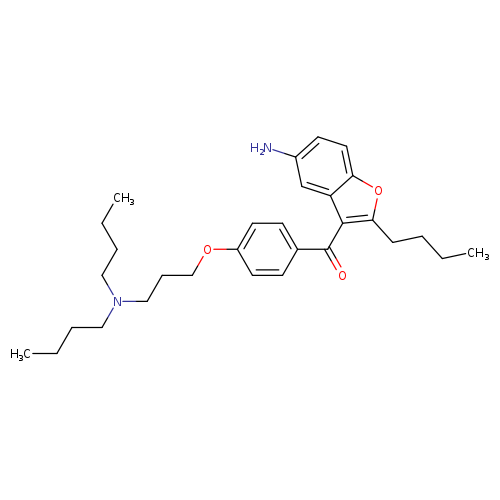
[1]CurrentPatentAssignee:ZYDUSLIFESCIENCESLTD-WO2012/32545,2012,A1Locationinpatent:Page/Pagecolumn36
[2]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-WO2012/52448,2012,A1Locationinpatent:Page/Pagecolumn13
[3]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-EP2447256,2012,A1Locationinpatent:Page/Pagecolumn9
[4]Madhasu,Madhu;Doda,SaiReddy;Begari,PremKumar;Dasari,KrishnaRao;Thalari,Gangadhar;Kadari,Sudhakar;Yadav,JhilluSingh[JournalofHeterocyclicChemistry,2021,vol.58,#9,p.1861-1866]
[5]CurrentPatentAssignee:SANOFI-US2004/10032,2004,A1Locationinpatent:Page/Pagecolumn6
[6]CurrentPatentAssignee:GLENMARKPHARMACEUTICALSLTD-WO2012/7959,2012,A1Locationinpatent:Page/Pagecolumn20-21
[7]CurrentPatentAssignee:GLENMARKPHARMACEUTICALSLTD-WO2012/7959,2012,A1Locationinpatent:Page/Pagecolumn23-24
[8]Locationinpatent:experimentalpartHivarekar,RaghvendraR.;Deshmukh,SanjayS.;Tripathy.,NarendraK.[OrganicProcessResearchandDevelopment,2012,vol.16,#4,p.677-681]
[9]CurrentPatentAssignee:SANOFI-WO2012/131408,2012,A1Locationinpatent:Page/Pagecolumn13
[10]CurrentPatentAssignee:ALEMBICPHARMACEUTICALSLIMITED-WO2012/153225,2012,A1Locationinpatent:Page/Pagecolumn3;13
[11]Klucznik,Tomasz;Mikulak-Klucznik,Barbara;McCormack,MichaelP.;Lima,Heather;Szymkuć,Sara;Bhowmick,Manishabrata;Molga,Karol;Zhou,Yubai;Rickershauser,Lindsey;Gajewska,EwaP.;Toutchkine,Alexei;Dittwald,Piotr;Startek,MichałP.;Kirkovits,GregoryJ.;Roszak,Rafał;Adamski,Ariel;Sieredzińska,Bianka;Mrksich,Milan;Trice,SarahL.J.;Grzybowski,BartoszA.[Chem,2018,vol.4,#3,p.522-532]
[1]CurrentPatentAssignee:SANOFI-US2004/10032,2004,A1Locationinpatent:Page/Pagecolumn5;6
[2]CurrentPatentAssignee:USVLTD.-EP2428511,2012,A1Locationinpatent:Page/Pagecolumn10
[3]CurrentPatentAssignee:USVLTD.-US2012/108828,2012,A1Locationinpatent:Page/Pagecolumn7
[4]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-WO2012/52448,2012,A1Locationinpatent:Page/Pagecolumn10-11
[5]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-EP2447256,2012,A1Locationinpatent:Page/Pagecolumn8
[6]Madhasu,Madhu;Doda,SaiReddy;Begari,PremKumar;Dasari,KrishnaRao;Thalari,Gangadhar;Kadari,Sudhakar;Yadav,JhilluSingh[JournalofHeterocyclicChemistry,2021,vol.58,#9,p.1861-1866]
[7]Chen,Hao;Chen,Xiuhui;Sun,Ping;Wu,Dan;Yue,Hu;Pan,Jintao;Li,Xinxuan;Zhang,Cheng;Wu,Xinyi;Hua,Lei;Hu,Wenhui;Yang,Zhongjin[BioorganicandMedicinalChemistryLetters,2021,vol.46]
[8]CurrentPatentAssignee:GUANGZHOUMEDICALUNIVERSITY-CN113200947,2021,ALocationinpatent:Paragraph0053-0055;0068-0069
[9]CurrentPatentAssignee:GLENMARKPHARMACEUTICALSLTD-WO2012/7959,2012,A1Locationinpatent:Page/Pagecolumn19-20
[10]CurrentPatentAssignee:FRICHEMPRIVATE-WO2012/4658,2012,A2Locationinpatent:Page/Pagecolumn18
[11]CurrentPatentAssignee:ZYDUSLIFESCIENCESLTD-WO2012/32545,2012,A1
[12]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-EP2447256,2012,A1
[13]CurrentPatentAssignee:SUNPHARMACEUTICALINDUSTRIESLIMITED-WO2012/120544,2012,A2Locationinpatent:Page/Pagecolumn18-19
[14]CurrentPatentAssignee:ALEMBICPHARMACEUTICALSLIMITED-WO2012/153225,2012,A1
[15]CurrentPatentAssignee:SANOFI-US2013/165675,2013,A1Locationinpatent:Paragraph0041;0042
[16]Mali,AnilC.;Ippar,SharadS.;Bodke,MahendraB.;Patil,NileshS.;Mathad,VijayavitthalT.[OrganicProcessResearchandDevelopment,2013,vol.17,#5,p.863-868]
[17]CurrentPatentAssignee:SANOFI-US2015/31901,2015,A1Locationinpatent:Paragraph0036;0037;0038;0039;0040
[18]Klucznik,Tomasz;Mikulak-Klucznik,Barbara;McCormack,MichaelP.;Lima,Heather;Szymkuć,Sara;Bhowmick,Manishabrata;Molga,Karol;Zhou,Yubai;Rickershauser,Lindsey;Gajewska,EwaP.;Toutchkine,Alexei;Dittwald,Piotr;Startek,MichałP.;Kirkovits,GregoryJ.;Roszak,Rafał;Adamski,Ariel;Sieredzińska,Bianka;Mrksich,Milan;Trice,SarahL.J.;Grzybowski,BartoszA.[Chem,2018,vol.4,#3,p.522-532]
[1]CurrentPatentAssignee:GLENMARKPHARMACEUTICALSLTD-WO2012/7959,2012,A1Locationinpatent:Page/Pagecolumn23-24
[1]CurrentPatentAssignee:GLENMARKPHARMACEUTICALSLTD-WO2012/7959,2012,A1
[2]CurrentPatentAssignee:USVLTD.-EP2428511,2012,A1
[3]CurrentPatentAssignee:ZYDUSLIFESCIENCESLTD-WO2012/32545,2012,A1
[4]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-WO2012/52448,2012,A1
[5]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-EP2447256,2012,A1
[6]CurrentPatentAssignee:PERMIRAHOLDINGSLIMITED-EP2447256,2012,A1
[7]CurrentPatentAssignee:USVLTD.-US2012/108828,2012,A1
[8]CurrentPatentAssignee:SUNPHARMACEUTICALINDUSTRIESLIMITED-WO2012/120544,2012,A2
[9]CurrentPatentAssignee:ALEMBICPHARMACEUTICALSLIMITED-WO2012/153225,2012,A1
[10]Mali,AnilC.;Ippar,SharadS.;Bodke,MahendraB.;Patil,NileshS.;Mathad,VijayavitthalT.[OrganicProcessResearchandDevelopment,2013,vol.17,#5,p.863-868]
[11]Klucznik,Tomasz;Mikulak-Klucznik,Barbara;McCormack,MichaelP.;Lima,Heather;Szymkuć,Sara;Bhowmick,Manishabrata;Molga,Karol;Zhou,Yubai;Rickershauser,Lindsey;Gajewska,EwaP.;Toutchkine,Alexei;Dittwald,Piotr;Startek,MichałP.;Kirkovits,GregoryJ.;Roszak,Rafał;Adamski,Ariel;Sieredzińska,Bianka;Mrksich,Milan;Trice,SarahL.J.;Grzybowski,BartoszA.[Chem,2018,vol.4,#3,p.522-532]
[12]Chen,Hao;Chen,Xiuhui;Sun,Ping;Wu,Dan;Yue,Hu;Pan,Jintao;Li,Xinxuan;Zhang,Cheng;Wu,Xinyi;Hua,Lei;Hu,Wenhui;Yang,Zhongjin[BioorganicandMedicinalChemistryLetters,2021,vol.46]
[13]CurrentPatentAssignee:GUANGZHOUMEDICALUNIVERSITY-CN113200947,2021,A
[1]CurrentPatentAssignee:USVLTD.-EP2428511,2012,A1Locationinpatent:Page/Pagecolumn10-11
[2]CurrentPatentAssignee:USVLTD.-US2012/108828,2012,A1Locationinpatent:Page/Pagecolumn7
[3]CurrentPatentAssignee:JIANGSUHENGRUIMEDICINECO.,LTD.-EP2581369,2013,A1Locationinpatent:Paragraph0050
[4]CurrentPatentAssignee:JIANGSUHENGRUIMEDICINECO.,LTD.-US2013/72697,2013,A1Locationinpatent:Paragraph0054
[5]Mali,AnilC.;Ippar,SharadS.;Bodke,MahendraB.;Patil,NileshS.;Mathad,VijayavitthalT.[OrganicProcessResearchandDevelopment,2013,vol.17,#5,p.863-868]
[6]CurrentPatentAssignee:FRICHEMPRIVATE-WO2012/4658,2012,A2Locationinpatent:Page/Pagecolumn18
[7]CurrentPatentAssignee:SUNPHARMACEUTICALINDUSTRIESLIMITED-WO2012/120544,2012,A2Locationinpatent:Page/Pagecolumn19
[8]CurrentPatentAssignee:SANOFI-US2013/165675,2013,A1Locationinpatent:Paragraph0043