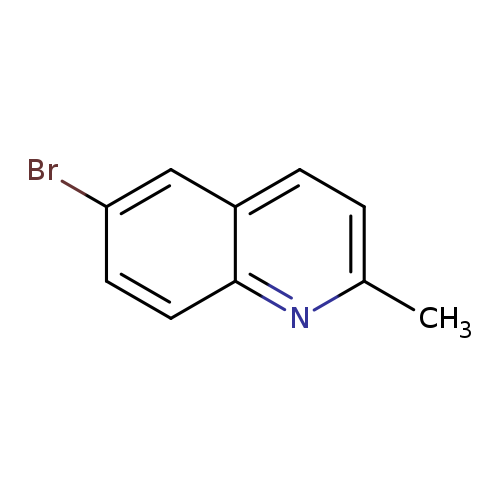
6-Bromo-2-methylquinolineCatalog No.:AA003N18 CAS No.:877-42-9 MDL No.:MFCD00079724 MF:C10H8BrN MW:222.0812 |
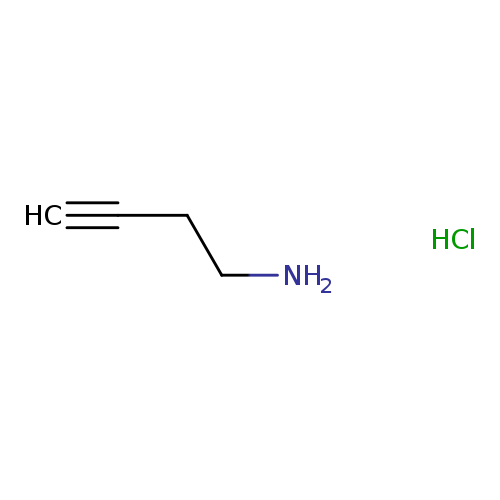
3-Butyn-1-amine, HClCatalog No.:AA00367X CAS No.:88211-50-1 MDL No.:MFCD00233043 MF:C4H8ClN MW:105.5660 |
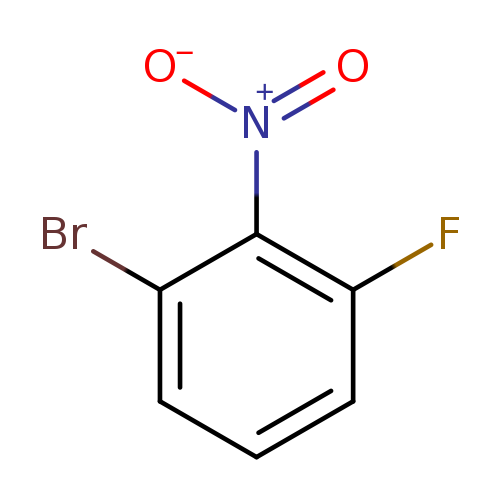
1-Bromo-3-fluoro-2-nitrobenzeneCatalog No.:AA003E1F CAS No.:886762-70-5 MDL No.:MFCD07368788 MF:C6H3BrFNO2 MW:219.9959 |
2-MethylbenzylamineCatalog No.:AA0036TY CAS No.:89-93-0 MDL No.:MFCD00008112 MF:C8H11N MW:121.1796 |
4-(Hydroxymethyl)-5-methyl-1,3-dioxol-2-oneCatalog No.:AA0038GQ CAS No.:91526-18-0 MDL No.:MFCD12165896 MF:C5H6O4 MW:130.0987 |
7-MethylindoleCatalog No.:AA0063O2 CAS No.:933-67-5 MDL No.:MFCD00005684 MF:C9H9N MW:131.1745 |
Methyl indole-3-carboxylateCatalog No.:AA003RHL CAS No.:942-24-5 MDL No.:MFCD00189407 MF:C10H9NO2 MW:175.1840 |
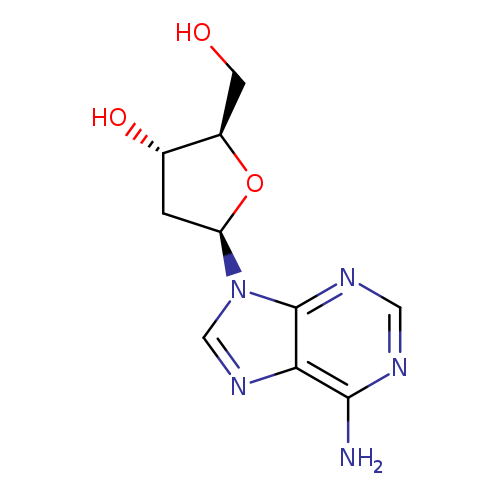
2'-DeoxyadenosineCatalog No.:AA0035TB CAS No.:958-09-8 MDL No.:MFCD00149364 MF:C10H13N5O3 MW:251.2419 |
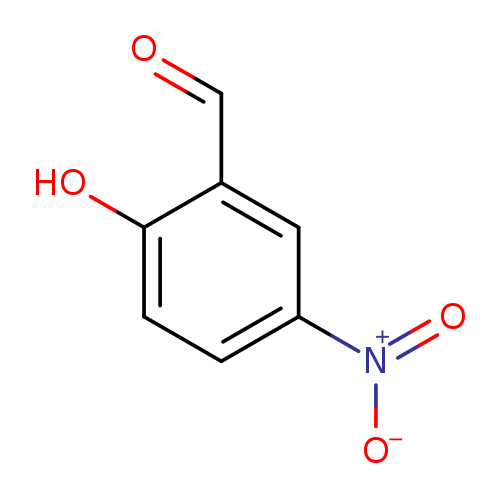
2-Hydroxy-5-nitrobenzaldehydeCatalog No.:AA006BP9 CAS No.:97-51-8 MDL No.:MFCD00007337 MF:C7H5NO4 MW:167.1189 |