2020-01-27 14:04:21
Charlotte Lorton and Arnaud Voituriez
1.Introduction
Tricyclic indole backbone is often encountered in natural products and pharmaceuticals. This class of heterocycles, and especially the pyrrolo[1,2-a]indole scaffolds, has attracted much at- tention due to their potent biological activities (Scheme 1a). As representative examples, mitomycin A and C (compounds 1 and 2) are two effective antitumor agents;[1a] the antiparasitic isoborreverine 3[1b–1c] and the antimalarial alkaloid flinderole C (4)[1d–1e] show interesting biological properties and compound5 acts as sphingosine-1 phosphate (S1P1) antagonist.[1f–1g] Moreover, compound 6 is a protein kinase C inhibitor selective for isozyme ß[1h–1i] and isatisine A (7) acts as an anti-HIV agent.[1j] Since the ‘60s, many synthetic pathways have been developed worldwide for the synthesis of the pyrrolo[1,2-a]- indoles, often driven by the interest of the organic chemistry community for the mitomycinoid alkaloids.
These strategies have been reviewed in 2016,[2] but in view of the renewed interest of the scientific community for this structure, it seemed to us interesting to make this update. In the context of this mini-review, among the three isomeric struc- tures of the pyrrolo[1,2-a]indoles, our attention will be focused towards the 9H-isomer 8 and its oxidized form, the 9H-pyr- rolo[1,2-a]indol-9-one 9, with respect to the 3H- and 1H-isomers (respectively products 10 and 11, Scheme 1b). The articles evoking the syntheses of 9H-pyrrolo[1,2-a]indoles will not be fully detailed in this review.[3] On the other hand, the synthetic methodologies (organometallic or non-metallic) for the synthe- sis of these three-membered nitrogen-containing rings will be detailed. First, the synthesis of 9H-pyrrolo[1,2-a]indoles will be resumed, and then, in the second part, the preparation of 9H- pyrrolo[1,2-a]indol-9-one derivatives. Before the conclusion, various applications in medicinal chemistry and materials chemistry of these molecules of interest will be summarized.
2.Synthesis of 9H-Pyrrolo[1,2-a]indoles
In this part, the synthetic pathways used for the isolation of 9H-pyrrolo[1,2-a]indole derivatives will be developed. First, the reactions promoted by non-metallic reagents will be presented. Both phosphonium and ammonium salts were used, but also acid-promotors, radical precursors, amine-catalyzed and ther- mal processes. Then will follow metal-catalyzed and metal-pro- moted reactions, classified according to the type of metal used (copper, palladium, silver, and others).
2.1.Non-Metal Promoted Reactions
In 1966, Schweizer and Light developed the synthesis of unsub- stituted 9H-pyrrolo[1,2-a]indole 8 from indole-2-carboxalde- hyde 12 and vinyltriphenylphosphonium bromide, in presence of sodium hydride (Scheme 2a).[4] The product was isolated in 58 % yield. It was noticed in this paper that the structure of this product was first incorrectly assigned to the 3H-pyrrolo- [1,2-a]indole 10.[5] As subsequently demonstrated in numerous examples, it turned out that the initially 3H-isomer formed after the intramolecular Wittig reaction isomerizes in situ to the most stable 9H-isomer.[6] This work is the logical extension of the methodologies used by the same team for the synthesis of 3H- pyrrolizine[5,7] and 2H-chromene derivatives.[8] The same strategy was later successfully applied to the synthesis of polysubsti- tuted 9H-pyrrolo[1,2-a]indole 14,[9] with methoxy- and benzyl- oxy-substituents in position 7 and 8 (Scheme 2b) or with sub- stituents in positions 6 and 7 (compound 16, Scheme 2c).[10]
With substituted-indole-2-carboxaldehydes 17 as starting ma- terials, in presence of methylvinylketone and a benzyltrimethyl- ammonium hydroxide solution, Matsui et al. synthesized five 9H- pyrrolo[1,2-a]indoles 18 in moderate yields (Scheme 3a).[11] In- oue described an original reaction of tris(isopropylthio)cyclopro- penylium perchlorate 20 with two different indoles 19, in pres- ence of sodium hydride.[12] Two pyrroloindoles 21 were isolated in 51–84 % yield (Scheme 3b).
Starting from 1-keto-1H-pyrrolo[1,2-a]indoles 22, Remers de- scribed a new rearrangement, giving direct access to the 9H- pyrrolo[1,2-a]indole structures 23, substituted with a chlorine atom and a methyl 2-oxoacetate group. The two-steps proce- dure first involves the reaction with oxalyl chloride and then the addition of methanol (Scheme 4).[13]
In 2009, Su et al. described the synthesis of twelve (9H- pyrrolo[1,2-a]indole-1,2-diyl)dimethanol analogs 26, using a three-steps methodology (Scheme 5).[14] Reaction of indoline- 2-carboxylic acids 24 with different alkoyl- or aroyl chlorides furnished the corresponding protected indolines 25. They were reacted then at 120 °C with dimethyl acetylenedicarboxylate (DMAD) in acetic anhydride. The corresponding pyrroloindoles 26 were isolated in 71–89 % yield, after recrystallization. Some post-functionalizations, included reduction of the diester func- tions to alcohols and reaction with methyl isocyanate, delivered the desired molecules 27. These products were specifically de- signed for biological evaluation as antitumor drugs. They are expected to act as DNA bifunctional alkylating agents. They exhibited cytotoxicity against human leukemia and tumor re- mission in nude mice bearing human breast carcinoma MX–1 xenograft. This work and the preliminary interesting biological results highlighted the interest to develop new methodologies for the synthesis of the 9H-pyrrolo[1,2-a]indole backbone (see part 4 for further applications).
Yavari and Esmaeili independently reported a halide and base-free phosphine-promoted Michael addition/intramolec- ular Wittig reaction (Scheme 6a).[15] Using a stoichiometric quantity of triphenylphosphine, the reaction pathway started with the addition of the phosphine to the dialkylacetylenedi- carboxylate 28 (DAAD), to form the zwitterionic species A. This intermediate deprotonates the indole-2-carboxaldehyde 29, which consequently performs a Michael addition on the corre- sponding vinylphosphonium salt B, to in situ form a phos- phorus ylide C. After the intramolecular Wittig reaction, the 3H- pyrrolo[1,2-a]indole derivatives 30 were isolated. In this process, the use of mild reaction conditions (room temperature for 15 minutes) allows isolating the 3H-pyrrolo[1,2-a]indole isomer. In fact, when the 9-chloro-3H-pyrrolo[1,2-a]indole-2,3-dicarboxyl- ate product was refluxed 24 h in toluene, the authors described the isomerization into the 9H-pyrrolo[1,2-a]indole. As a poten- tial derivatization of these compounds, we can notice that the hydrolysis of 9-chloro-9H-pyrrolo[1,2-a]indole product occurred in a mixture CHCl3/H2O, 24 h at reflux. Even if this synthetic protocol proves to be very efficient in term of conversion rate (96–98 % yield), only three examples using 3-chloro-1H-indole- 2-carbaldehydes were showed and at least 1.0 equiv. of triphen- ylphosphine oxide is formed during the reaction.
Mainly to facilitate the purification issue, we proposed re- cently to use in this reaction a sub-stoichiometric quantity of trivalent phosphine (Scheme 6b). The in situ chemoselective reduction of the phosphine oxide could be achieved efficiently using 1.0 equiv. of a reducing agent, such as phenylsilane.[16] Even if this catalytic protocol was previously used in different venerable reactions (Wittig, Mitsunobu, Staudinger), that was to the best of our knowledge, the first example of a tandem Michael addition/Wittig reaction, catalytic in phosphine. Using this protocol, it was possible to realize a wide range of Michael addition/intramolecular Wittig reactions, using electron-rich (such as 5-methyl, 5-methoxy and 5-benzyloxy-indoles) and electron-poor substrates (such as 5-fluoro, 5-nitro, and 5-triflate- indole-2-carbaldehydes).[17] Using these reaction conditions (60 °C in toluene for 16 h), the most stable 9H-pyrrolo[1,2-a]- indole isomer 31 was directly isolated. In our hand, the use of microwave heating allowed to shorten the reaction time to 2 h, while maintaining excellent yield (93 % yield for the synthesis of the diethyl 7-bromo-9H-pyrrolo[1,2-a]indole-2,3-dicarboxyl- ate product). The structural assignment of this molecule was ascertained by single-crystal X-ray analysis, which verified the formation of the 9H-pyrroloindole isomer.
In our hand, with the 3-chloro-1H-indole-2-carbaldehyde substrate 29, the hydrolysis of the chlorinated intermediate was directly observed (Scheme 7). Subsequent oxidation into the 9H-pyrrolo[1,2-a]indol-9-one derivative 33 was accomplished in 90 % yield, using pyridinium chlorochromate (PCC). The proce- dure (cyclization/isomerization/hydrolysis/oxidation) could be achieved sequentially in one pot, with 90 % overall yield. In the following, we fully expanded the scope of this catalytic one-pot Michael addition/intramolecular Wittig reaction to the synthesis of numerous nitrogen-containing heterocycles. Notably, pyrrol- izine, pyrroloquinoline, dihydroquinoline, and benzothieno- pyridine backbones were easily obtained using this catalytic protocol.
In the continuity of this study, we introduced alkyl buta-2,3- dienoates as substrate, to open the structural diversity in the 9H-pyrrolo[1,2-a]indole derivatives (Scheme 8a).[19] Different 1H-indole-2-carbaldehydes 34 reacted with allenoates 35, in presence of stoichiometric amounts of triphenylphosphine. The corresponding 9H-pyrrolo[1,2-a]indole products 36 were syn- thesized in good yields, with either electron-rich or electron- withdrawing groups on the indole backbone (13 examples, 51– 88 % isolated yield). In this transformation, the substitution pat- tern of the indole substrate and the allenoate partners seems not to have a huge impact on the reaction outcome. Interest- ingly, this reaction could be extended to the use of α-substi- tuted allenoates 37 (Scheme 8b). Indeed, ethyl 2-benzylbuta- 2,3-dienoate (R5 = Ph) and diethyl 2-vinylidenesuccinate (R5 = CO2Et) 37 gave the annulation products 38 in moderate to good yields (4 examples, 43–82 % yield). Only the ethyl 2-meth- ylbuta-2,3-dienoate substrate (R5 = H) gave disappointing re- sults (32 % yield). To simplify the purification step, it was em- phasized that polymer-supported PPh3 could be used in this process (56 % yield). Unfortunately, up to now, the develop- ment of the catalytic version of this reaction failed, presumably because of the poor stability of the allenoate partner in the reaction conditions. Finally, a mechanism was proposed. After the addition of the trivalent phosphine to the allenoate 35, the zwitterionic species D was formed. The latter was subsequently protonated by the N-H proton of the indole, to give the corre- sponding vinylphosphonium salt F. The addition of the conju- gate base of the indole E to F furnished the ylide G, which undergoes an intramolecular Wittig reaction to form the 3H- pyrrolo[1,2-a]indole isomer H. After isomerization, the stable 9H-isomer was isolated.
Some research groups have developed simple transforma- tions for the synthesis of pyrroloindole structure, with the use of Brønsted acid such as hydrochloric acid (Scheme 9a). Starting from 4,6-dimethoxy-3-methyl (or 3-aryl)-1H-indoles 39, in reac- tion with chalcone derivatives 40, the 9H-pyrrolo[1,2-a]indoles 41 are directly synthesized in good yields, after recrystallization from acetonitrile (18 examples, up to 95 % yield).[20] As ex- pected, this reaction proceeds via a Michael addition of the indole C2-carbon to the chalcone double bond, followed by the addition of the nitrogen atom to form the pyrroloindole backbone, with the elimination of one molecule of water. Later on, Zu expanded this methodology by the use of α,ß-unsatu- rated ketone derivatives 43 and tryptamines or 3-methylindole substrates 42, in presence of catalytic amounts of p-TSA (p- toluenesulfonic acid) (Scheme 9b).[21] Numerous highly func- tionalized products 44 were easily obtained, in generally good yields (16 examples, 42–90 % yield). It was noticed that at 110 °C, this transformation could be catalyzed with other Brøn- sted acid or Lewis acid (such as FeCl3 or ZnCl2), albeit with lower yields.
9,9-Disubstituted 9H-pyrrolo[1,2-a]indoles 46 were also effi- ciently obtained via hydroiodic acid (HI) promoted (or cata- lyzed) cyclization of 1-[2-(1-arylvinyl)phenyl]-1H-pyrrole sub- strates 45 (Scheme 9c).[22] Alkyl-substituted substrates (R2 = Me) gave a lower yield in the desired compound, even using stoichiometric amounts of HI. This lower reactivity can be attrib- uted to the less stabilized benzylic carbocation intermediate in the case of an alkyl substituent (R2 = Me), compared to the aryl moieties (R2 = Ar).
In 2018, the microwave-assisted condensation of 3H- indolium chloride salt 47 with 2.0 equiv. of pyridine or quinol- ine-carboxaldehyde derivatives, in acetic acid, led to the isola- tion of polysubstituted 9H-pyrrolo[1,2-a]indole-3-carboxamides 48 (5 examples, 60–76 % yield).[23] In the presence of piperidine, the dihydro-1H-imidazo[1,2-a]indol-2(3H)-one derivatives 49 were isolated in good yields (Scheme 10). The reaction of this compound in acidic media (glacial acetic acid at 100 °C using microwave heating) furnished the pyrroloindole product 50 in 74 % isolated yield. Furthermore, the reaction of 49 with pyrid- ine-4-carboxaldehyde produced the highly functionalized prod- uct 51 in 80 % yield. Overall, this synthetic strategy gives straightforward access to highly functionalized 9H-pyrrolo- [1,2-a]indole-3-carboxamides. A careful NMR analysis of these new compounds and a tentative explanation of the different reaction pathways were proposed by the authors and resumed in Scheme 10.
During the studies on the formation of 3H-pyrrolo[1,2-a]- indole-2-carbaldehydes 55 using enantioselective organocata- lytic methodologies, Enders and Wang independently found that the corresponding product isomerizes directly to furnish the most stable 9H-isomer 56 (Scheme 11).[24] This isomeriza- tion proceeds more particularly with the use of the furylacrolein substrate. The authors noticed that instead of the diphenyl- prolinol ether catalyst 53, pyrrolidine 54 could be used to cata- lyze the reaction. With the use of other aryl acroleins, if the 3H- isomer-products are enough stable in the air at room tempera- ture and could be purified using silica gel chromatography, the isomerization into the 9H-isomer took place easily in CDCl3 at room temperature within one day. This transformation can even be accelerated in presence of a strong acid such as p-TSA.
In 2017, Tang described an approach for the synthesis of the 9H-pyrrolo[1,2-a]indole backbone, based on a radical addition/ cyclization/isomerization cascade process (Scheme 12a).[25a] Us- ing NaI as a catalyst and tert-butyl hydroperoxide (TBHP) as an oxidant, different N-propargyl indoles 57 reacted smoothly with arylsulfonohydrazide (ArSO2NHNH2) derivatives 58 to furnish the corresponding 2-sulfonated-pyrroloindoles 59. If different iodine source (such as tetrabutylammonium iodide, KI and I2) could be used in this reaction, TBHP proved to be the best oxidant. The reaction scope was extended to more than 30 ex- amples, including substitutions on different positions of the indole backbone and the propargyl functional groups. Interest- ingly, different sulfonohydrazide substrates were efficient in this transformation, forming in situ either alkyl or aryl sulfonyl radi- cals. The reaction mechanism started with the iodine-assisted decomposition of TBHP, which after reaction with the hydrazide generated a sulfonyl radical I. The addition of the latter on the N-propargyl group of the substrate 57 furnished the corre- sponding alkenyl radical J, which undergoes an intramolecular cyclization to form the pyrroloindole ring. After oxidation and isomerization steps, the desired 9H-isomer was delivered. In 2019, Wu, Qiu et al. reported another tandem radical process starting from 1-(prop-2-yn-1-yl)indoles 57, aryldiazonium tetra- fluoroborates and sulfur dioxide surrogate of DABCO (1,4-di- azabicyclo[2.2.2]octane) [DABCO·(SO2)2], for the synthesis of 9H- pyrrolo[1,2-a]indole derivatives 59 (Scheme 12b, 19 examples, 53–77 % yield).[25b] The mechanism remains globally the same as that previously described, namely the combination of DABCO·(SO2)2 with aryldiazonium tetrafluoroborates generates in situ the arylsulfonyl radical, which performs a radical addition on the alkyne of the substrate giving the corresponding alkenyl radical intermediate. Subsequently, an intramolecular addition occurs, followed by oxidation assisted by a tertiary amine radi- cal cation, and then the deprotonation and tautomerization steps. To avoid the use of an excess amount of oxidant (such as TBHP), Zhang, Lin et al. developed a facile route for the syn- thesis of 2-sulfonated 9H-pyrrolo[1,2-a]indoles 59 via a photo- redox-catalyzed cascade radical annulation of N-propargyl- indoles 57 and sulfonyl chlorides (Scheme 12c, 25 examples, 31–81 % yield).[25c] In this study, the activated arylsulfonyl radi- cal species is generated from the combination of visible light and fac-Ir(bpy)3 as a photocatalyst.
Feldman reported an original thermal rearrangement of 2- (allenyl)phenyl azides 60 for the formation of 3H-pyrrolo[1,2-a]- indoles 62, which in most cases undergo isomerization to the most stable 9H-isomer 63 after purification on silica gel (Scheme 13).[26] During this thermolysis, the concomitant for- mation of the 1,4-dihydrocyclopenta[b]indole product 61 was always observed, with a ratio 61:62 of 2.7:1 to 1:1.5.
2.2.Organometallic-Promoted Reactions
2.2.1.Copper-Catalyzed Transformations
In 2016, Chen et al. developed a tandem Friedel-Crafts alkyl- ation/annulation transformation, catalyzed by Cu(OTf)2 (Scheme 14a).[27] The reaction of N-unprotected indole deriva- tives 64 with 1,2-dicarbonyl-3-enes 65 furnished the 9H- pyrrolo[1,2-a]indoles 66 in moderate to good isolated yields (31 examples, 38–87 % yield). This reaction occurred also in pres- ence of AgOTf or Zn(OTf)2, albeit with lower yields (52 % and 69 % respectively). Only 3-substituted indoles (R2 = Me, allyl, 3- cyanomethyl) should be used in this reaction, presumably to promote the exclusive formation of the 9H-isomer of the pyrrol- oindole product. The proposed mechanism involves an inter- molecular Friedel-Crafts alkylation, followed by the annulation reaction and an isomerization to finally deliver the 9H-pyrr- olo[1,2-a]indole product.
Later, Zhang et al. described another Michael addition-con- densation cascade reaction between α,ß-unsaturated ketimines 68 and 3-substituted indoles (Scheme 14b).[28] With 5 mol-% of CuBr2 as a catalyst, the reaction provides 9H-pyrrolo[1,2-a]ind- ole derivatives 69 in moderate to good isolated yields (25 ex- amples, 50–99 % yield). The reaction can also be carried out both with Lewis acids (FeCl3, 91 %) and Brønsted acids (TfOH, 82 %). It seems that many aryls on the ketamine partner are well tolerated (R3 and R4 groups) with good yields, even though no reaction occurs with a strong electron-withdrawing group such as R3 = 4-nitrophenyl. Moderate yields were obtained with 3-methylindoles bearing various substituents on the 4, 5, and 6-positions (R1 and R2 substituents). Here again, no substrate with R2 = H has been tested. A plausible mechanism begins with a Michael addition followed by an intramolecular nucleo- philic addition and then an isomerization to provide the desired product.
In 2017, copper(II) was also used by Zhu et al. in a tandem radical cyclization of diphenylphosphine oxide 71 with 1-(3-ar- ylprop-2-yn-1-yl)-1H-indoles 70 (Scheme 15).[29] With the opti- mized conditions in hand (25 mol-% of CuSO4 as a catalyst and
2.0 equiv. of K2S2O8 as oxidant), a series of 2-phosphinoyl-9H- pyrrolo[1,2-a]indoles 72 was obtained in moderate yields (21 examples, 38–76 %). The outcomes of the reaction were not significantly affected by functional groups either on indoles (R1 and R2) or on the aryls linked to the alkyne. However, when dialkylphosphites were used, no reaction was detected. After some control experiments, a mechanism has been suggested which is initiated by addition of the P-centered radical [formed by the oxidation of Ar2P(O)H by CuII] on the alkyne, leading to a vinyl radical which then undergoes an intramolecular cycliza- tion. After an oxidation step, the isomerization of 3H-isomer leads to 9H-pyrrolo[1,2-a]indole derivatives.
In 2017, Bu et al. described a Friedel-Crafts alkylation/cycliza- tion with C3-substituted indoles 73 and aromatic aldehyde- derived oxodienes 74, catalyzed by Cu(OTf)2 (Scheme 16).[30] For this tandem reaction, this catalyst leads to a quantitative yield, while both Brønsted acid (TfOH, 81 % yield) and Lewis acid [Sc(OTf)3, 97 % yield] can be used. Several substrates were investigated to synthesize 9H-pyrrolo[1,2-a]indole derivatives 75 (20 examples, 59–88 %). It appeared that steric hindrance of substituents in para-position on the phenyl ring (Ar1) influenced the results, whereas, in meta-position on the same aryl group, the reaction outcome was affected by electronic effects. The reaction could work when R2 is different than a methyl group (CH2CO2Et, CH2CH2OH, ...). Concerning the mechanism, the first step is a Friedel-Crafts alkylation followed by a 1,2-addition. After a dehydration step, isomerization occurs.
In the continuity of their previous work, Bu et al. extended their methodology to 3-arylcarbonylcoumarins 77 to increase the structural complexity of 9H-pyrrolo[1,2-a]indoles 78 (21 ex- amples, 34–99 %) (Scheme 17).[31] The mechanism remains globally the same, while only 3-methyl indole seems to be a suitable partner. Additionally, the yields drop when R2 is differ- ent than a phenyl group, and the reaction can tolerate a wide range of R1 substituents. It seems that electron-withdrawing groups improve the reactivity of the corresponding substrates. A Suzuki cross-coupling post-functionalization has been devel- oped to obtain more complex pentacyclic core, in good yields.
2.2.2.Palladium-Catalyzed Transformations
Knochel et al. developed the first synthesis of 9H-pyrrolo[1,2-a]- indoles via a palladium-catalyzed chemoselective benzylic C–H activation of pyrroles (Scheme 18).[32] Based on the optimized conditions, several 9H-pyrrolo[1,2-a]indoles were prepared starting from symmetric halogeno-N-arylpyrrole substrates 79 (11 examples, 33–86 %, Scheme 18a) and non-symmetric sub- strates 81 and 83 (10 examples, 50–81 %, Scheme 18b and 18c). With symmetric pyrroles, starting from iodine- or bromine- substituted substrates provides similar yields. Overall, esters, ketones, and aldehydes are well-tolerated whereas strong elec- tron-withdrawing group such as nitro groups or strong elec- tron-donating groups affects considerably the isolated yield. With non-symmetrical pyrrole, regioselectivity issue of C–H acti- vation may occur. In this study for the 2-substituted mono- bromo derivatives 81, only the C–H activation of the methyl- substituent of the pyrrole has been observed due to the steric hindrance of the substrate. Based on this analysis, oxidative addition of Pd0 first occurs following by concomitant C–H activa- tion and HX elimination. Then the palladium catalyst is regener- ated after a reductive elimination. In the case of asymmetric 2,5-substituted dibromo derivatives 83, only the benzylic C–H activation occurs. If the reaction time is prolonged in presence of more base (2.2 equiv., 24 h), a series of C–H activation occurs leading to more complex heterocyclics 84 (3 examples, 50– 61 %, Scheme 18c). In this case, after the oxidative addition of Pd0, the N-(2-haloaryl)pyrrole undergoes an electrophilic substi- tution to furnish an eight-membered palladacycle. The interme- diate is then generated after an acid-base reaction and a reduc- tive elimination. Subsequently, a second oxidative addition oc- curs following by a C–H activation/HX elimination and then a reductive elimination to provide the desired pentacyclic com- pound 84.
In 2008, a Pd-catalyzed intramolecular C–H activation was described by Chang et al. (Scheme 19). In this study, the C–H activation occurs between the C–H bond of the pyrrole and a benzylic chloride to furnish a broad range of 9H-pyrrolo[1,2-a]indoles 86 (11 examples, 60–97 %).[33] Unlike the previous study, the reaction could be dramatically affected by the type of (pseudo)halides used. The benzylic chloride gave the best results, in comparison with other halogens or (pseudo)halides such as mesylate or tosylate groups. In addi- tion, it seems that the reaction occurs faster with substrates bearing electron-withdrawing group while bulky substrates re- quired higher reaction temperatures with more catalyst. This dependence on the acidity of the C–H bond seems to indicate that the cyclization proceeds through a base-assisted deproto- native metalation pathway.
In 2012, Lautens et al. described a Pd-catalyzed cascade reac- tion involving a series of C–H functionalizations to synthesize a broad range of 9H-pyrrolo[1,2-a]indoles.[34] The reaction scope was first investigated using substrate 88 and variously substi- tuted aryl iodides 87. Both electron-donating and withdrawing groups furnished the desired racemic products 89 in good yields (7 examples, 60–75 %, Scheme 20a). Starting from the enantiopure alkyne substrate 91 and 2-iodotoluene 90, the re- action afforded the chiral alkenes 92 with excellent diastereo- selectivity (2 examples, 88–92 %, d.r. = 10:1, Scheme 20b). In addition, using 1-{2-[(2-bromophenyl)ethynyl]phenyl}-1H-pyrr- oles 94 and several substituted aryl iodides 93, more sterically hindered products were isolated (5 examples, 39–85 %, Scheme 20c). These domino reactions proceed via a Pd-cata- lyzed norbornene-mediated cascade of C–H activation.
(But-3-en-1-yloxy)-tert-butyldimethylsilaneCatalog No.:AA007BV4 CAS No.:108794-10-1 MDL No.:MFCD09033284 MF:C10H22OSi MW:186.3666 |
5H-Benzo[b]phosphindole, 5-phenyl-Catalog No.:AA00836F CAS No.:1088-00-2 MDL No.:MFCD00046900 MF:C18H25P MW:272.3649 |
LumiflavineCatalog No.:AA0082YS CAS No.:1088-56-8 MDL No.:MFCD00042742 MF:C13H12N4O2 MW:256.2600 |
2-(3,4-Dimethoxyphenyl)-2-(pyrazin-2-yl)acetonitrileCatalog No.:AA01F8BL CAS No.:1088-67-1 MDL No.:MFCD00958043 MF:C14H13N3O2 MW:255.2719 |
1-Acetyl-2,6-dihydroxynaphthaleneCatalog No.:AA003DTH CAS No.:108804-50-8 MDL No.:MFCD24725628 MF:C12H10O3 MW:202.2060 |
5-chloro-1,2-benzoxazole-3-carboxylic acidCatalog No.:AA01BU57 CAS No.:108805-37-4 MDL No.:MFCD30731611 MF:C8H4ClNO3 MW:197.5753 |
N-Methyl-3-(3-methyl-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)anilineCatalog No.:AA00836A CAS No.:108810-87-3 MDL No.:MFCD00277901 MF:C13H13N5 MW:239.2758 |
3-(6-Chloropyridazin-3-yl)anilineCatalog No.:AA01DX7N CAS No.:108810-90-8 MDL No.:MFCD22199835 MF:C10H8ClN3 MW:205.6436 |
1-chlorocyclopropane-1-carboxylic acidCatalog No.:AA01AKRM CAS No.:108817-35-2 MDL No.:MFCD19228827 MF:C4H5ClO2 MW:120.5343 |
Methyl 1,5-diphenyl-4,5-dihydro-1h-pyrazole-3-carboxylateCatalog No.:AA01ABF0 CAS No.:1088200-27-4 MDL No.:MFCD10660990 MF:C17H16N2O2 MW:280.3211 |
N-Cyclopropyl-4-(methylsulfanyl)benzamideCatalog No.:AA01BE1Y CAS No.:1088204-17-4 MDL No.:MFCD10651321 MF:C11H13NOS MW:207.2920 |
4,9-Dibromo-2,7-bis(2-ethylhexyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2h,7h)-tetraoneCatalog No.:AA0091E0 CAS No.:1088205-02-0 MDL No.:MFCD27923032 MF:C30H36Br2N2O4 MW:648.4258 |
3-(Piperidin-1-yl)butan-2-olCatalog No.:AA008VJL CAS No.:1088238-06-5 MDL No.:MFCD12827437 MF:C9H19NO MW:157.2533 |
2-methyl-1,2,3,4-tetrahydroquinolin-5-olCatalog No.:AA01DUVT CAS No.:108825-11-2 MDL No.:MFCD01685093 MF:C10H13NO MW:163.2163 |
Acetamide,N-methyl-N-[3-(3-methyl-1,2,4-triazolo[4,3-b]pyridazin-6-yl)phenyl]-Catalog No.:AA007BTD CAS No.:108825-65-6 MDL No.:MFCD01567008 MF:C15H15N5O MW:281.3125 |
6-Methylthieno[2,3-d]pyrimidin-4-olCatalog No.:AA007TWN CAS No.:108831-66-9 MDL No.:MFCD01570294 MF:C7H6N2OS MW:166.2003 |
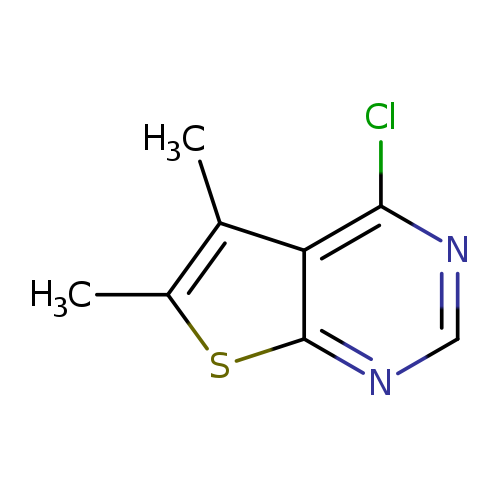
4-Chloro-5,6-dimethylthieno[2,3-d]pyrimidineCatalog No.:AA0033WS CAS No.:108831-68-1 MDL No.:MFCD00464832 MF:C8H7ClN2S MW:198.6726 |
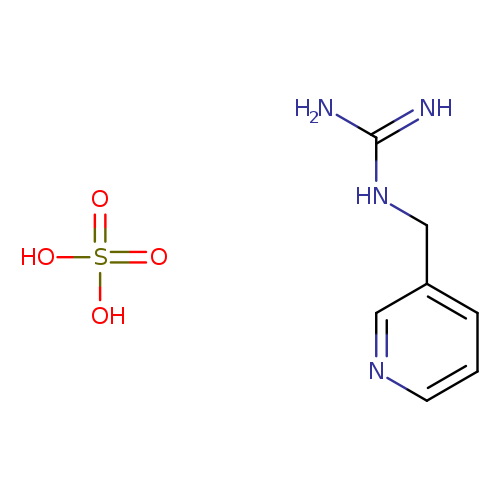
N-(3-Pyridinylmethyl)guanidine sulfateCatalog No.:AA00J1F5 CAS No.:108833-95-0 MDL No.:MFCD13193928 MF:C7H12N4O4S MW:248.2596 |
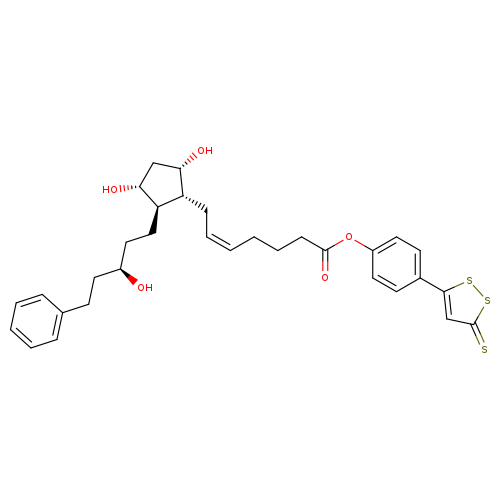
ACS 67Catalog No.:AA008SOF CAS No.:1088434-86-9 MDL No.:MFCD18428027 MF:C32H38O5S3 MW:598.8361 |
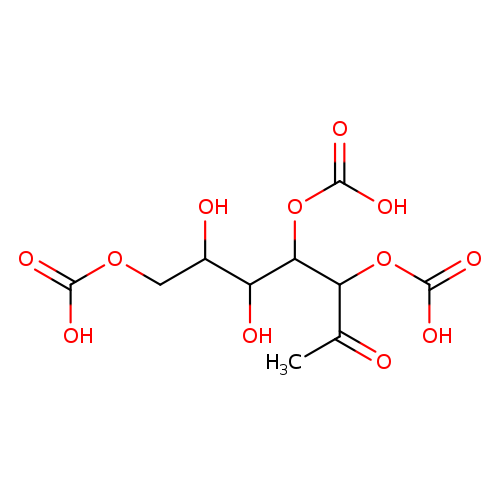
2,3,6-Tri-O-Carboxymethyl-D-glucoseCatalog No.:AA01EAWG CAS No.:108844-55-9 MDL No.: MF:C12H18O12 MW:354.2641 |
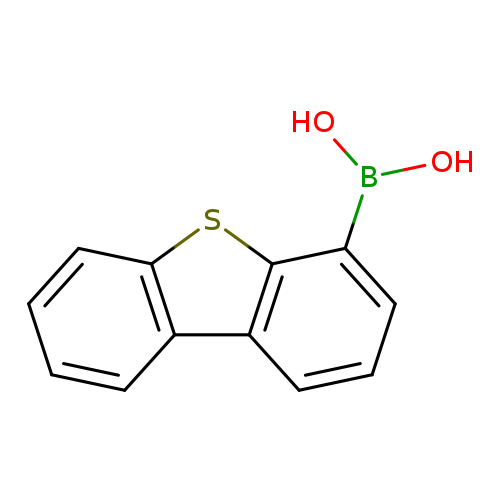
Dibenzo[b,d]thiophene-4-boronic acidCatalog No.:AA0033XV CAS No.:108847-20-7 MDL No.:MFCD01318182 MF:C12H9BO2S MW:228.0747 |
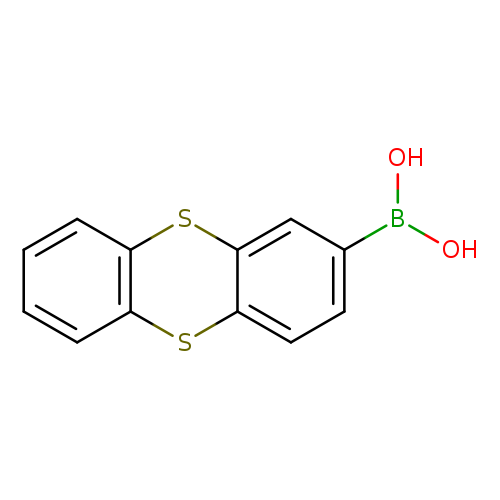
Thianthren-2-ylboronic acidCatalog No.:AA007TWF CAS No.:108847-21-8 MDL No.:MFCD06801664 MF:C12H9BO2S2 MW:260.1397 |
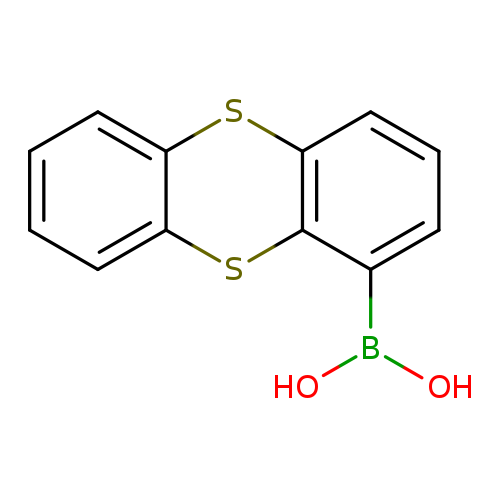
Thianthrene-1-boronic acidCatalog No.:AA003EL2 CAS No.:108847-76-3 MDL No.:MFCD00093039 MF:C12H9BO2S2 MW:260.1397 |
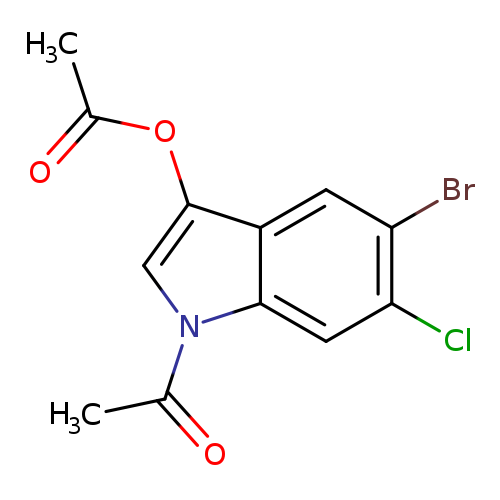
5-Bromo-6-chloroindolyl 1,3-diacetateCatalog No.:AA008XHW CAS No.:108847-96-7 MDL No.:MFCD06797509 MF:C12H9BrClNO3 MW:330.5618 |
2-(Methylsulfonyl)pyridine-5-boronic acidCatalog No.:AA008SRO CAS No.:1088496-41-6 MDL No.:MFCD12964553 MF:C6H8BNO4S MW:201.0080 |
3-Chloro-5-(4-methylphenyl)-4-phenyl-4h-1,2,4-triazoleCatalog No.:AA019MKK CAS No.:108850-11-9 MDL No.:MFCD11099415 MF:C15H12ClN3 MW:269.7289 |
diperdipineCatalog No.:AA009OE0 CAS No.:108852-42-2 MDL No.:MFCD09833170 MF:C24H31N3O6 MW:457.5194 |
NemorubicinCatalog No.:AA008TFR CAS No.:108852-90-0 MDL No.:MFCD00871079 MF:C32H37NO13 MW:643.6351 |
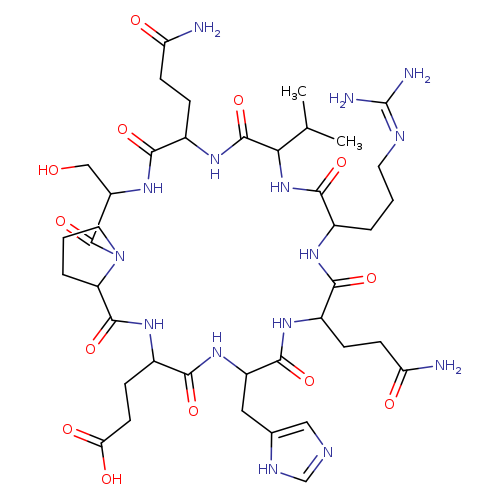
AZP-531Catalog No.:AA01EOK0 CAS No.:1088543-62-7 MDL No.:MFCD31544369 MF:C40H63N15O13 MW:962.0209 |
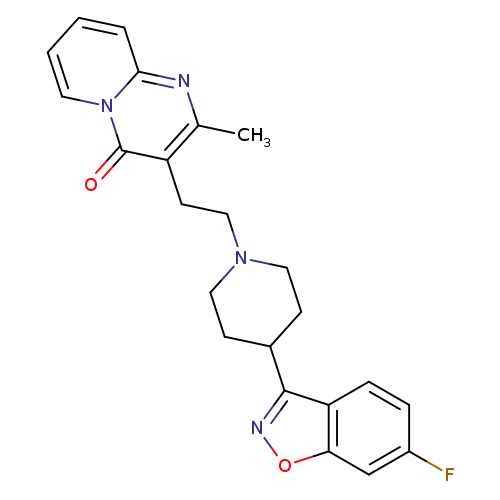
4H-Pyrido[1,2-a]pyrimidin-4-one,3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-2-methyl-Catalog No.:AA01CCIO CAS No.:108855-18-1 MDL No.:MFCD31621070 MF:C23H23FN4O2 MW:406.4527 |
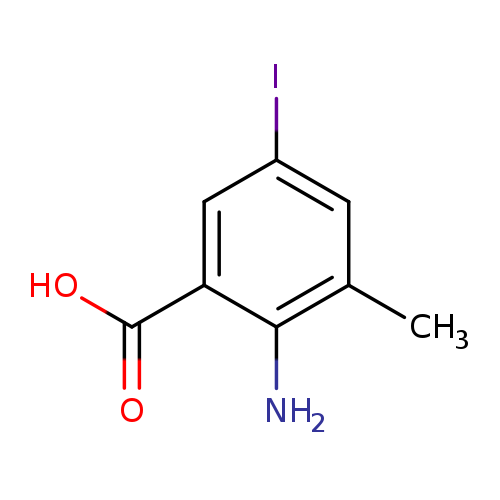
2-Amino-5-iodo-3-methylbenzoic acidCatalog No.:AA007BM0 CAS No.:108857-24-5 MDL No.:MFCD09029816 MF:C8H8INO2 MW:277.0591 |
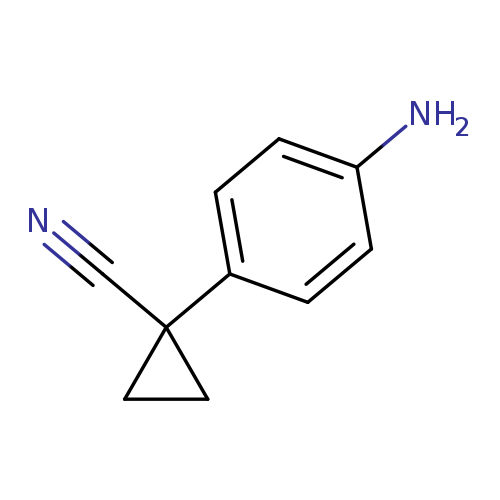
1-(4-Aminophenyl)cyclopropanecarbonitrileCatalog No.:AA008XHV CAS No.:108858-86-2 MDL No.:MFCD16622255 MF:C10H10N2 MW:158.1998 |
1,1'-[1,4-Phenylenebis(methylene)]bis(4,4'-bipyridinium)bis(hexafluorophosphate)Catalog No.:AA003D24 CAS No.:108861-20-7 MDL No.:MFCD02063876 MF:C28H24F12N4P2 MW:706.4453 |
2-Chloro-n-(3,4-dimethylphenyl)propanamideCatalog No.:AA00HB67 CAS No.:108871-73-4 MDL No.:MFCD09939207 MF:C11H14ClNO MW:211.6880 |
2-(piperazin-1-ylmethyl)-4-(propan-2-yl)morpholineCatalog No.:AA01AB7O CAS No.:1088710-28-4 MDL No.:MFCD19381847 MF:C12H25N3O MW:227.3464 |
LY2510924Catalog No.:AA01EOIE CAS No.:1088715-84-7 MDL No.:MFCD31728513 MF:C62H88N14O10 MW:1189.4499 |
3-(propan-2-yl)-1H,4H-indeno[1,2-c]pyrazol-4-oneCatalog No.:AA00IX4O CAS No.:108874-23-3 MDL No.:MFCD00170429 MF:C13H12N2O MW:212.2472 |
2-(6,7,8,9-Tetrahydro-5h-[1,2,4]triazolo[4,3-a]azepin-3-yl)-phenolCatalog No.:AA008V9Q CAS No.:108877-44-7 MDL No.:MFCD02701039 MF:C13H15N3O MW:229.2777 |
3,5-Dichloro-1-(tetrahydro-2H-pyran-2-yl)-1H-1,2,4-triazoleCatalog No.:AA008VI7 CAS No.:1088834-41-6 MDL No.:MFCD16556141 MF:C7H9Cl2N3O MW:222.0719 |
Z-(r,s)-3-amino-2-oxo-5-phenyl-1,4-benzodiazepineCatalog No.:AA008TIS CAS No.:108895-98-3 MDL No.:MFCD01074700 MF:C23H19N3O3 MW:385.4153 |
[(3-chlorophenyl)methyl](2-{[(3-chlorophenyl)methyl]amino}ethyl)amineCatalog No.:AA00IXFB CAS No.:108896-77-1 MDL No.:MFCD00243906 MF:C16H18Cl2N2 MW:309.2335 |
Gsk-923295Catalog No.:AA0082YC CAS No.:1088965-37-0 MDL No.:MFCD16038931 MF:C32H38ClN5O4 MW:592.1282 |
(2R,3R)-1-benzyl-2,3-dimethylpiperidin-4-oneCatalog No.:AA00HB68 CAS No.:108897-26-3 MDL No.:MFCD03768188 MF:C14H19NO MW:217.3068 |
Mk-6096Catalog No.:AA008TFF CAS No.:1088991-73-4 MDL No.:MFCD28100674 MF:C24H25FN4O2 MW:420.4793 |
5-Methyl-2-(pyrimidin-2-yl)benzoic acidCatalog No.:AA0037WE CAS No.:1088994-22-2 MDL No.:MFCD14706695 MF:C12H10N2O2 MW:214.2200 |
4-(4-METHYLPHENOXY)PHENYLHYDRAZINE HYDROCHLORIDECatalog No.:AA008V4N CAS No.:108902-83-6 MDL No.:MFCD03426043 MF:C13H15ClN2O MW:250.7240 |
trans/cis Resveratrol-D4Catalog No.:AA0099B1 CAS No.:1089051-56-8 MDL No.:MFCD11976910 MF:C14H8D4O3 MW:232.2679 |
trans-trismethoxy Resveratrol-d4Catalog No.:AA008SPY CAS No.:1089051-64-8 MDL No.:MFCD11976909 MF:C17H14D4O3 MW:274.3477 |
1H-Indole-2-carboxamide,2,3-dihydro-(9CI)Catalog No.:AA009POJ CAS No.:108906-13-4 MDL No.:MFCD09743255 MF:C9H10N2O MW:162.1885 |
N-Aminosuccinimide HClCatalog No.:AA003SNE CAS No.:108906-15-6 MDL No.:MFCD08276265 MF:C4H7ClN2O2 MW:150.5636 |
Aripiprazole-d8 (Butyl-d8)Catalog No.:AA007TW0 CAS No.:1089115-04-7 MDL No.:MFCD08460937 MF:C23H19Cl2D8N3O2 MW:456.4347 |
Aripiprazole-d8Catalog No.:AA008TIO CAS No.:1089115-06-9 MDL No.:MFCD08460937 MF:C23H19Cl2D8N3O2 MW:456.4347 |
3-[4-(BROMOMETHYL)PHENYL]THIOPHENECatalog No.:AA008ROE CAS No.:108912-09-0 MDL No.:MFCD08435895 MF:C11H9BrS MW:253.1582 |
3-(Boc-amino)-2-(4-bromophenyl)-propanolCatalog No.:AA00HB6G CAS No.:1089131-54-3 MDL No.:MFCD28405199 MF:C14H20BrNO3 MW:330.2175 |
Z-Dbu(boc)-ohCatalog No.:AA003VCH CAS No.:108919-51-3 MDL No.:MFCD03788646 MF:C17H24N2O6 MW:352.3823 |
rac-(1R,2R)-2-methanesulfonylcyclohexan-1-ol, transCatalog No.:AA01DUVV CAS No.:108920-21-4 MDL No.:MFCD28954081 MF:C7H14O3S MW:178.2493 |
4-Bromo-3-(difluoromethyl)-1-methyl-1H-pyrazoleCatalog No.:AA008Z1V CAS No.:1089212-38-3 MDL No.:MFCD23703127 MF:C5H5BrF2N2 MW:211.0074 |
4-bromo-5-(difluoromethyl)-1-methyl-1H-pyrazoleCatalog No.:AA01BS38 CAS No.:1089212-57-6 MDL No.:MFCD28528999 MF:C5H5BrF2N2 MW:211.0074 |
U0124Catalog No.:AA0082VG CAS No.:108923-79-1 MDL No.:MFCD02179218 MF:C8H10N4S2 MW:226.3218 |
3-(1-Methyl-1H-pyrazol-4-yl)anilineCatalog No.:AA008S0H CAS No.:1089278-81-8 MDL No.:MFCD10703509 MF:C10H11N3 MW:173.2144 |
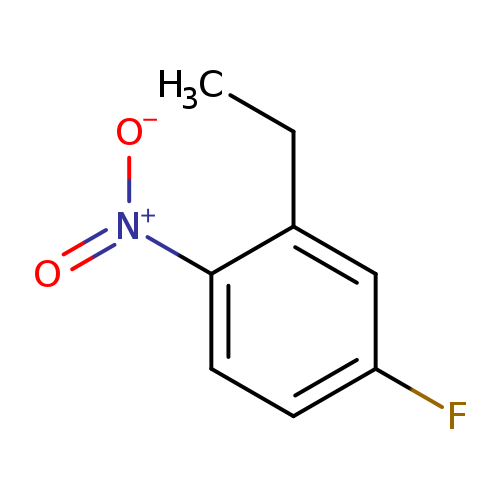
2-Ethyl-4-fluoro-1-nitrobenzeneCatalog No.:AA00HB6L CAS No.:1089279-29-7 MDL No.:MFCD16658616 MF:C8H8FNO2 MW:169.1530 |
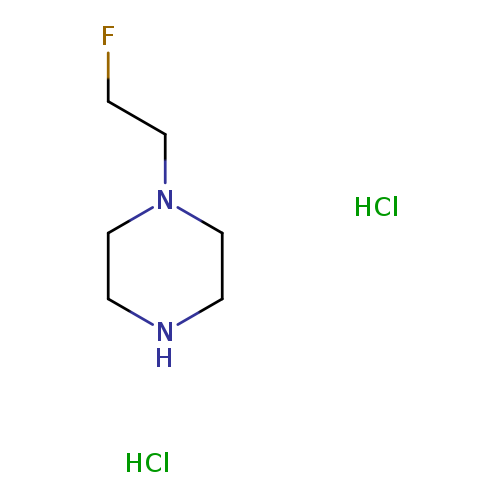
1-(2-Fluoroethyl)piperazine dihydrochlorideCatalog No.:AA008YYQ CAS No.:1089279-64-0 MDL No.:MFCD21602355 MF:C6H15Cl2FN2 MW:205.1011 |
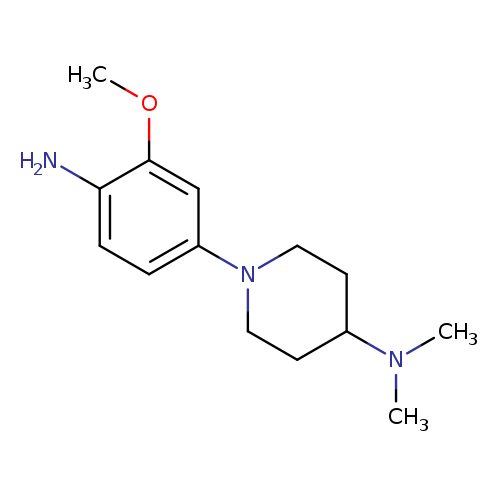
1-(4-Amino-3-methoxyphenyl)-n,n-dimethylpiperidin-4-amineCatalog No.:AA0091YY CAS No.:1089279-91-3 MDL No.:MFCD18995922 MF:C14H23N3O MW:249.3519 |
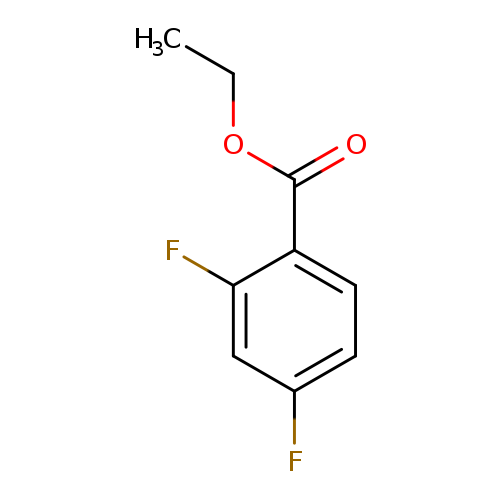
Ethyl 2,4-difluorobenzoateCatalog No.:AA003PZI CAS No.:108928-00-3 MDL No.:MFCD00153149 MF:C9H8F2O2 MW:186.1554 |
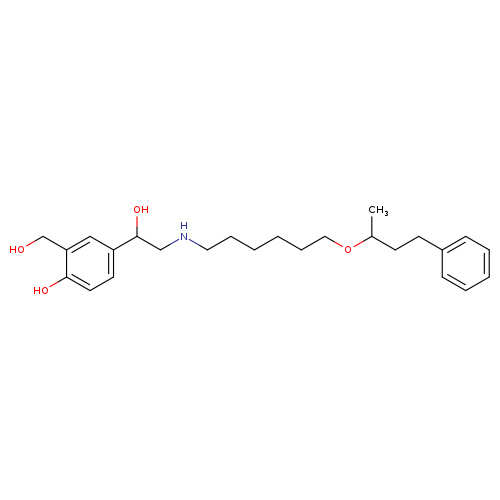
4-(1-Hydroxy-2-((6-((4-phenylbutan-2-yl)oxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenolCatalog No.:AA008WMQ CAS No.:108928-81-0 MDL No.:MFCD28134528 MF:C25H37NO4 MW:415.5656 |
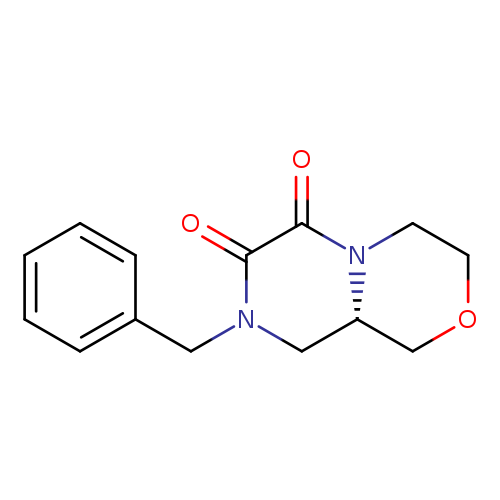
(S)-8-Benzylhexahydropyrazino[2,1-c][1,4]oxazine-6,7-dioneCatalog No.:AA00HB6M CAS No.:1089280-11-4 MDL No.:MFCD20526988 MF:C14H16N2O3 MW:260.2884 |
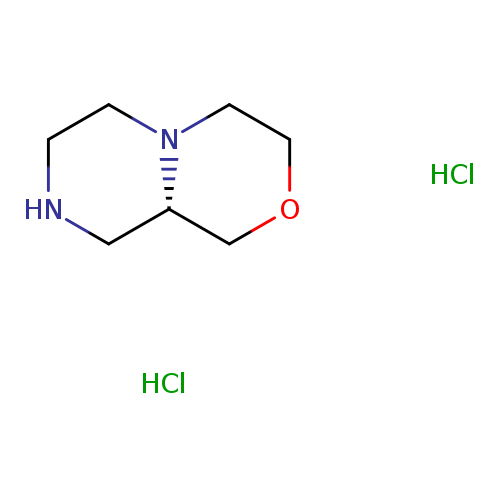
(S)-Octahydropyrazino[2,1-c][1,4]oxazine dihydrochlorideCatalog No.:AA0092O0 CAS No.:1089280-14-7 MDL No.:MFCD23105798 MF:C7H16Cl2N2O MW:215.1207 |
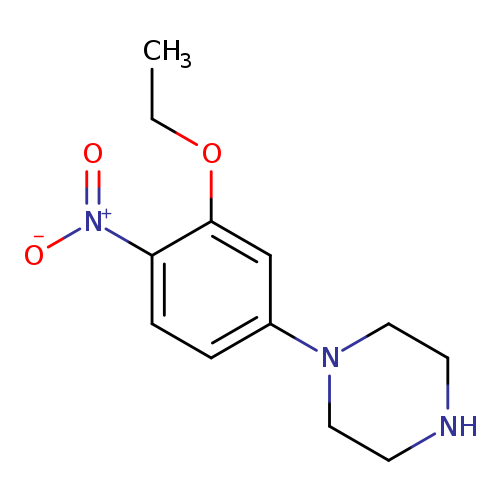
1-(3-ethoxy-4-nitrophenyl)piperazineCatalog No.:AA01BGXY CAS No.:1089280-28-3 MDL No.:MFCD18605294 MF:C12H17N3O3 MW:251.2817 |
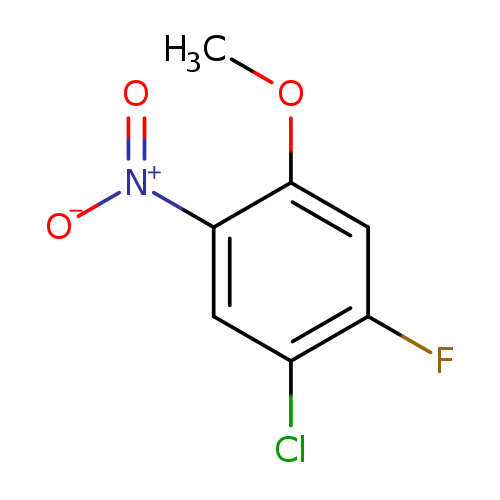
1-Chloro-2-fluoro-4-methoxy-5-nitrobenzeneCatalog No.:AA0094H5 CAS No.:1089280-66-9 MDL No.:MFCD11847613 MF:C7H5ClFNO3 MW:205.5709 |
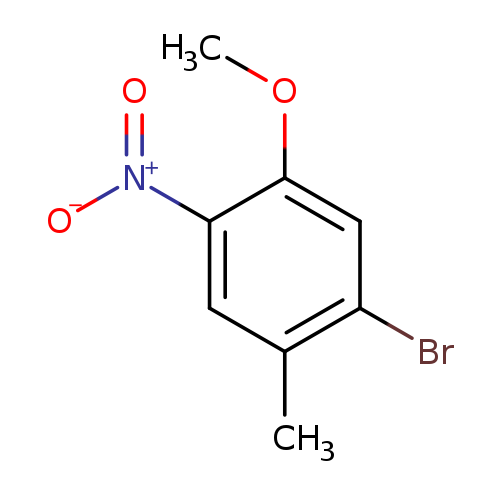
1-Bromo-5-methoxy-2-methyl-4-nitrobenzeneCatalog No.:AA003MGQ CAS No.:1089281-86-6 MDL No.:MFCD20482626 MF:C8H8BrNO3 MW:246.0580 |
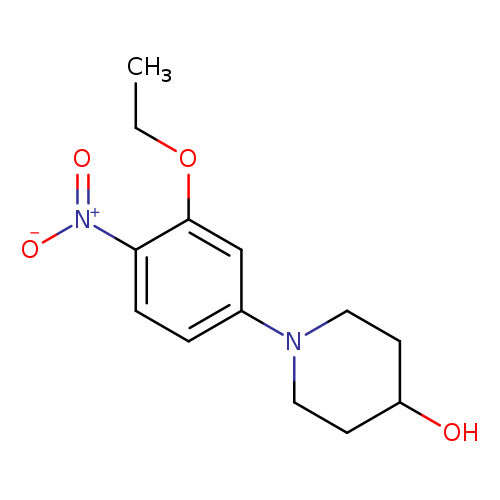
1-(3-ethoxy-4-nitrophenyl)piperidin-4-olCatalog No.:AA01AKW9 CAS No.:1089283-25-9 MDL No.:MFCD19175034 MF:C13H18N2O4 MW:266.2930 |
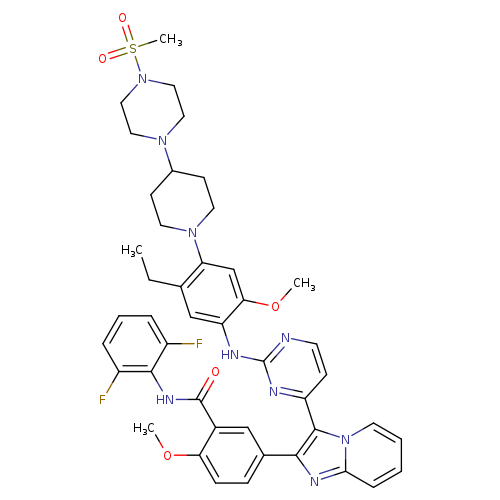
GSK1904529ACatalog No.:AA008TDW CAS No.:1089283-49-7 MDL No.:MFCD17010271 MF:C44H47F2N9O5S MW:851.9631 |
Epinastine HClCatalog No.:AA003PUU CAS No.:108929-04-0 MDL No.:MFCD00933434 MF:C16H16ClN3 MW:285.7713 |
Isoquinoline,5-[(2-methyl-1-piperazinyl)sulfonyl]-,hydrochloride (1:2)Catalog No.:AA007TQD CAS No.:108930-17-2 MDL No.:MFCD00036961 MF:C14H17N3O2S MW:291.3687 |
4-(4-chlorophenyl)-3,3-dimethylbutanoic acidCatalog No.:AA01AG8Y CAS No.:1089303-25-2 MDL No.:MFCD19320004 MF:C12H15ClO2 MW:226.6993 |
2-(dimethylamino)-1-(4-methoxyphenyl)-1-propanone,monohydrochlorideCatalog No.:AA01EOWE CAS No.:1089307-23-2 MDL No.: MF:C12H18ClNO2 MW:243.7298 |
ethyl 3-propylhexanoateCatalog No.:AA01FP7Z CAS No.:1089315-43-4 MDL No.:MFCD31661337 MF:C11H22O2 MW:186.2912 |
4-methyl-4-(2-methylpropyl)piperidine hydrochlorideCatalog No.:AA01BBKA CAS No.:1089317-11-2 MDL No.:MFCD28506061 MF:C10H22ClN MW:191.7414 |
Ethyl 5,6-dihydro-2h-pyran-3-carboxylateCatalog No.:AA00J2QW CAS No.:1089317-19-0 MDL No.:MFCD22398673 MF:C8H12O3 MW:156.1791 |
[5-(Ethanesulfonyl)thiophen-2-yl]methanamine hydrochlorideCatalog No.:AA01BGEJ CAS No.:1089317-54-3 MDL No.:MFCD28125129 MF:C7H12ClNO2S2 MW:241.7587 |
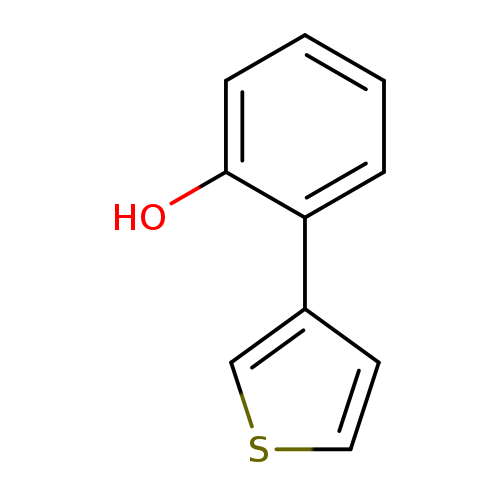
2-(Thiophen-3-yl)phenolCatalog No.:AA0091GS CAS No.:108932-41-8 MDL No.:MFCD06801794 MF:C10H8OS MW:176.2349 |
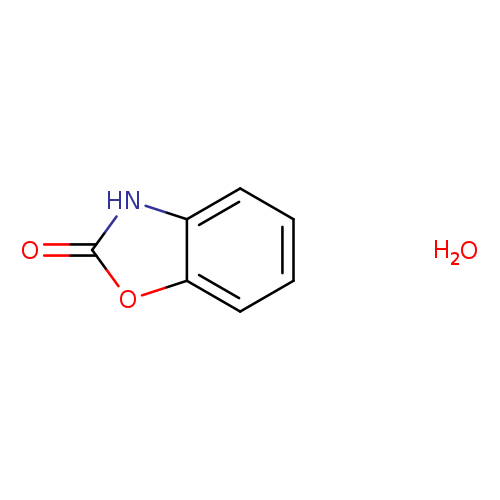
2-Benzoxazolinone hydrateCatalog No.:AA01EH2Z CAS No.:1089327-13-8 MDL No.:MFCD31726446 MF:C7H7NO3 MW:153.1354 |